The Study of Variation of Metabolites by Sleep Deficiency, and Intervention Possibility of Aerobic Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
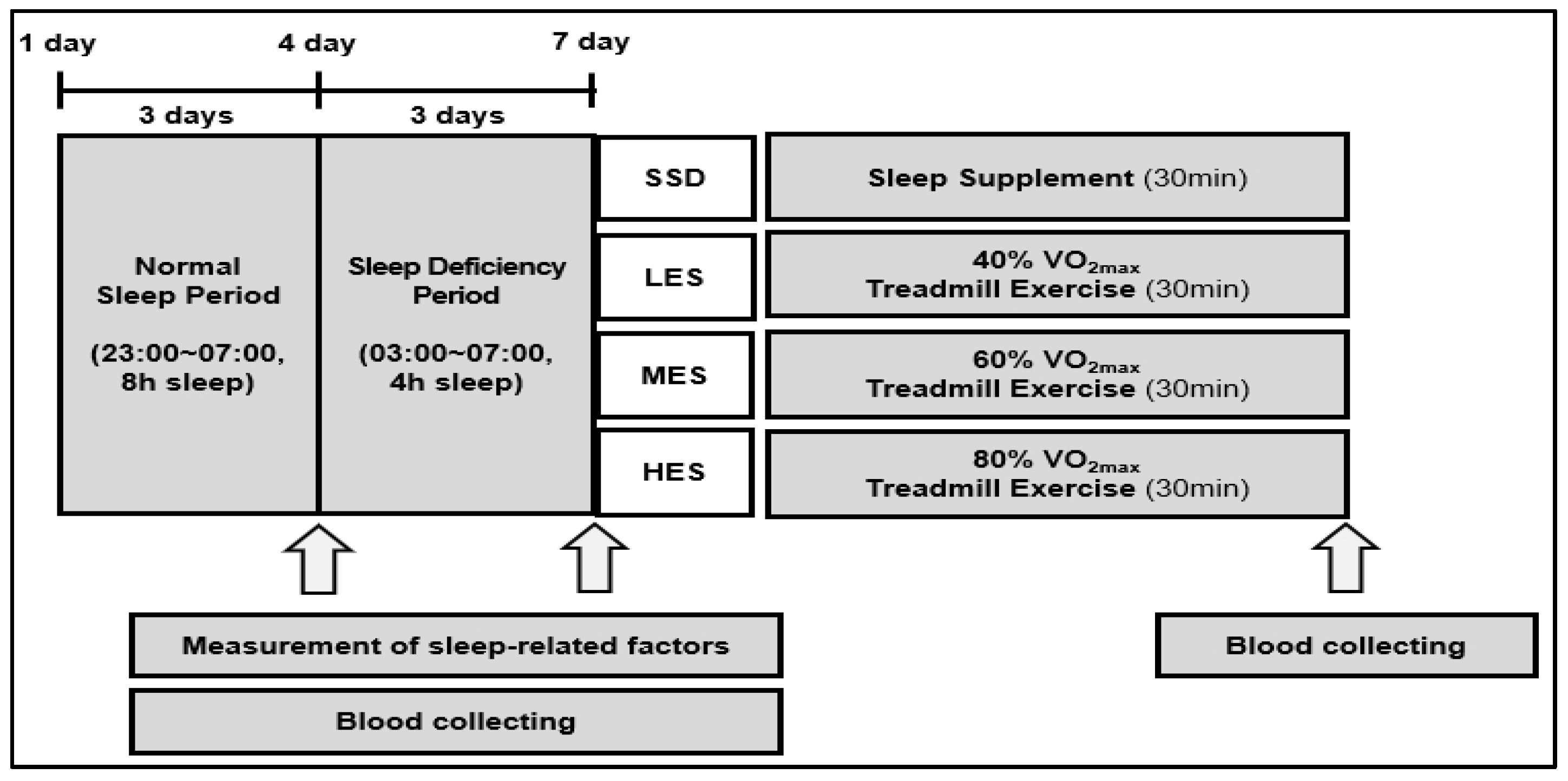
2.2. Experimental Protocol
2.3. Treadmill Test
2.4. Measurement of Sleep-Related Factors
2.5. Treatment of Groups
2.6. Metabolomics
2.6.1. Blood Sample Collection
2.6.2. Chemicals and Materials
2.6.3. Metabolite Extraction
2.6.4. Gas Chromatography-Time-of-Flight-Mass Spectrometry (GC-TOF-MS) Analysis
2.7. Data Processing and Statistical Analysis
3. Results
3.1. The Differences in Sleep-Related Factors between NS and SD
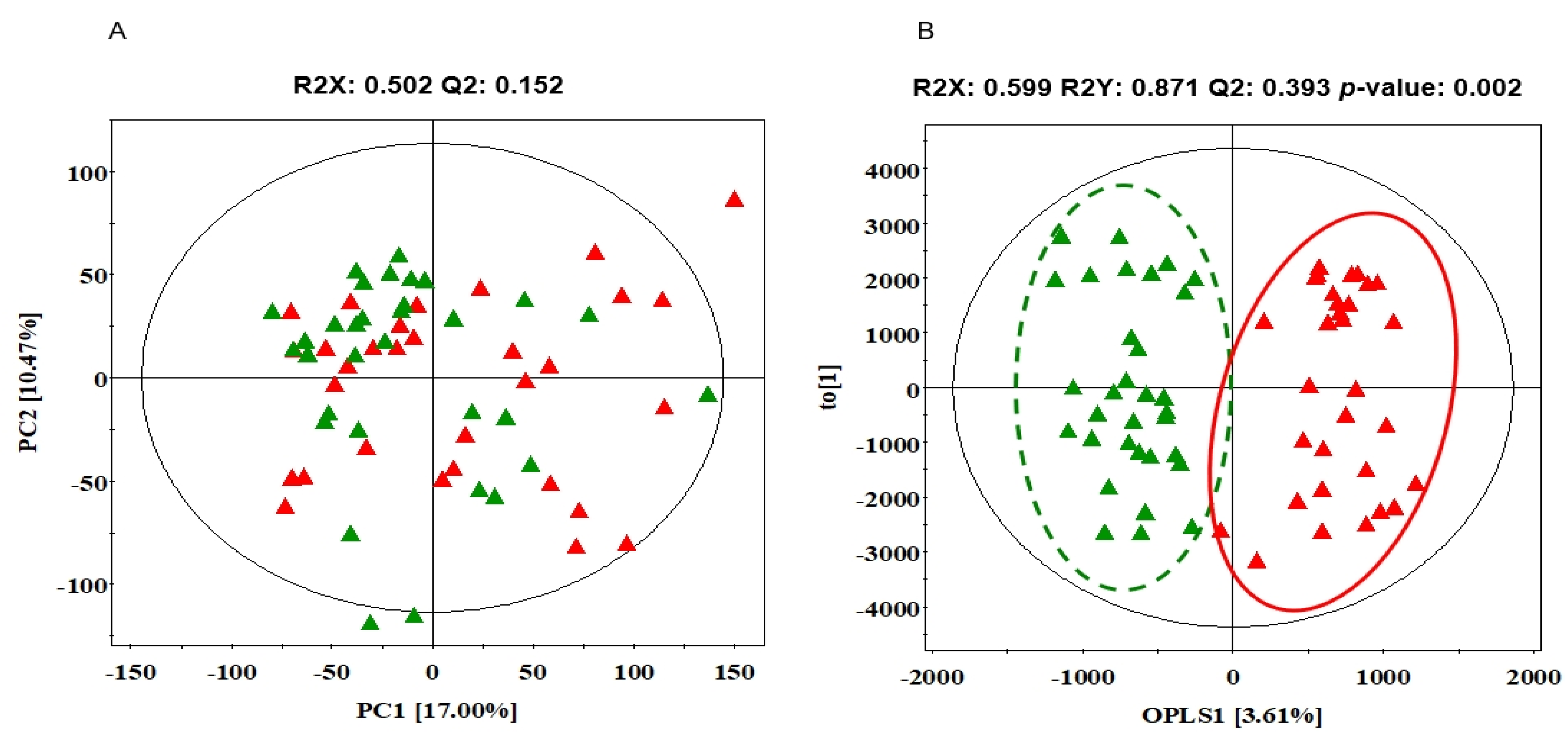
3.2. The Differences in Serum Metabolites between NS and SD
3.3. The Differences in Exercise-Related Factors among Exercise Groups
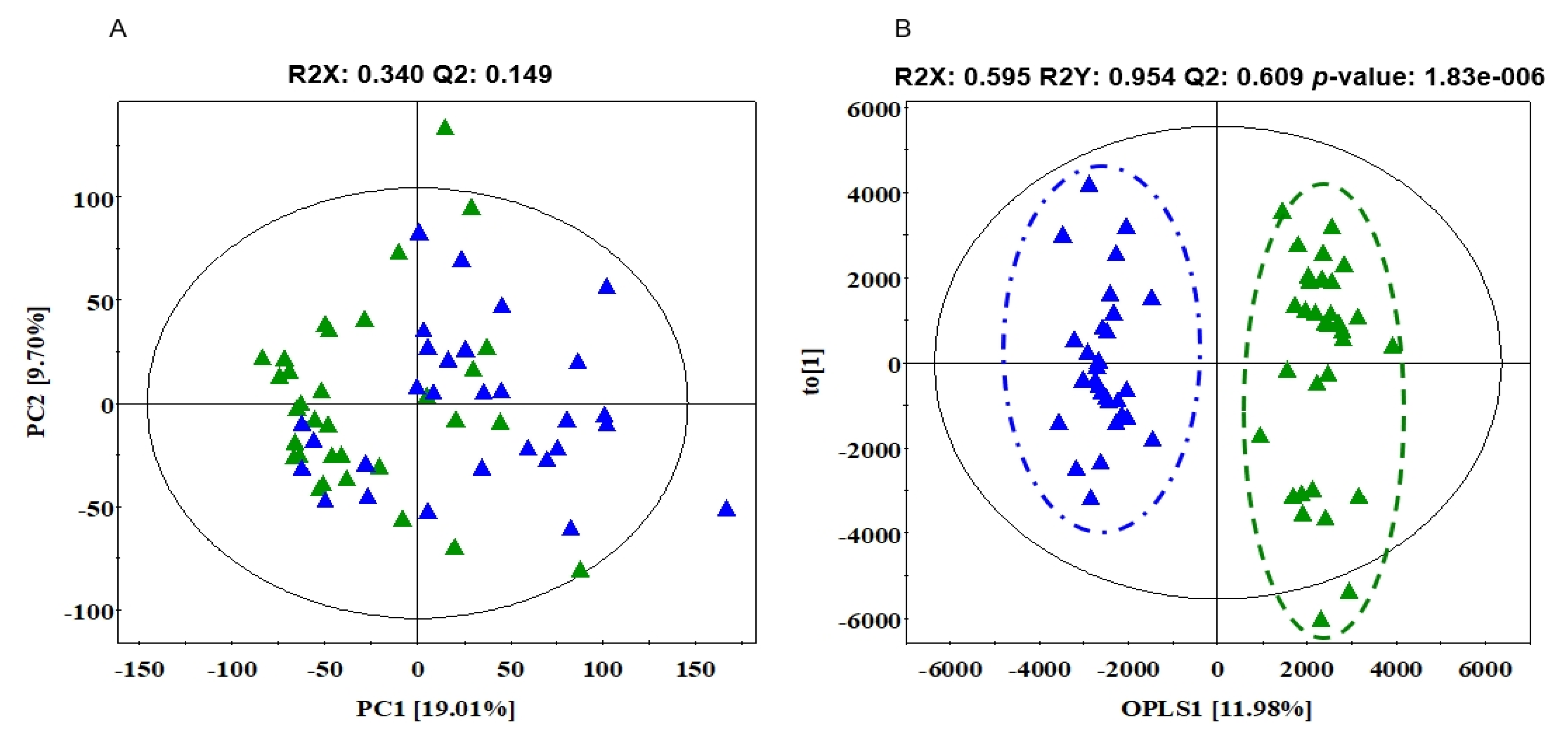
3.4. The Differences in Serum Metabolites between SD and AT
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cirelli, C. Cellular consequences of sleep deprivation in the brain. Sleep Med. Rev. 2006, 10, 307–321. [Google Scholar] [CrossRef]
- Schmidt, M.H.; Valatx, J.-L.; Sakai, K.; Fort, P.; Jouvet, M. Role of the Lateral Preoptic Area in Sleep-Related Erectile Mechanisms and Sleep Generation in the Rat. J. Neurosci. 2000, 20, 6640–6647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diekelmann, S.; Born, J. The memory function of sleep. Nat. Rev. Neurosci. 2010, 11, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Heo, N.B.; Byun, Y.H.; Hwang, M.H.; Kim, S.H. The Relation Analysis of Obesity and Blood Lipid on Total Physical Activity Level in Short Sleeping Adults. J. Sport Leis. Stud. 2014, 57, 879–892. [Google Scholar] [CrossRef]
- Ayas, N.T.; White, D.P.; Manson, J.E.; Stampfer, M.J.; Speizer, F.E.; Malhotra, A.; Hu, F.B. A Prospective Study of Sleep Duration and Coronary Heart Disease in Women. Arch. Intern. Med. 2003, 163, 205–209. [Google Scholar] [CrossRef]
- Gangwisch, J.E.; Heymsfield, S.B.; Boden-Albala, B.; Buijs, R.M.; Kreier, F.; Pickering, T.G.; Rundle, A.G.; Zammit, G.K.; Malaspina, D. Short Sleep Duration as a Risk Factor for Hypertension. Hypertension 2006, 47, 833–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiegel, K.; Knutson, K.; Leproult, R.; Tasali, E.; Van Cauter, E. Sleep loss: A novel risk factor for insulin resistance and Type 2 diabetes. J. Appl. Physiol. 2005, 99, 2008–2019. [Google Scholar] [CrossRef]
- Stefani, K.M.; Kim, H.C.; Kim, J.; Oh, K.; Suh, I. The influence of sex and age on the relationship between sleep duration and metabolic syndrome in Korean adults. Diabetes Res. Clin. Pract. 2013, 102, 250–259. [Google Scholar] [CrossRef]
- Williams, C.J.; Hu, F.B.; Patel, S.R.; Mantzoros, C.S. Sleep Duration and Snoring in Relation to Biomarkers of Cardiovascular Disease Risk Among Women With Type 2 Diabetes. Diabetes Care 2007, 30, 1233–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basner, M.; Fomberstein, K.M.; Razavi, F.M.; Banks, S.; William, J.H.; Rosa, R.R.; Dinges, D.F. American Time Use Survey: Sleep Time and Its Relationship to Waking Activities. Sleep 2007, 30, 1085–1095. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.; Li, X.; Li, H.; Jia, W. Toward Personalized Nutrition: Comprehensive Phytoprofiling and Metabotyping. J. Proteome Res. 2013, 12, 1547–1559. [Google Scholar] [CrossRef]
- Pechlivanis, A.; Kostidis, S.; Saraslanidis, P.; Petridou, A.; Tsalis, G.; Mougios, V.; Gika, H.G.; Mikros, E.; Theodoridis, G.A. 1H NMR-Based Metabonomic Investigation of the Effect of Two Different Exercise Sessions on the Metabolic Fingerprint of Human Urine. J. Proteome Res. 2010, 9, 6405–6416. [Google Scholar] [CrossRef]
