Abstract
The use of pre-procedural rinses has been investigated to reduce the number of viral particles and bacteria in aerosols, potentially decreasing the risk of cross-infection from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during medical and dental procedures. This review aims to confirm whether there is evidence in the literature describing a reduction in salivary load of SARS-CoV-2 when povidone-iodine (PVP-I) is used as a pre-intervention mouthwash. An search of the MEDLINE, Embase, SCOPUS, and the Cochrane library databases was conducted. The criteria used followed the PRISMA® Statement guidelines. Randomized controlled trials investigating the reduction of salivary load of SARS-CoV-2 using PVP-I were included. Ultimately, four articles were included that met the established criteria. According to the current evidence, PVP-I is effective against SARS-CoV-2 in saliva and could be implemented as a rinse before interventions to decrease the risk of cross-infection in healthcare settings.
1. Introduction
Aerosols are defined as particles of liquid or solid in gas and are ≤5 μm in diameter [1]. Due to their small size, they are inspirable and can remain suspended in the air for hours [2]. These particles are generated by aerosol-generating procedures (AGPs) in medical settings, including airway suctioning, bronchoscopies, and high-flow oxygen therapy, among many others [3]. Subsequently, aerosols in dental offices are generated by the frequent use of high-speed handpieces, ultrasonic devices, and 3-in-1 air-water syringes. For this reason, dentists are one of the collectives that have the highest risk of infection of COVID-19 due to the close proximity with the patients’ oral cavities and the numerous AGPs performed routinely [4]. Saliva and blood are main components for viral and bacterial spread; therefore, procedures that generate aerosols should be minimized. However, dental clinicians have a particularly limited range of options regarding treatments and armamentarium that do not generate aerosols [4].
The primary mode of transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is through aerosols and respiratory droplets generated during daily activities, such as speaking or coughing [5]. Several factors, including the immune response of the host, the pathogenicity of the virus, and the amount of infected particles, determine the susceptibility of being infected via an aerosol [6,7,8]. Furthermore, it has been demonstrated that COVID-19-positive patients present high viral loads in saliva [9,10]; therefore, healthcare professionals performing AGPs have a greater risk of becoming infected with SARS-CoV-2 [11].
For this reason, there have been several investigations attempting to mitigate the negative effects of aerosols during AGPs. Pre-procedural rinses have been explored to reduce the salivary load of different microorganisms and the colony-forming units (CFUs) in aerosols, which could potentially decrease the risk of cross-infection during medical and dental procedures [9,12,13,14,15]. One of the most predominant mouthwash solutions studied is povidone-iodine (PVP-I). This molecule is an iodophor globally used due to its broad-spectrum antiseptic properties with a low number of contraindications, including allergy to iodine, thyroid disease, and pregnancy [16]. Several in vitro studies and, more recently, randomized controlled trials (RCTs) using PVP-I as pre-procedural mouthwash have published their results.
Therefore, this study aimed to investigate the effectiveness of PVP-I used as a mouthwash to decrease the salivary viral load of SARS-CoV-2.
2. Materials and Methods
2.1. Protocol
This review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA®) Statement [17,18]. The protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42022303756.
2.2. Focused Question
A PICO (P, population/patient/problem; I, intervention; C, comparison; O, outcome) question was formulated based on the PRISMA® guidelines:
“In patients diagnosed with COVID-19 (P), does the use of PVP-I mouthwashes (I) compared to not prescribing them (C) reduce the viral load present in saliva (O)?”
2.3. Eligibility Criteria
Prior to the search, inclusion and exclusion criteria were defined:
2.3.1. Inclusion Criteria
Included studies were (a) RCTs; (b) studies in which the participants had a reverse-transcription polymerase chain reaction (RT-PCR) examination positive for SARS-CoV-2; (c) studies that used PVP-I as a form of intervention; and (d) studies published in English.
2.3.2. Exclusion Criteria
Excluded studies were the following: (a) animal studies; (b) experimental laboratory studies; (c) studies whose study base focused on other areas besides the oral cavity and/or oropharynx; (d) studies that did not evaluate the reduction of viral load in saliva; (e) non-RCTs; (f) systematic reviews and meta-analyses; (g) literature review studies; (h) case reports; (i) letters to the editor; (j) abstracts or conference papers; (k) comments; and (l) unpublished articles.
2.4. Information Sources and Search Strategy
The search was conducted in four different electronic databases: MEDLINE (via PubMed), Embase, SCOPUS, and the Cochrane library database.
