Trends in the Epidemiology and Outcomes of Pneumocystis Pneumonia among Human Immunodeficiency Virus (HIV) Hospitalizations
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Design
2.2. Study Groups and Outcomes
2.3. Statistical Analyses
3. Results
3.1. PCP Incidence, Demographic Characteristics and Prevelance
3.2. Prevalence
3.3. Length of Stay Outcome
3.4. In-Hospital Mortality Outcome
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNAIDS. 90-90-90: Treatment for All. Available online: https://www.unaids.org/en/resources/909090 (accessed on 12 February 2022).
- Frank, T.D.; Carter, A.; Jahagirdar, D.; Biehl, M.H.; Douwes-Schultz, D.; Larson, S.L.; Arora, M.; Dwyer-Lindgren, L.; Steuben, K.M.; Abbastabar, H.; et al. Global, Regional, and National Incidence, Prevalence, and Mortality of HIV, 1980–2017, and Forecasts to 2030, for 195 Countries and Territories: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV 2019, 6, e831–e859. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Centers for Disease Control and Prevention. Estimated HIV Incidence and Prevalence in the United States, 2015–2019. HIV Surveillance Supplemental Report 2021, 26 (No. 1). Published May 2021. Available online: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (accessed on 13 February 2022).
- Pneumocystis Pneumonia—Los Angeles. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/june_5.htm (accessed on 12 February 2022).
- Kaplan, J.E.; Hanson, D.L.; Navin, T.R.; Jones, J.L. Risk Factors for PrimaryPneumocystis CariniiPneumonia in Human Immunodeficiency Virus-Infected Adolescents and Adults in the United States: Reassessment of Indications for Chemoprophylaxis. J. Infect. Dis. 1998, 178, 1126–1132. [Google Scholar] [CrossRef]
- Kaplan, J.E.; Hanson, D.L.; Jones, J.L.; Dworkin, M.S. Viral Load as an Independent Risk Factor for Opportunistic Infections in HIV-Infected Adults and Adolescents. AIDS 2001, 15, 1831–1836. [Google Scholar] [CrossRef]
- Selwyn, P.A.; Pumerantz, A.S.; Durante, A.; Alcabes, P.G.; Gourevitch, M.N.; Boiselle, P.G.; Elmore, J.G. Clinical Predictors of Pneumocystis Carinii Pneumonia, Bacterial Pneumonia and Tuberculosis in HIV-Infected Patients. AIDS 1998, 12, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Palella, F.J.; Delaney, K.M.; Moorman, A.C.; Loveless, M.O.; Fuhrer, J.; Satten, G.A.; Aschman, D.J.; Holmberg, S.D. Declining Morbidity and Mortality among Patients with Advanced Human Immunodeficiency Virus Infection. N. Engl. J. Med. 1998, 338, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Crum-Cianflone, N.F.; Grandits, G.; Echols, S.; Ganesan, A.; Landrum, M.; Weintrob, A.; Barthel, R.; Agan, B. Trends and Causes of Hospitalizations among HIV-Infected Persons during the Late HAART Era: What Is the Impact of CD4 Counts and HAART Use? JAIDS J. Acquir. Immune Defic. Syndr. 2010, 54, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Buchacz, K.; Baker, R.K.; Moorman, A.C.; Richardson, J.T.; Wood, K.C.; Holmberg, S.D.; Brooks, J.T. Rates of Hospitalizations and Associated Diagnoses in a Large Multisite Cohort of HIV Patients in the United States, 1994–2005. AIDS 2008, 22, 1345–1354. [Google Scholar] [CrossRef]
- Limper, A.H.; Offord, K.P.; Smith, T.F.; Martin, W.J. Pneumocystis CariniiPneumonia: Differences in Lung Parasite Number and Inflammation in Patients with and without AIDS. Am. Rev. Respir. Dis. 1989, 140, 1204–1209. [Google Scholar] [CrossRef]
- Roux, A.; Canet, E.; Valade, S.; Gangneux-Robert, F.; Hamane, S.; Lafabrie, A.; Maubon, D.; Debourgogne, A.; Le Gal, S.; Dalle, F.; et al. Pneumocystis Jirovecii Pneumonia in Patients with or without AIDS, France. Emerg. Infect. Dis. 2014, 20, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, A.-L.; Traore, K.; Plekhanova, I.; Bouchrik, M.; Bossard, C.; Picot, S. Pneumocystis Pneumonia Suspected Cases in 604 Non-HIV and HIV Patients. Int. J. Infect. Dis. 2016, 46, 11–17. [Google Scholar] [CrossRef] [PubMed]
- HCUP-US NIS Overview. Available online: https://www.hcup-us.ahrq.gov/nisoverview.jsp (accessed on 14 January 2022).
- NIS Trend Weights. Available online: https://www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp (accessed on 14 January 2022).