- Davies, S.K.; Ang, J.E.; Revell, V.L.; Holmes, B.; Mann, A.; Robertson, F.P.; Cui, N.; Middleton, B.; Ackermann, K.; Kayser, M.; et al. Effect of sleep deprivation on the human metabolome. Proc. Natl. Acad. Sci. USA 2014, 111, 10761–10766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehrman, P.; Sengupta, A.; Harders, E.; Ubeydullah, E.; Pack, A.I.; Weljie, A. Altered diurnal states in insomnia reflect peripheral hyperarousal and metabolic desynchrony: A preliminary study. Sleep 2018, 41, zsy043. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.; Derkach, A.; Moore, S.C.; Zheng, W.; Shu, X.-O.; Gu, F.; Caporaso, N.E.; Sampson, J.N.; Matthews, C.E. Habitual sleep and human plasma metabolomics. Metabolomics 2017, 13, 63. [Google Scholar] [CrossRef]
- van den Berg, R.; Mook-Kanamori, D.O.; Donga, E.; van Dijk, M.; van Dijk, J.G.; Lammers, G.-J.; van Kralingen, K.W.; Prehn, C.; Adamski, J.; Romijn, J.A.; et al. A single night of sleep curtailment increases plasma acylcarnitines: Novel insights in the relationship between sleep and insulin resistance. Arch. Biochem. Biophys. 2016, 589, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Wu, H.-W.; Song, G.-Q.; Lu, C.; Li, Y.-H.; Qu, L.-N.; Chen, S.-G.; Liu, X.-M.; Chang, Q. Chronical sleep interruption-induced cognitive decline assessed by a metabolomics method. Behav. Brain Res. 2016, 302, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.N.; Kilkus, J.M.; Booth, J.N., 3rd; Bromley, L.E.; Imperial, J.G.; Penev, P.D. Effects of sleep restriction on the human plasma metabolome. Physiol. Behav. 2013, 122, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depner, C.M.; Cogswell, D.T.; Bisesi, P.J.; Markwald, R.R.; Cruickshank-Quinn, C.; Quinn, K.; Melanson, E.L.; Reisdorph, N.; Wright, K.P., Jr. Developing preliminary blood metabolomics-based biomarkers of insufficient sleep in humans. Sleep 2020, 43, zsz321. [Google Scholar] [CrossRef] [PubMed]
- Weljie, A.M.; Meerlo, P.; Goel, N.; Sengupta, A.; Kayser, M.; Abel, T.; Birnbaum, M.; Dinges, D.F.; Sehgal, A. Oxalic acid and diacylglycerol 36:3 are cross-species markers of sleep debt. Proc. Natl. Acad. Sci. USA 2015, 112, 2569–2574. [Google Scholar] [CrossRef] [Green Version]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 8th ed.; Lippincot Williams & Wilkins: Baltimore, MD, USA, 2010. [Google Scholar]
- Zagaar, M.; Dao, A.; Levine, A.; Alhaider, I.; Alkadhi, K. Regular Exercise Prevents Sleep Deprivation Associated Impairment of Long-Term Memory and Synaptic Plasticity in The CA1 Area of the Hippocampus. Sleep 2013, 36, 751–761. [Google Scholar] [CrossRef] [Green Version]
- Saadati, H.; Sheibani, V.; Esmaeili-Mahani, S.; Hajali, V.; Mazhari, S. Prior regular exercise prevents synaptic plasticity impairment in sleep deprived female rats. Brain Res. Bull. 2014, 108, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-Y.; Ho, K.-H.; Chen, H.-C.; Chien, M.-Y. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: A systematic review. J. Physiother. 2012, 58, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Dinges, D.F.; Pack, F.; Williams, K.; Gillen, K.A.; Powell, J.W.; Ott, G.E.; Aptowicz, C.; Pack, A. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep 1997, 20, 267–277. [Google Scholar] [PubMed]
- van Leeuwen, W.M.A.; Lehto, M.; Karisola, P.; Lindholm, H.; Luukkonen, R.; Sallinen, M.; Härmä, M.; Porkka-Heiskanen, T.; Alenius, H. Sleep Restriction Increases the Risk of Developing Cardiovascular Diseases by Augmenting Proinflammatory Responses through IL-17 and CRP. PLoS ONE 2009, 4, e4589. [Google Scholar] [CrossRef] [Green Version]
- Montgomery-Downs, H.E.; Insana, S.P.; Bond, J.A. Movement toward a novel activity monitoring device. Sleep Breath. 2012, 16, 913–917. [Google Scholar] [CrossRef]
- Buysse, D.J.; Hall, M.L.; Strollo, P.J.; Kamarck, T.W.; Owens, J.; Lee, L.; Reis, S.; Matthews, K.A. Relationships Between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and Clinical/Polysomnographic Measures in a Community Sample. J. Clin. Sleep Med. 2008, 4, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Levy, O.; Amit-Vazina, M.; Segal, R.; Tishler, M. Visual Analogue Scales of Pain, Fatigue and Function in Patients with Various Rheumatic Disorders Receiving Standard Care. Isr. Med. Assoc. J. IMAJ 2015, 17, 691–696. [Google Scholar]