The search strategy was carried out by two researchers independently (A.G.-S. and A.-O.S.-P.). The search was not time-restricted and was updated to January 2022. MeSH (Medical Subjects Headings) terms, keywords, and other free terms were used with Boolean operators (OR, AND) to merge searches: (‘povidone’ OR ‘povidone-iodine’ OR ‘polyvidone iodine’ OR iodopovidone’ OR ‘PVP-I’ OR ‘iodine’) AND (‘COVID-19’ OR ‘SARS-CoV-2’ OR ‘SARS’). These keywords were implemented in all databases according to the syntax rules of each database.
2.5. Study Records
The results were independently compared by two authors (A.G.-S. and A.-O.S.-P.) to guarantee completeness and removal of duplicates. Next, the title and abstract of the remaining articles were screened individually. Ultimately, full-text papers to be included in this study were selected following the criteria previously described. Disagreements over eligible articles were resolved by including a third author (J.-F.P.-C.) to reach a consensus.
2.6. Risk of Bias Assessment
The methodology of eligible studies was evaluated following the Joanna Briggs Institute (JBI) Critical Appraisal Tool [19] by two independent authors (A.G.-S. and A.-O.S.-P.). The studies were categorized as low-quality (0–7 domains) or high-quality assessment (8–13 domains). A third author (J.-F.P.-C) was included to resolve any disagreements between the two authors.
3. Results
3.1. Study Selection
The search strategy resulted in 630 articles. There were 218 duplicates; therefore, 412 remained. Then, two authors (A.G.-S. and A.-O.S.-P.) independently examined the titles and abstracts and excluded 375 articles that were beyond the scope of this study. Therefore, we obtained 37 possible references. After reading the full text of those 37 papers, 33 were excluded because they investigated areas other than oral cavity saliva and/or oropharyngeal saliva (n = 6) or were experimental laboratory studies (n = 9), systematic reviews (n = 1), literature reviews (n = 2), letters to the editor (n = 12), and commentaries (n = 3). Therefore, four studies were included in our systematic review (Figure 1).
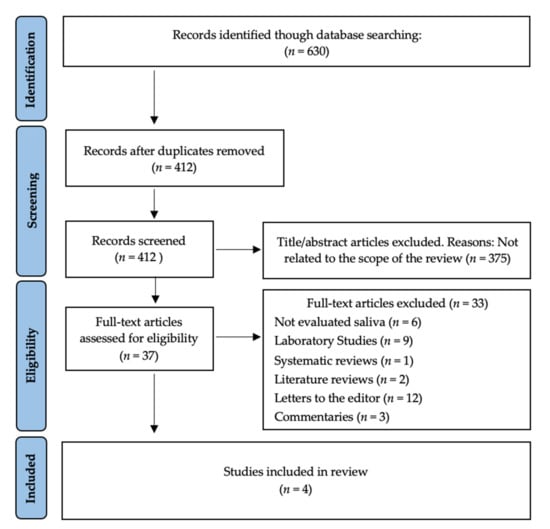
Figure 1.
PRISMA® flow diagram of the search processes and results.
3.2. Study Characteristics
All the studies included were RCTs published in 2020 and 2021. There are some discrepancies in the sample size of the different articles (ranging from 36 to 84). Due to the low number of studies available, it was decided that there would not be exclusion criteria set for a minimum of participants. The total number of patients included within the studies was 221. All patients recruited had a positive RT-PCR examination for SARS-CoV-2.
In these studies, rinsing times ranged between 30 s and 1 min. In the placebo groups, distilled water [20,21,22] and saline [23] were used. In the test group, several concentrations of PVP-I were used: 0.50% [21,23], 1% [22], and 2% [20]. All saliva samples were evaluated with RT-PCR. Baseline samples were collected immediately before the interventions. The number of saliva samples after interventions varies among the studies. One study collected one sample of saliva after intervention [22], one collected two samples [23], and two collected three samples [20,21].
A summary of the findings of the included articles is described in Table 1.

Table 1.
Results of the included RCTs.
The central findings of the resulting articles are described as follows:
Chaudhary et al. [23] (2021) evaluated the effect of 0.50% PVP-I, 1% hydrogen peroxide (HP), 0.12% chlorhexidine (CHX), and normal saline. Forty patients were randomly allocated into groups, and saliva samples were collected and tested with RT-PCR. In the PVP-I group, there was a median reduction of 61% and 97% at 15 and 45 min, respectively.