- HCUP Methods Series Calculating National Inpatient Sample (NIS) Variances for Data Years 2012 and Later. Available online: https://www.hcup-us.ahrq.gov/reports/methods/2015_09.jsp (accessed on 14 January 2022).
- Elixhauser Comorbidity Software, Version 3.7. Available online: https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp (accessed on 14 January 2022).
- Khera, R.; Angraal, S.; Couch, T.; Welsh, J.W.; Nallamothu, B.K.; Girotra, S.; Chan, P.S.; Krumholz, H.M. Adherence to Methodological Standards in Research Using the National Inpatient Sample. JAMA 2017, 318, 2011. [Google Scholar] [CrossRef]
- Kovacs, J.A.; Masur, H. Evolving Health Effects of Pneumocystis. JAMA 2009, 301, 2578. [Google Scholar] [CrossRef]
- Variable Impact on Mortality of AIDS-Defining Events Diagnosed during Combination Antiretroviral Therapy: Not All AIDS-Defining Conditions Are Created Equal. Clin. Infect. Dis. 2009, 48, 1138–1151. [CrossRef]
- Walzer, P.D.; Evans, H.E.R.; Copas, A.J.; Edwards, S.G.; Grant, A.D.; Miller, R.F. Early Predictors of Mortality from Pneumocystis Jirovecii Pneumonia in HIV-Infected Patients: 1985–2006. Clin. Infect. Dis. 2008, 46, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Radhi, S.; Alexander, T.; Ukwu, M.; Saleh, S.; Morris, A. Outcome of HIV-Associated Pneumocystis Pneumonia in Hospitalized Patients from 2000 through 2003. BMC Infect. Dis. 2008, 8, 118. [Google Scholar] [CrossRef]
- Fei, M.W.; Sant, C.A.; Kim, E.J.; Swartzman, A.; Davis, J.L.; Jarlsberg, L.G.; Huang, L. Severity and Outcomes of Pneumocystis Pneumonia in Patients Newly Diagnosed with HIV Infection: An Observational Cohort Study. Scand. J. Infect. Dis. 2009, 41, 672–678. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakanjako, D.; Kiragga, A.N.; Musick, B.S.; Yiannoutsos, C.T.; Wools-Kaloustian, K.; Diero, L.; Oyaro, P.; Lugina, E.; Ssali, J.C.; Kambugu, A.; et al. Frequency and Impact of Suboptimal Immune Recovery on First-Line Antiretroviral Therapy within the International Epidemiologic Databases to Evaluate AIDS in East Africa. AIDS 2016, 30, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- López-Sánchez, C.; Falcó, V.; Burgos, J.; Navarro, J.; Martín, M.T.; Curran, A.; Miguel, L.; Ocaña, I.; Ribera, E.; Crespo, M.; et al. Epidemiology and Long-Term Survival in HIV-Infected Patients with Pneumocystis Jirovecii Pneumonia in the HAART Era. Medicine 2015, 94, e681. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.E.; Hanson, D.; Dworkin, M.S.; Frederick, T.; Bertolli, J.; Lindegren, M.L.; Holmberg, S.; Jones, J.L. Epidemiology of Human Immunodeficiency Virus-Associated Opportunistic Infections in the United States in the Era of Highly Active Antiretroviral Therapy. Clin. Infect. Dis. 2000, 30 (Suppl. 1), S5–S14. [Google Scholar] [CrossRef] [PubMed]
- Sepkowitz, K.A. Pneumocystis Carinii Pneumonia without Acquired Immunodeficiency Syndrome. More Patients, Same Risk. Arch. Intern. Med. 1995, 155, 1125–1128. [Google Scholar] [CrossRef]
- Kamanfu, G.; Mlika-Cabanne, N.; Girard, P.-M.; Nimubona, S.; Mpfizi, B.; Cishako, A.; Roux, P.; Coulaud, J.-P.; Larouzé, B.; Aubry, P.; et al. Pulmonary Complications of Human Immunodeficiency Virus Infection in Bujumbura, Burundi. Am. Rev. Respir. Dis. 1993, 147, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Batungwanayo, J.; Taelman, H.; Dhote, R.; Bogaerts, J.; Allen, S.; van de Perre, P. Pulmonary Tuberculosis in Kigali, Rwanda: Impact of Human Immunodeficiency Virus Infection on Clinical and Radiographic Presentation. Am. Rev. Respir. Dis. 1992, 146, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, S.; Engel, M.E.; Griesel, R.; Mendelson, M. Burden of Pneumocystis Pneumonia in HIV-Infected Adults in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2016, 16, 482. [Google Scholar] [CrossRef] [PubMed]