- Dhand, R.; Sohal, H. Good sleep, bad sleep! The role of daytime naps in healthy adults. Curr. Opin. Intern. Med. 2007, 6, 91–94. [Google Scholar] [CrossRef]
- Faraut, B.; Andrillon, T.; Vecchierini, M.-F.; Leger, D. Napping: A public health issue. From epidemiological to laboratory studies. Sleep Med. Rev. 2017, 35, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.S.; Park, H.M.; Lee, K.-E.; Shin, J.-H.; Mun, S.; Kim, J.K.; Lee, S.J.; Liu, K.-H.; Hwang, J.-K.; Lee, C.H. A metabolomics approach shows that catechin-enriched green tea attenuates ultraviolet B-induced skin metabolite alterations in mice. Metabolomics 2014, 11, 861–871. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axelsson, J.; Sundelin, T.; Ingre, M.; Van Someren, E.J.W.; Olsson, A.; Lekander, M. Beauty sleep: Experimental study on the perceived health and attractiveness of sleep deprived people. BMJ 2010, 341, c6614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanlon, E.E.; Tasali, E.E.; Leproult, R.; Stuhr, K.K.; Doncheck, E.E.; De Wit, H.H.; Hillard, C.C.; Van Cauter, E. Sleep Restriction Enhances the Daily Rhythm of Circulating Levels of Endocannabinoid 2-Arachidonoylglycerol. Sleep 2016, 39, 653–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, H.G.; Reider, B.D.; Whiting, A.B.; Prichard, J.R. Sleep Patterns and Predictors of Disturbed Sleep in a Large Population of College Students. J. Adolesc. Health 2010, 46, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Philip, P.; Sagaspe, P.; Prague, M.; Tassi, P.; Capelli, A.; Bioulac, B.; Commenges, D.; Taillard, J. Acute Versus Chronic Partial Sleep Deprivation in Middle-Aged People: Differential Effect on Performance and Sleepiness. Sleep 2012, 35, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Pikovsky, O.; Oron, M.; Shiyovich, A.; Perry, Z.; Nesher, L. The impact of sleep deprivation on sleepiness, risk factors and professional performance in medical residents. Isr. Med. Assoc. J. IMAJ 2013, 15, 739–744. [Google Scholar]
- Purim, K.S.M.; Guimarães, A.T.B.; Titski, A.C.K.; Leite, N. Sleep deprivation and drowsiness of medical residents and medical students. Rev. Colégio Bras. Cir. 2016, 43, 438–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shechter, A.; O’Keeffe, M.; Roberts, A.L.; Zammit, G.K.; RoyChoudhury, A.; St-Onge, M.-P. Alterations in sleep architecture in response to experimental sleep curtailment are associated with signs of positive energy balance. Am. J. Physiol. Integr. Comp. Physiol. 2012, 303, R883–R889. [Google Scholar] [CrossRef]
- Macotela, Y.; Emanuelli, B.; Bång, A.M.; Espinoza, D.O.; Boucher, J.; Beebe, K.; Gall, W.; Kahn, C.R. Dietary Leucine—An Environmental Modifier of Insulin Resistance Acting on Multiple Levels of Metabolism. PLoS ONE 2011, 6, e21187. [Google Scholar] [CrossRef] [Green Version]
- Ferrando, A.A.; Williams, B.D.; Stuart, C.A.; Lane, H.W.; Wolfe, R.R. Oral Branched-Chain Amino Acids Decrease Whole-Body Proteolysis. J. Parenter. Enter. Nutr. 1995, 19, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Everson, C.A.; Szabo, A. Repeated Exposure to Severely Limited Sleep Results in Distinctive and Persistent Physiological Imbalances in Rats. PLoS ONE 2011, 6, e22987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedeltcheva, A.V.; Kilkus, J.M.; Imperial, J.; Schoeller, D.A.; Penev, P.D. Insufficient Sleep Undermines Dietary Efforts to Reduce Adiposity. Ann. Intern. Med. 2010, 153, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.J.; Pack, S.H.; Shin, M.H. The effect of the meridian massage on the change of the low back pain RPE (rating of perceived exertion) and the changes of serum cortisol as well as stress index of middle aged women. J. Korean Soc. Cosm. 2013, 19, 751–756. [Google Scholar]