Seneviratne et al. [21] (2020) recruited 36 patients that were randomly allocated to four different groups: distilled water (control), 0.50% PVP-I, 0.20% CHX, and 0.075% cetylpyridinium chloride (CPC). Samples were collected before rinsing, 5 min, and 3 and 6 h post-rinse. Cycle threshold (Ct) changes were estimated at each time-point value. The PVP-I group exhibited greater changes in Ct values after 5 min and 3 h; however, there was a significant increase in the virucidal activity only at 6 h when compared to distilled water.
Elzein et al. [22] (2021) performed a triple-blinded RCT evaluating 1% PVP-I, 0.20% CHX, and distilled water (control) in 61 patients. Saliva samples were collected at baseline and 5 min post-rinse. The delta Ct values (4.72 ± 0.89) indicated a statistically significant reduction in the salivary viral load after using 1% PVP-I for 30 s compared to distilled water (0.519 ± 0.519).
Ferrer et al. [20] (2021) evaluated the differences in viral load of SARS-CoV-2 in 80 participants using 2% PVP-I, 1% HP, 0.07% CPC, 0.12% CHX, and distilled water (control). Samples were collected at baseline, 30, 60, and 120 min post-rinse. There was not a statistically significant reduction in salivary load when 2% PVP-I was used compared with the control group.
3.3. Risk Bias Assessment
Using the JBI Critical Appraisal Tool for RCTs [19], we determined that none of the articles presented a low-quality assessment (0–7 domains), and all of the articles included [20,21,22,23] had a high-quality assessment (8–13 domains). Table 2 shows a detailed description of the studies included.

Table 2.
JBI Critical Appraisal Tool [19] for RCTs. Reprinted with permission from JBI. Copyright 2020.
4. Discussion
The surface of SARS-CoV-2 presents a spike protein (S) involved in the receptor recognition and cell membrane fusion process. The S protein mediates cell entry when it contacts the angiotensin-converting enzyme 2 (ACE2) receptors [24], and oral mucosa and salivary gland epithelium present a great amount of these receptors [25,26,27]. In a study by Huang et al. [28], RNA molecules of SARS-CoV-2 were consistently found in ACE2-expressing ducts of salivary glands and in epithelial cells of the oral mucosa. They also proposed that the virus replicating in infected glands and the shedding of the infected oral mucosa are the sources of SARS-CoV-2 in saliva [28].
Patients undergoing medical and dental procedures were thoroughly screened for COVID-19 signs and symptoms as a means to prevent the risk of infection of healthcare providers. However, SARS-CoV-2 mRNA was detected in saliva of asymptomatic/pre-symptomatic patients [28]. For that reason, it might be beneficial to use mouthwashes, such as PVP-I, to decrease the risk of cross-infection in healthcare settings where AGPs are performed in both confirmed COVID-19 and asymptomatic patients.
PVP-I efficacy in reducing the salivary viral load was compared to other solutions in the articles included in this review. Chaudhary et al. [23] found that reduction in viral load at 15 and 45 min did not differ among 1% HP, 0.12% CHX, and 0.50% PVP-I. Similarly, Elzein et al. [22] did not find any significant difference in the reduction of salivary load between 0.20% CHX and 1% PVP-I, and both were significantly effective compared to distilled water. In the RCT by Seneviratne et al. [21], salivary Ct values within all groups at the different time periods did not demonstrate any significant differences. Nonetheless, compared to distilled water, CPC was significantly more effective at 5 min and 6 h, while PVP-I was only significantly more effective at the 6-h mark. Ferrer et al. [20] found no statistically significant changes in salivary load of SARS-CoV-2 in any of the mouthwashes evaluated. However, comparing the loads at baseline and after intervention, PVP-I and CPC groups showed mean reductions of 30%, with the highest activity 2 h after intervention. None of the articles included showed any complications after oral PVP-I use at different concentrations.
Various in vitro studies have also assessed the virucidal activity of PVP-I. Many studies used the logarithmic reductions scales of viral load in their results. As a reference, a 3log10 reduction equals a 99.90% reduction, and a 4log10 reduction equals a 99.99% reduction in viral load. Xu et al. [29] found a virucidal activity of >3log10 with a contact time of 30 min. Hassandarvish et al. [30] evaluated the reduction in salivary viral load using PVP-I at concentrations of 1% and 0.50%, which resulted in virucidal activities of >5log10 (> 99.99%) at 15 and 30 s, respectively. Similar studies found virucidal activities of >4log10 at 15 [31], 30 [32], and 60 s [33,34].