- Fischi, M.A. Safety and Efficacy of Sulfamethoxazole and Trimethoprim Chemoprophylaxis for Pneumocystis Carinii Pneumonia in AIDS. JAMA 1988, 259, 1185. [Google Scholar] [CrossRef]
- Schneider, M.M.E.; Hoepelman, A.I.M.; Schattenkerk, J.K.M.E.; Nielsen, T.L.; van der Graaf, Y.; Frissen, J.P.H.J.; van der Ende, I.M.E.; Kolsters, A.F.P.; Borleffs, J.C.C. A Controlled Trial of Aerosolized Pentamidine or Trimethoprim–Sulfamethoxazole as Primary Prophylaxis AgainstPneumocystis CariniiPneumonia in Patients with Human Immunodeficiency Virus Infection. N. Engl. J. Med. 1992, 327, 1836–1841. [Google Scholar] [CrossRef] [PubMed]
- Hardy, W.D.; Feinberg, J.; Finkelstein, D.M.; Power, M.E.; He, W.; Kaczka, C.; Frame, P.T.; Holmes, M.; Waskin, H.; Fass, R.J.; et al. A Controlled Trial of Trimethoprim–Sulfamethoxazole or Aerosolized Pentamidine for Secondary Prophylaxis of Pneumocystis Carinii Pneumonia in Patients with the Acquired Immunodeficiency Syndrome. N. Engl. J. Med. 1992, 327, 1842–1848. [Google Scholar] [CrossRef]
- Hanna, D.B.; Hessol, N.A.; Golub, E.T.; Cocohoba, J.M.; Cohen, M.H.; Levine, A.M.; Wilson, T.E.; Young, M.; Anastos, K.; Kaplan, R.C. Increase in Single-Tablet Regimen Use and Associated Improvements in Adherence-Related Outcomes in HIV-Infected Women. JAIDS J. Acquir. Immune Defic. Syndr. 2014, 65, 587–596. [Google Scholar] [CrossRef]
- Mannheimer, S.B.; Wang, L.; Wilton, L.; Van Tieu, H.; del Rio, C.; Buchbinder, S.; Fields, S.; Glick, S.; Connor, M.B.; Cummings, V.; et al. Infrequent HIV Testing and Late HIV Diagnosis Are Common among a Cohort of Black Men Who Have Sex with Men in 6 US Cities. JAIDS J. Acquir. Immune Defic. Syndr. 2014, 67, 438–445. [Google Scholar] [CrossRef]
- Dailey, A.F.; Hoots, B.E.; Hall, H.I.; Song, R.; Hayes, D.; Fulton, P.; Prejean, J.; Hernandez, A.L.; Koenig, L.J.; Valleroy, L.A. Vital Signs: Human Immunodeficiency Virus Testing and Diagnosis Delays—United States. MMWR. Morb. Mortal. Wkly. Rep. 2017, 66, 1300–1306. [Google Scholar] [CrossRef]
- HIV Risk, Prevention, and Testing Behaviors Among Heterosexuals at Increased Risk for HIV Infection—National HIV Behavioral Surveillance System, 21 U.S. Cities. 2010. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/ss6314a1.htm (accessed on 15 February 2022).
- Centers for Disease Control and Prevention. HIV Surveillance Report; 2015; Volume 27, Published November 2016. Available online: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (accessed on 13 February 2022).
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; Gamble, T.; Hosseinipour, M.C.; Kumarasamy, N.; Hakim, J.G.; Kumwenda, J.; Grinsztejn, B.; Pilotto, J.H.S.; et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N. Engl. J. Med. 2016, 375, 830–839. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Z.; Wang, Y.; Hao, Y.; Wang, F.; Gao, G.; Yang, D.; Xiao, J.; Zhao, H. A Model to Predict In-Hospital Mortality in HIV/AIDS Patients with Pneumocystis Pneumonia in China: The Clinical Practice in Real World. BioMed Res. Int. 2019, 2019, 6057028. [Google Scholar] [CrossRef] [PubMed]
- Alvaro-Meca, A.; Palomares-Sancho, I.; Diaz, A.; Resino, R.; De Miguel, A.G.; Resino, S. Pneumocystis Pneumonia in HIV-Positive Patients in Spain: Epidemiology and Environmental Risk Factors. J. Int. AIDS Soc. 2015, 18, 19906. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-W.; Lin, C.-C.; Kuo, C.-F.; Liu, C.-P.; Lee, C.-M. Mortality Predictors of Pneumocystis Jirovecii Pneumonia in Human Immunodeficiency Virus-Infected Patients at Presentation: Experience in a Tertiary Care Hospital of Northern Taiwan. J. Microbiol. Immunol. Infect. 2011, 44, 274–281. [Google Scholar] [CrossRef][Green Version]