- Buxton, O.M.; Pavlova, M.; Reid, E.W.; Wang, W.; Simonson, D.C.; Adler, G.K. Sleep Restriction for 1 Week Reduces Insulin Sensitivity in Healthy Men. Diabetes 2010, 59, 2126–2133. [Google Scholar] [CrossRef] [Green Version]
- Donga, E.; Van Dijk, M.; Van Dijk, J.G.; Biermasz, N.R.; Lammers, G.-J.; Van Kralingen, K.W.; Corssmit, E.P.M.; Romijn, J.A. A Single Night of Partial Sleep Deprivation Induces Insulin Resistance in Multiple Metabolic Pathways in Healthy Subjects. J. Clin. Endocrinol. Metab. 2010, 95, 2963–2968. [Google Scholar] [CrossRef] [Green Version]
- Nedeltcheva, A.V.; Kessler, L.; Imperial, J.; Penev, P.D. Exposure to Recurrent Sleep Restriction in the Setting of High Caloric Intake and Physical Inactivity Results in Increased Insulin Resistance and Reduced Glucose Tolerance. J. Clin. Endocrinol. Metab. 2009, 94, 3242–3250. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, K.A.; Punjabi, N.M. Effects of Sleep Fragmentation on Glucose Metabolism in Normal Subjects. Chest 2010, 137, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Spiegel, K.; Leproult, R.; Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Spiegel, K.; Leproult, R.; L’Hermite-Balériaux, M.; Copinschi, G.; Penev, P.D.; Van Cauter, E. Leptin Levels Are Dependent on Sleep Duration: Relationships with Sympathovagal Balance, Carbohydrate Regulation, Cortisol, and Thyrotropin. J. Clin. Endocrinol. Metab. 2004, 89, 5762–5771. [Google Scholar] [CrossRef] [Green Version]
- Mullington, J.M.; Haack, M.; Toth, M.; Serrador, J.M.; Meier-Ewert, H.K. Cardiovascular, Inflammatory, and Metabolic Consequences of Sleep Deprivation. Prog. Cardiovasc. Dis. 2009, 51, 294–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penev, P.D. Sleep deprivation and energy metabolism: To sleep, perchance to eat? Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 374–381. [Google Scholar] [CrossRef]
- Buijs, R.M.; Wortel, J.; Van Heerikhuize, J.J.; Feenstra, M.G.P.; Ter Horst, G.J.; Romijn, H.J.; Kalsbeek, A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur. J. Neurosci. 1999, 11, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Challet, E.; Malan, A.; Turek, F.W.; Van Reeth, O. Daily variations of blood glucose, acid-base state and PCO2 in rats: Effect of light exposure. Neurosci. Lett. 2003, 355, 131–135. [Google Scholar] [CrossRef]
- Kurotani, K.; Sato, M.; Ejima, Y.; Nanri, A.; Yi, S.; Pham, N.M.; Akter, S.; Poudel-Tandukar, K.; Kimura, Y.; Imaizumi, K.; et al. High levels of stearic acid, palmitoleic acid, and dihomo-γ-linolenic acid and low levels of linoleic acid in serum cholesterol ester are associated with high insulin resistance. Nutr. Res. 2012, 32, 669–675.e3. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, L.; Yang, X.; Shi, H.; Zhang, L. α-Linolenic acid prevents endoplasmic reticulum stress-mediated apoptosis of stearic acid lipotoxicity on primary rat hepatocytes. Lipids Health Dis. 2011, 10, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacca, A.; Xiao, C.; Oprescu, A.I.; Carpentier, A.C.; Lewis, G.F. Lipid-induced pancreatic β-cell dysfunction: Focus on in vivo studies. Am. J. Physiol. Metab. 2011, 300, E255–E262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodson, D.J.; Mitchell, R.K.; Bellomo, E.A.; Sun, G.; Vinet, L.; Meda, P.; Li, D.; Li, W.-H.; Bugliani, M.; Marchetti, P.; et al. Lipotoxicity disrupts incretin-regulated human β cell connectivity. J. Clin. Investig. 2013, 123, 4182–4194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, A.J.G.; Frederico, M.J.S.; Cazarolli, L.H.; Mendes, C.P.; Bretanha, L.C.; Schmidt, E.C.; Bouzon, Z.L.; de Medeiros Pinto, V.A.; da Fonte Ramos, C.; Pizzolatti, M.G. The mechanism of action of ursolic acid as insulin secretagogue and insu-linomimetic is mediated by cross-talk between calcium and kinases to regulate glucose balance. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2015, 1850, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.P.; Yoon, J.H.; Jung, I.G.; Kim, J.O.; Seo, T.B.; Kim, H.; Kim, C.J.; Chae, J.R. The effect of exercise intensity on blood insulin and GLUT-4 protein expression of skeletal muscle in STZ-diabetic rats. Exerc. Sci. 2004, 13, 211–221. [Google Scholar]