When evaluating PVP-I as a nasal rinse, Frank et al. [35] showed a complete inactivation of the virus using 0.50% PVP-I with a contact time of 15 s. Furthermore, a RCT study by Guenezan et al. [14] evaluated the reductions of viral titers in the nasopharynx using a 1% PVP-I rinse followed by 1% PVP-I nasal spray and an application of a 10% PVP-I balm over nasal mucosa during 7 days. The mean reductions in salivary load were 75% at day 1 compared with a reduction of 32% in the placebo group. However, there was no difference in the reduction of viral load over 7 days.
The results of this systematic review show that PVP-I is a potentially effective pre-procedural mouthwash to decrease the salivary viral load of symptomatic and asymptomatic COVID-19-positive patients. The prevention of the asymptomatic transmission of SARS-CoV-2 is still one of the biggest challenges [36], and the implementation of protocols to reduce the salivary load of SARS-CoV-2 before AGPs could play a significant role in decreasing the risk of cross-infection in healthcare settings.
4.1. Strengths and Limitations
This systematic review presents several strengths, including an unrestricted search in the literature, the search protocol, data retrieval, and risk assessment analysis performed in duplicate.
However, COVID-19 is a disease that is continuously being investigated, and multiple RCTs are evaluating the use of different mouthwashes in progress at this moment. In addition, this systematic review only included four RCTs; therefore, our results must be interpreted with caution, and further investigations must be carried out soon.
4.2. Recommendations for Further Research
This study systematically reviewed the first RCTs investigating PVP-I as a pre-procedural rinse. Further in vitro studies evaluating potential new mouthwash solutions and additional RCTs are needed to demonstrate the safety and efficacy of different mouthwashes.
5. Conclusions
Within the limitations of this study, PVP-I presents a significant virucidal activity against SARS-CoV-2 in saliva with concentrations ranging from 0.5% to 1%. On the other hand, concentrations of 2% did not show statistically significant changes in salivary load in one of the included studies. In clinical practice, a 30- or 60-s pre-procedural rinse of 0.50/1% PVP-I could be beneficial to reduce the risk of cross-infection in healthcare settings performing AGPs in diagnosed, suspected, or asymptomatic COVID-19-positive patients. However, these results should be taken with caution, as this review included a low number of studies, and additional RCTs are essential to confirm the validity of these findings.
Author Contributions
Conceptualization, A.G.-S. and A.-O.S.-P.; methodology, A.-O.S.-P.; validation, J.-F.P.-C., D.V. and N.K.; formal analysis, A.G.-S. and W.K.; investigation, D.V. and G.P.; resources, E.O.-F.; data curation, A.G. and L.-I.G.-G.; writing—original draft preparation, A.G.-S. and J.-F.P.-C.; writing—review and editing, E.O.-F. and M.M.-A.; visualization, E.O.-F.; supervision, A.-O.S.-P.; project administration, A.G.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tellier, R. Aerosol Transmission of Influenza A Virus: A Review of New Studies. J. R. Soc. Interface 2009, 6, 783–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.; Deibert, D.; Wyatt, G.; Durand-Moreau, Q.; Adisesh, A.; Khunti, K.; Khunti, S.; Smith, S.; Chan, X.H.S.; Ross, L.; et al. Classification of Aerosol-Generating Procedures: A Rapid Systematic Review. BMJ Open Respir. Res. 2020, 7, e000730. [Google Scholar] [CrossRef] [PubMed]