- Adeva-Andany, M.; Souto-Adeva, G.; Ameneiros-Rodríguez, E.; Fernández-Fernández, C.; Donapetry-García, C.; Domínguez-Montero, A. Insulin resistance and glycine metabolism in humans. Amino Acids 2017, 50, 11–27. [Google Scholar] [CrossRef]
- Grandy, D.K.; Marchionni, M.A.; Makam, H.; Stofko, R.E.; Alfano, M.; Frothingham, L.; Fischer, J.B.; Burke-Howie, K.J.; Bunzow, J.R.; Server, A.C. Cloning of the cDNA and gene for a human D2 dopamine receptor. Proc. Natl. Acad. Sci. USA 1989, 86, 9762–9766. [Google Scholar] [CrossRef] [Green Version]
- Gainetdinov, R.R.; Caron, M.G. Monoamine Transporters: From Genes to Behavior. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 261–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Swinderen, B.; Andretic, R. Dopamine in Drosophila: Setting arousal thresholds in a miniature brain. Proc. R. Soc. B Boil. Sci. 2011, 278, 906–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruddick, J.P.; Evans, A.K.; Nutt, D.J.; Lightman, S.L.; Rook, G.A.; Lowry, C.A. Tryptophan metabolism in the central nervous system: Medical implications. Expert Rev. Mol. Med. 2006, 8, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Turner, E.H.; Loftis, J.M.; Blackwell, A.D. Serotonin a la carte: Supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol. Ther. 2006, 109, 325–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Abri, M.A. Sleep Deprivation and Depression: A bi-directional association. Sultan Qaboos Univ. Med. J. 2015, 15, e4. [Google Scholar]
- Hemmeter, U.-M.; Hemmeter-Spernal, J.; Krieg, J.-C. Sleep deprivation in depression. Expert Rev. Neurother. 2010, 10, 1101–1115. [Google Scholar] [CrossRef]
- Christiansen, L.; Tan, Q.; Iachina, M.; Bathum, L.; Kruse, T.A.; McGue, M.; Christensen, K. Candidate Gene Polymorphisms in the Serotonergic Pathway: Influence on Depression Symptomatology in an Elderly Population. Biol. Psychiatry 2007, 61, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Amini-Khoei, H.; Mohammadi-Asl, A.; Alijanpour, S.; Haj-Mirzaian, A.; Rahimi-Balaei, M.; Razmi, A.; Olson, C.O.; Rastegar, M.; Mehdizadeh, M.; et al. Involvement of D1 and D2 dopamine receptors in the antidepressant-like effects of selegiline in maternal separation model of mouse. Physiol. Behav. 2016, 163, 107–114. [Google Scholar] [CrossRef]
- Klumpers, U.M.H.; Veltman, D.J.; Van Tol, M.-J.; Kloet, R.W.; Boellaard, R.; Lammertsma, A.A.; Hoogendijk, W.J.G. Neurophysiological Effects of Sleep Deprivation in Healthy Adults, a Pilot Study. PLoS ONE 2015, 10, e0116906. [Google Scholar] [CrossRef] [Green Version]
- Melancon, M.O.; Lorrain, D.; Dionne, I.J. Exercise and sleep in aging: Emphasis on serotonin. Pathol. Biol. 2014, 62, 276–283. [Google Scholar] [CrossRef]
- Meeusen, R.; Piacentini, M.F.; Kempenaers, F.; Busschaert, B.; De Schutter, G.; Buyse, L.; De Meirleir, K. Brain neuro-transmitter levels during exercise. Deut. Z. Sportmed. 2001, 52, 361–368. [Google Scholar]
- Yoon, M.-C.; Shin, M.-S.; Kim, T.-S.; Kim, B.-K.; Ko, I.-G.; Sung, Y.-H.; Kim, S.-E.; Lee, H.-H.; Kim, Y.-P.; Kim, C.-J. Treadmill exercise suppresses nigrostriatal dopaminergic neuronal loss in 6-hydroxydopamine-induced Parkinson’s rats. Neurosci. Lett. 2007, 423, 12–17. [Google Scholar] [CrossRef]
- Chiappelli, J.; Rowland, L.; Wijtenburg, S.A.; Muellerklein, F.; Tagamets, M.; McMahon, R.P.; Gaston, F.; Kochunov, P.; Hong, L.E. Evaluation of Myo-Inositol as a Potential Biomarker for Depression in Schizophrenia. Neuropsychopharmacology 2015, 40, 2157–2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.-P.; Dai, H.-Y.; Dai, Z.-Z.; Xu, C.-T.; Wu, R.-H. Anterior cingulate cortex and cerebellar hemisphere neurometabolite changes in depression treatment: A1H magnetic resonance spectroscopy study. Psychiatry Clin. Neurosci. 2014, 68, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Ranaei, A.; Najafizadeh-Sari, S. Anti-Depressant Effects of Palmitic Acid: The Necessity of Focusing on New Agents. Int. J. Med. Rev. 2018, 4, 93–94. [Google Scholar] [CrossRef]
- Thomas, K.C.; Hynes, S.H.; Ingledew, W.M. Influence of medium buffering capacity on inhibition of Saccharomyces cerevisiae growth by acetic and lactic acids. Appl. Environ. Microbiol. 2002, 68, 1616–1623. [Google Scholar] [CrossRef] [Green Version]
| Variables | SSD (n = 8) | LES (n = 8) | MES (n = 8) | HES (n = 8) | F | p |
|---|---|---|---|---|---|---|
| Age (years) | 20.38 ± 0.52 | 21.88 ± 2.10 | 21.38 ± 2.00 | 21.50 ± 1.77 | 1.172 | 0.361 |
| Height (cm) | 176.88 ± 4.36 | 176.88 ± 3.27 | 174.63 ± 4.47 | 174.13 ± 5.19 | 0.887 | 0.460 |
| Weight (kg) | 75.23 ± 8.82 | 78.68 ± 11.78 | 70.38 ± 7.13 | 70.78 ± 5.74 | 1.657 | 0.199 |
| BMI (kg/m2) | 24.03 ± 2.14 | 25.06 ± 2.95 | 23.05 ± 1.63 | 23.36 ± 2.21 | 1.216 | 0.322 |
| Muscle mass (kg) | 35.26 ± 3.41 | 35.51 ± 3.22 | 33.38 ± 3.79 | 33.70 ± 2.65 | 0.863 | 0.472 |
| Body fat percentage (%) | 17.40 ± 3.50 | 19.90 ± 5.16 | 16.40 ± 2.23 | 16.09 ± 6.03 | 1.193 | 0.331 |
| SBP (mmHg) | 114.75 ± 6.25 | 114.00 ± 9.61 | 115.63 ± 9.01 | 116.50 ± 6.33 | 1.051 | 0.385 |
| DBP (mmHg) | 74.50 ± 5.86 | 71.63 ± 7.87 | 74.13 ± 5.72 | 71.38 ± 7.44 | 0.464 | 0.710 |
| VO2max (ml/kg/min) | 53.33 ± 4.57 | 52.12 ± 6.77 | 51.79 ± 5.37 | 53.29 ± 7.40 | 0.134 | 0.939 |
| PSQI (point) | 4.13 ± 0.64 | 3.25 ± 1.28 | 3.25 ± 0.71 | 3.25 ± 1.48 | 1.285 | 0.299 |
| ESS (point) | 4.00 ± 0.93 | 3.13 ± 1.46 | 4.25 ± 1.03 | 3.63 ± 0.92 | 1.567 | 0.219 |
| Variables | NS | SD | t | p |
|---|---|---|---|---|
| TST (min) | 400.90 ± 17.32 | 216.28 ± 16.56 | 54.710 | <0.001 |
| Duration of REM (min) | 80.89 ± 17.51 | 37.16 ± 11.26 | 13.905 | <0.001 |
| Duration of light sleep (min) | 248.33 ± 28.52 | 138.14 ± 18.11 | 23.941 | <0.001 |
| Duration of deep sleep (min) | 71.68 ± 18.28 | 40.99 ± 11.58 | 10.449 | <0.001 |
| Steps during the day (steps) | 10,637.06 ± 3549.80 | 10,961.25 ± 2644.61 | −0.514 | 0.611 |
| Calorie consumption (kcal) | 2884.61 ± 455.95 | 2907.91 ± 343.72 | −0.371 | 0.713 |
| PSQI (points) | 3.13 ± 1.39 | 6.16 ± 1.35 | −11.333 | <0.001 |
| ESS (points) | 4.34 ± 2.31 | 11.00 ± 4.56 | −9.012 | <0.001 |
| VAS (points) | 3.64 ± 1.50 | 7.32 ± 1.17 | −13.118 | <0.001 |
| No | Ret (min) | Metabolites | Unique Mass | Fragment Pattern (m/z) | ID | Fold Change (SD/NS) |
|---|---|---|---|---|---|---|
| Amino acids | ||||||
| 1 | 6.78 | Valine | 218 | 73, 144, 218, 147, 100, 74, 146, 219, 156 | STD/MS | 1.034 |
| 2 | 7.32 | Leucine | 158 | 73, 158, 147, 159, 75, 103, 117, 160 | STD/MS | 1.115 *** |
| 3 | 7.54 | Isoleucine | 158 | 73, 158, 218, 147, 159, 100, 75, 74 | STD/MS | 1.105 ** |
| 4 | 7.59 | Proline | 142 | 142, 73, 143, 75, 147, 74, 70, 66, 144 | STD/MS | 1.187 |
| 5 | 7.67 | Glycine | 86 | 73, 174, 86, 147, 100, 175, 59, 248, 176 | STD/MS | 0.953 |
| 6 | 8.13 | Serine | 218 | 73, 204, 218, 100, 147, 75, 205, 74 | STD/MS | 1.082 |
| 7 | 8.40 | Threonine | 219 | 73, 57, 117, 101, 219, 147, 100, 129 | STD/MS | 1.042 |
| 8 | 9.58 | trans-4-hydoxyl-L-proline | 156 | 156, 73, 147, 157, 230, 75, 258, 14, 158 | STD/MS | 0.971 |
| 9 | 10.27 | Ornithine | 142 | 73, 70, 142, 74, 75, 102, 147, 144 | STD/MS | 1.288 |
| 10 | 10.41 | Phenylalanine | 218 | 73, 218, 192, 100, 147, 75, 74, 219, 193 | STD/MS | 1.041 |