- Li, R.W.K.; Leung, K.W.C.; Sun, F.C.S.; Samaranayake, L.P. Severe Acute Respiratory Syndrome (SARS) and the GDP. Part II: Implications for GDPs. Br. Dent. J. 2004, 197, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ayeh, S.K.; Chidambaram, V.; Karakousis, P.C. Modes of Transmission of SARS-CoV-2 and Evidence for Preventive Behavioral Interventions. BMC Infect. Dis. 2021, 21, 496. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High Expression of ACE2 Receptor of 2019-NCoV on the Epithelial Cells of Oral Mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef]
- Bennett, A.M.; Fulford, M.R.; Walker, J.T.; Bradshaw, D.J.; Martin, M.V.; Marsh, P.D. Microbial Aerosols in General Dental Practice. Br. Dent. J. 2000, 189, 664–667. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; et al. Aerodynamic Analysis of SARS-CoV-2 in Two Wuhan Hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef]
- Vergara-Buenaventura, A.; Castro-Ruiz, C. Use of Mouthwashes against COVID-19 in Dentistry. Br. J. Oral Maxillofac. Surg. 2020, 58, 924–927. [Google Scholar] [CrossRef]
- To, K.K.-W.; Tsang, O.T.-Y.; Leung, W.-S.; Tam, A.R.; Wu, T.-C.; Lung, D.C.; Yip, C.C.-Y.; Cai, J.-P.; Chan, J.M.-C.; Chik, T.S.-H.; et al. Temporal Profiles of Viral Load in Posterior Oropharyngeal Saliva Samples and Serum Antibody Responses during Infection by SARS-CoV-2: An Observational Cohort Study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Van der Valk, J.P.M.; in ’t Veen, J.C.C.M. SARS-Cov-2: The Relevance and Prevention of Aerosol Transmission. J. Occup. Environ. Med. 2021, 63, e395–e401. [Google Scholar] [CrossRef] [PubMed]
- Carrouel, F.; Conte, M.P.; Fisher, J.; Gonçalves, L.S.; Dussart, C.; Llodra, J.C.; Bourgeois, D. COVID-19: A Recommendation to Examine the Effect of Mouthrinses with β-Cyclodextrin Combined with Citrox in Preventing Infection and Progression. J. Clin. Med. 2020, 9, 1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marui, V.C.; Souto, M.L.S.; Rovai, E.S.; Romito, G.A.; Chambrone, L.; Pannuti, C.M. Efficacy of Preprocedural Mouthrinses in the Reduction of Microorganisms in Aerosol. J. Am. Dent. Assoc. 2019, 150, 1015–1026.e1. [Google Scholar] [CrossRef] [PubMed]
- Guenezan, J.; Garcia, M.; Strasters, D.; Jousselin, C.; Lévêque, N.; Frasca, D.; Mimoz, O. Povidone Iodine Mouthwash, Gargle, and Nasal Spray to Reduce Nasopharyngeal Viral Load in Patients With COVID-19. JAMA Otolaryngol.–Head Neck Surg. 2021, 147, 400. [Google Scholar] [CrossRef]
- Rodríguez-Casanovas, H.J.; de la Rosa, M.; Bello-Lemus, Y.; Rasperini, G.; Acosta-Hoyos, A.J. Virucidal Activity of Different Mouthwashes Using a Novel Biochemical Assay. Healthcare 2021, 10, 63. [Google Scholar] [CrossRef]
- Chen, S.; Chen, J.W.; Guo, B.; Xu, C.C. Preoperative Antisepsis with Chlorhexidine Versus Povidone-Iodine for the Prevention of Surgical Site Infection: A Systematic Review and Meta-Analysis. World J. Surg. 2020, 44, 1412–1424. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- The Joanna Briggs Institute Checklist for Randomized Controlled Trials. Available online: http://joannabriggs.org/research/critical-appraisal-tools.html (accessed on 3 January 2022).
- Ferrer, M.D.; Barrueco, Á.S.; Martinez-Beneyto, Y.; Mateos-Moreno, M.V.; Ausina-Márquez, V.; García-Vázquez, E.; Puche-Torres, M.; Giner, M.J.F.; González, A.C.; Coello, J.M.S.; et al. Clinical Evaluation of Antiseptic Mouth Rinses to Reduce Salivary Load of SARS-CoV-2. Sci. Rep. 2021, 11, 24392. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Balan, P.; Ko, K.K.K.; Udawatte, N.S.; Lai, D.; Ng, D.H.L.; Venkatachalam, I.; Lim, K.S.; Ling, M.L.; Oon, L.; et al. Efficacy of Commercial Mouth-Rinses on SARS-CoV-2 Viral Load in Saliva: Randomized Control Trial in Singapore. Infection 2021, 49, 305–311. [Google Scholar] [CrossRef]