| Sugars and Sugar alcohols | ||||||
| 11 | 12.09 | Saccharide 1 | 73 | 73, 147, 217, 191, 103, 129, 218, 75 | MS | 0.977 |
| 12 | 12.26 | Fructose | 103 | 73, 103, 217, 147, 307, 74, 133, 75 | STD/MS | 0.727 |
| 13 | 12.39 | Galactose | 204 | 73, 204, 191, 147, 205, 217, 129, 75, 103 | STD/MS | 0.734 * |
| 14 | 12.48 | Glucose | 205 | 73, 147, 205, 160, 103, 217, 319, 74 | STD/MS | 1.046 |
| 15 | 12.60 | Glucose | 205 | 73, 147, 103, 205, 160, 129, 217, 157, 319 | STD/MS | 1.129 * |
| 16 | 12.81 | Saccharide 2 | 217 | 73, 217, 75, 147, 103, 129, 74, 117, 59 | MS | 0.877 |
| 17 | 12.94 | Saccharide 3 | 204 | 73, 204, 147, 191, 217, 75, 205, 74, 129 | MS | 0.779 * |
| 18 | 13.27 | Saccharide 4 | 204 | 73, 204, 147, 205, 75, 217, 74, 129 | MS | 1.317 *** |
| Fatty acids | ||||||
| 19 | 13.22 | Palmitic acid | 313 | 117, 75, 73, 132, 129, 131, 118, 133, 313 | STD/MS | 1.097 |
| 20 | 14.40 | Stearic acid | 341 | 117, 73, 132, 129, 145, 131, 133, 118, 341 | STD/MS | 1.120 * |
| Non-identification | ||||||
| 21 | 4.14 | N.I.1 | 151 | 171, 73, 78 64, 172, 151, 173, 186 | - | 0.709 |
| 22 | 6.03 | N.I.2 | 86 | 73, 147, 133, 59, 86, 100, 72, 89 | - | 0.794 ** |
| 23 | 6.20 | N.I.3 | 147 | 147, 73, 117, 75, 66, 191, 88, 148 | - | 1.086 |
| 24 | 7.64 | N.I.4 | 107 | 107, 73, 77, 256, 55, 84, 140 | - | 0.901 |
| 25 | 7.94 | N.I.5 | 184 | 184, 73, 134, 77, 86, 59, 100, 285 | - | 0.848 |
| 26 | 9.89 | N.I.6 | 147 | 73, 147, 177, 292, 220, 103, 102, 130 | - | 0.247 * |
| 27 | 10.16 | N.I.7 | 227 | 73, 227, 147, 155, 154, 139, 59, 75 | - | 1.280 ** |
| 28 | 11.44 | N.I.8 | 299 | 73, 299, 147, 357, 103, 101, 75, 129 | - | 0.730 ** |
| Variables | LES (a) | MES (b) | HES (c) | F | p | Post Hoc |
|---|---|---|---|---|---|---|
| VO2 (ml/kg/min) | 21.25 ± 2.74 | 33.71 ± 2.96 | 43.31 ± 4.93 | 72.320 | <0.001 | a < b < c |
| HR (beat) | 120.60 ± 9.36 | 145.41 ± 9.28 | 172.15 ± 2.83 | 87.783 | <0.001 | a < b < c |
| RER | 0.82 ± 0.02 | 0.85 ± 0.02 | 0.89 ± 0.04 | 12.760 | <0.001 | a < b < c |
| Running speed (mph) | 2.32 ± 0.32 | 3.08 ± 0.22 | 4.16 ± 0.65 | 35.651 | <0.001 | a < b < c |
| Calorie consumption (kcal) | 250.25 ± 25.52 | 352.75 ± 56.53 | 455.88 ± 48.32 | 41.033 | <0.001 | a < b < c |
| Running distance (m) | 2297.25 ± 250.89 | 2912.25 ± 142.36 | 3651.38 ± 457.08 | 37.766 | <0.001 | a < b < c |
| No | Ret (min) | Metabolites | Unique Mass | Fragment Pattern (m/z) | ID | Fold Change | |||
|---|---|---|---|---|---|---|---|---|---|
| SSD/SD | LES/SD | MES/SD | HES/SD | ||||||
| Organic acids | |||||||||
| 1 | 5.19 | Lactic acid | 73 | 73, 117, 147, 66, 75, 191, 88, 59 | STD/MS | 1.116 | 1.050 | 1.074 | 1.338 * |
| 2 | 5.88 | Oxalic acid | 131 | 73, 131, 147, 75, 66, 74, 132, 148 | STD/MS | 1.480 * | 0.955 | 0.955 | 1.501 * |
| 3 | 7.41 | Phosphoric acid | 299 | 73, 299, 133, 300, 74, 314, 207, 193, 75 | STD/MS | 0.821 | 0.705 * | 0.784 * | 0.632 * |
| Amino acids | |||||||||
| 4 | 6.78 | Valine | 218 | 73, 144, 218, 147, 100, 74, 146, 219, 156 | STD/MS | 1.185 * | 1.103 | 1.089 | 1.118 |
| 5 | 7.32 | Leucine | 158 | 73, 158, 147, 159, 75, 103, 117, 160 | STD/MS | 1.554 | 2.029 | 1.392 | 1.610 |
| 6 | 7.54 | Isoleucine | 158 | 73, 158, 218, 147, 159, 100, 75, 74 | STD/MS | 0.975 | 0.939 | 0.922 | 0.957 |
| 7 | 7.67 | Glycine | 86 | 73, 174, 86, 147, 100, 175, 59, 248, 176 | STD/MS | 1.200 | 1.403 | 1.350 * | 1.365 * |
| 8 | 8.13 | Serine | 218 | 73, 204, 218, 100, 147, 75, 205, 74 | STD/MS | 1.112 | 0.947 | 1.167 | 1.200 |
| 9 | 8.40 | Threonine | 219 | 73, 57, 117, 101, 219, 147, 100, 129 | STD/MS | 1.029 | 1.030 | 1.089 | 1.121 |
| 10 | 10.41 | Phenylalanine | 218 | 73, 218, 192, 100, 147, 75, 74, 219, 193 | STD/MS | 1.054 | 1.111 | 1.032 | 1.104 |
| 11 | 12.53 | Lysine | 156 | 73, 174, 156, 147, 75, 59, 128, 205 | STD/MS | 1.071 * | 1.368 *** | 1.282 * | 1.539 *** |