- Elzein, R.; Abdel-Sater, F.; Fakhreddine, S.; Hanna, P.A.; Feghali, R.; Hamad, H.; Ayoub, F. In Vivo Evaluation of the Virucidal Efficacy of Chlorhexidine and Povidone-Iodine Mouthwashes against Salivary SARS-CoV-2. A Randomized-Controlled Clinical Trial. J. Evid.-Based Dent. Pract. 2021, 21, 101584. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Melkonyan, A.; Meethil, A.; Saraswat, S.; Hall, D.L.; Cottle, J.; Wenzel, M.; Ayouty, N.; Bense, S.; Casanova, F.; et al. Estimating Salivary Carriage of Severe Acute Respiratory Syndrome Coronavirus 2 in Nonsymptomatic People and Efficacy of Mouthrinse in Reducing Viral Load: A Randomized Controlled Trial. J. Am. Dent. Assoc. 2021, 152, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Letko, M.; Marzi, A.; Munster, V. Functional Assessment of Cell Entry and Receptor Usage for SARS-CoV-2 and Other Lineage B Betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Carrouel, F.; Gonçalves, L.S.; Conte, M.P.; Campus, G.; Fisher, J.; Fraticelli, L.; Gadea-Deschamps, E.; Ottolenghi, L.; Bourgeois, D. Antiviral Activity of Reagents in Mouth Rinses against SARS-CoV-2. J. Dent. Res. 2021, 100, 124–132. [Google Scholar] [CrossRef]
- Cavalcante-Leão, B.L.; de Araujo, C.; Basso, I.; Schroder, A.; Guariza-Filho, O.; Ravazzi, G.; Gonçalves, F.; Zeigelboim, B.; Santos, R.; Stechman-Neto, J. Is There Scientific Evidence of the Mouthwashes Effectiveness in Reducing Viral Load in Covid-19? A Systematic Review. J. Clin. Exp. Dent. 2021, 13, e179–e189. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Stamatelopoulos, K.; Terpos, E.; Tsitsilonis, O.E.; Aivalioti, E.; Paraskevis, D.; Kastritis, E.; Pavlakis, G.N.; Dimopoulos, M.A. Insights to SARS-CoV-2 Life Cycle, Pathophysiology, and Rationalized Treatments That Target COVID-19 Clinical Complications. J. Biomed. Sci. 2021, 28, 9. [Google Scholar] [CrossRef]
- Huang, N.; Pérez, P.; Kato, T.; Mikami, Y.; Okuda, K.; Gilmore, R.C.; Conde, C.D.; Gasmi, B.; Stein, S.; Beach, M.; et al. SARS-CoV-2 Infection of the Oral Cavity and Saliva. Nat. Med. 2021, 27, 892–903. [Google Scholar] [CrossRef]
- Xu, C.; Wang, A.; Hoskin, E.R.; Cugini, C.; Markowitz, K.; Chang, T.L.; Fine, D.H. Differential Effects of Antiseptic Mouth Rinses on SARS-CoV-2 Infectivity In Vitro. Pathogens 2021, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Hassandarvish, P.; Tiong, V.; Mohamed, N.A.; Arumugam, H.; Ananthanarayanan, A.; Qasuri, M.; Hadjiat, Y.; Abubakar, S. In Vitro Virucidal Activity of Povidone Iodine Gargle and Mouthwash against SARS-CoV-2: Implications for Dental Practice. Br. Dent. J. 2020. [Google Scholar] [CrossRef]
- Bidra, A.S.; Pelletier, J.S.; Westover, J.B.; Frank, S.; Brown, S.M.; Tessema, B. Comparison of In Vitro Inactivation of SARS CoV-2 with Hydrogen Peroxide and Povidone-Iodine Oral Antiseptic Rinses. J. Prosthodont. 2020, 29, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E.; Sivalingam, V.; Kang, A.E.Z.; Ananthanarayanan, A.; Arumugam, H.; Jenkins, T.M.; Hadjiat, Y.; Eggers, M. Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease. Infect. Dis. Ther. 2020, 9, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.S.; Tessema, B.; Frank, S.; Westover, J.B.; Brown, S.M.; Capriotti, J.A. Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations Against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2). Ear Nose Throat J. 2021, 100, 192S–196S. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Grover, V.; Singh, C.; Sharma, A.; Das, D.K.; Singh, P.; Thakur, K.G.; Ringe, R.P. Chlorhexidine: An Effective Anticovid Mouth Rinse. J. Indian Soc. Periodontol. 2021, 25, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Brown, S.M.; Capriotti, J.A.; Westover, J.B.; Pelletier, J.S.; Tessema, B. In Vitro Efficacy of a Povidone-Iodine Nasal Antiseptic for Rapid Inactivation of SARS-CoV-2. JAMA Otolaryngol.–Head Neck Surg. 2020, 146, 1054. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.; Yokoe, D.S.; Havlir, D.V. Asymptomatic Transmission, the Achilles’ Heel of Current Strategies to Control Covid-19. N. Engl. J. Med. 2020, 382, 2158–2160. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