| 12 | 12.65 | Tyrosine | 218 | 73, 218, 100, 75, 147, 74, 219, 179, 103 | STD/MS | 1.057 | 1.251 * | 1.031 | 1.029 |
| 13 | 14.44 | Tryptophan | 202 | 74, 291, 204, 147, 218, 100, 117, 131, 129 | STD/MS | 1.152 | 1.267 * | 1.031 | 1.029 |
| Sugars and Sugar alcohols | |||||||||
| 14 | 12.09 | Saccharide 1 | 73 | 73, 147, 217, 191, 103, 129, 218, 75 | MS | 1.042 | 1.001 | 0.911 | 0.773 |
| 15 | 12.26 | Fructose | 103 | 73, 103, 217, 147, 307, 74, 133, 75 | STD/MS | 1.303 * | 1.695 *** | 1.358 | 1.409 * |
| 16 | 12.39 | Galactose | 204 | 73, 204, 191, 147, 205, 217, 129, 75, 103 | STD/MS | 0.991 | 1.139 | 1.195 | 1.428 * |
| 17 | 12.48 | Glucose | 205 | 73, 147, 205, 160, 103, 217, 319, 74 | STD/MS | 0.783 | 0.651 * | 0.762 | 0.611 * |
| 18 | 12.60 | Glucose | 205 | 73, 147, 103, 205, 160, 129, 217, 157, 319 | STD/MS | 0.954 | 0.861 * | 0.923 | 0.787 * |
| 19 | 12.81 | Saccharide 2 | 217 | 73, 217, 75, 147, 103, 129, 74, 117, 59 | MS | 1.357 * | 1.809 *** | 1.434 * | 1.493 * |
| 20 | 12.94 | Saccharide 3 | 204 | 73, 204, 147, 191, 217, 75, 205, 74, 129 | MS | 0.960 | 1.111 | 1.102 | 1.168 |
| 21 | 13.27 | Saccharide 4 | 204 | 73, 204, 147, 205, 75, 217, 74, 129 | MS | 0.827 * | 0.962 * | 1.051 | 0.790 * |
| 22 | 13.70 | myo-Inositol | 217 | 73, 147, 217, 305, 129, 133, 103 | STD/MS | 1.568 | 1.433 * | 1.537 * | 1.672 * |
| Fatty acids and Lipids | |||||||||
| 23 | 13.22 | Palmitic acid | 313 | 117, 75, 73, 132, 129, 131, 118, 133, 313 | STD/MS | 1.034 | 1.246 * | 1.267 * | 1.143 |
| 24 | 14.40 | Stearic acid | 341 | 117, 73, 132, 129, 145, 131, 133, 118, 341 | STD/MS | 1.175 | 1.207 | 1.195 | 1.014 |
| 25 | 15.40 | Oleamide | 338 | 116, 128, 55, 69, 115, 132, 198, 145, 338 | STD/MS | 0.954 | 1.071 * | 1.002 | 1.221 |
| 26 | 19.92 | Cholesterol | 129 | 81, 55, 107, 57, 105, 91, 121, 119, 93 | STD/MS | 1.455 | 1.399 | 1.252 | 1.824 * |
| Non-identification | |||||||||
| 27 | 5.45 | N.I.9 | 72 | 72, 55, 75, 146, 130, 156 | - | 0.654 | 0.556 * | 0.594 | 0.773 |
| 28 | 5.62 | N.I.10 | 116 | 116, 73, 147, 117, 75, 59, 118, 103 | - | 1.312 | 1.154 | 1.265 | 1.587 *** |
| 29 | 6.03 | N.I.11 | 86 | 73, 147, 133, 59, 86, 100, 72, 89 | - | 1.011 | 0.819 | 1.136 | 1.111 |
| 30 | 7.64 | N.I.12 | 107 | 107, 73, 77, 256, 55, 84, 140 | - | 1.181 * | 1.256 * | 1.125 | 1.150 |
| 31 | 7.94 | N.I.13 | 184 | 184, 73, 134, 77, 86, 59, 100, 285 | - | 0.864 | 0.856 | 0.823 | 0.771 |
| 32 | 11.44 | N.I.14 | 299 | 73, 299, 147, 357, 103, 101, 75, 129 | - | 1.222 | 1.208 | 1.161 | 1.358 * |
| 33 | 13.51 | N.1.15 | 122 | 55, 69, 122, 136, 56, 83, 67, 54, 70 | - | 1.182 | 1.527 * | 1.055 | 0.998 |
| 34 | 14.98 | N.1.16 | 203 | 55, 216, 148, 69, 131, 74, 204, 67, 54 | - | 1.372 | 2.213 * | 1.321 | 1.750 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-S.; Kim, Y.-J.; Heo, W.; Kim, S. The Study of Variation of Metabolites by Sleep Deficiency, and Intervention Possibility of Aerobic Exercise. Int. J. Environ. Res. Public Health 2022, 19, 2774. https://doi.org/10.3390/ijerph19052774
Park J-S, Kim Y-J, Heo W, Kim S. The Study of Variation of Metabolites by Sleep Deficiency, and Intervention Possibility of Aerobic Exercise. International Journal of Environmental Research and Public Health. 2022; 19(5):2774. https://doi.org/10.3390/ijerph19052774
Chicago/Turabian StylePark, Jong-Suk, Young-Jun Kim, Wan Heo, and Sangho Kim. 2022. "The Study of Variation of Metabolites by Sleep Deficiency, and Intervention Possibility of Aerobic Exercise" International Journal of Environmental Research and Public Health 19, no. 5: 2774. https://doi.org/10.3390/ijerph19052774
APA StylePark, J.-S., Kim, Y.-J., Heo, W., & Kim, S. (2022). The Study of Variation of Metabolites by Sleep Deficiency, and Intervention Possibility of Aerobic Exercise. International Journal of Environmental Research and Public Health, 19(5), 2774. https://doi.org/10.3390/ijerph19052774