- Sun, J.; Su, J.; Xie, Y.; Yin, M.T.; Huang, Y.; Xu, L.; Zhou, Q.; Zhu, B. Plasma IL-6/IL-10 Ratio and IL-8, LDH, and HBDH Level Predict the Severity and the Risk of Death in AIDS Patients WithPneumocystisPneumonia. J. Immunol. Res. 2016, 2016, 1583951. [Google Scholar] [CrossRef]
- Cowell, A.; Shenoi, S.V.; Kyriakides, T.C.; Friedland, G.; Barakat, L.A. Trends in Hospital Deaths among Human Immunodeficiency Virus-Infected Patients during the Antiretroviral Therapy Era, 1995 to 2011. J. Hosp. Med. 2015, 10, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Psevdos, G.; Gonzalez, E.; Singh, S.; Kilayko, M.C.; Sharp, V. All-Cause Mortality in Hospitalized HIV-Infected Patients at an Acute Tertiary Care Hospital with a Comprehensive Outpatient HIV Care Program in New York City in the Era of Highly Active Antiretroviral Therapy (HAART). Infection 2012, 41, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Huson, M.A.M.; Grobusch, M.P.; van der Poll, T. The Effect of HIV Infection on the Host Response to Bacterial Sepsis. Lancet Infect. Dis. 2015, 15, 95–108. [Google Scholar] [CrossRef]
- Jordano, Q.; Falco, V.; Almirante, B.; Planes, A.M.; del Valle, O.; Ribera, E.; Len, O.; Pigrau, C.; Pahissa, A. Invasive Pneumococcal Disease in Patients Infected with HIV: Still a Threat in the Era of Highly Active Antiretroviral Therapy. Clin. Infect. Dis. 2004, 38, 1623–1628. [Google Scholar] [CrossRef]
- Lombardi, F. Weekend Effect on Acute MI Mortality. Eur. Heart J. 2018, 39, 2698. [Google Scholar] [CrossRef]
- Liu, L.; Hao, D.; Liu, W.; Wang, L.; Wang, X. Does Weekend Hospital Admission Affect Upper Gastrointestinal Hemorrhage Outcomes? J. Clin. Gastroenterol. 2020, 54, 55–62. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; McGinley, E.L.; Saeian, K. Outcomes of Weekend Admissions for Upper Gastrointestinal Hemorrhage: A Nationwide Analysis. Clin. Gastroenterol. Hepatol. 2009, 7, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, K.; Murthi, S.; Elango, K.; Rahi, M.S.; Thilagar, B.; Ramalingam, S.; Voruganti, D.; Paramasivam, V.K.; Kolandaivel, K.P.; Arora, A.; et al. The Impact of Diabetes Mellitus in Patients with Chronic Obstructive Pulmonary Disease (COPD) Hospitalization. J. Clin. Med. 2021, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, K.; Voruganti, D.C.; Singh Rahi, M.; Elango, K.; Ramalingam, S.; Geeti, A.; Kwon, J. Trends in Prevalence and Outcomes of Cannabis Use among Chronic Obstructive Pulmonary Disease Hospitalizations: A Nationwide Population-Based Study 2005–2014. Cannabis Cannabinoid Res. 2021, 6, 340–348. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | All HIV (n = 3,011,724) | HIV without PCP (n = 2,863,099) | HIV with PCP (n = 148,624) | p-Value |
|---|---|---|---|---|
| Age | 45.18 ± 11.109 | 45.35 ± 11.133 | 42.08 ± 10.141 | <0.0001 |
| Length of stay | 6.54 ± 8.938 | 6.33 ± 8.742 | 10.59 ± 11.378 | <0.0001 |
| Total charges | 38,243.79 ± 66,337 | 36,860.45 ± 63,561 | 65,090.83 ± 103,153 | <0.0001 |
| Sex | ||||
| Male | 1,981,195 (65.8%) | 1,876,654 (65.5%) | 104,542 (70.3%) | <0.0001 |
| Female | 1,030,528 (34.2%) | 986,446 (34.5%) | 44,083 (29.7%) | <0.0001 |
| Race (uniform) | ||||
| White | 745,951 (24.8%) | 711,292 (27.9%) | 34,659 (26.8%) | <0.0001 |
| Black | 1,426,627 (47.4%) | 1,358,620 (53.3%) | 68,006 (52.7%) | <0.0001 |
| Hispanic | 370,092 (12.3%) | 350,334 (13.8%) | 19,758 (15.3%) | <0.0001 |
| Asian or Pacific Islander | 15,160 (0.5%) | 13,781 (0.5%) | 1379 (1.1%) | <0.0001 |
| Native American | 9370 (0.3%) | 8853 (0.3%) | 517 (0.4%) | <0.0001 |
| Others | 108,755 (3.6%) | 103,914 (4.1%) | 4841 (3.7%) | <0.0001 |
| Region of Hospital | ||||
| Northeast | 991,494 (32.9%) | 957,493 (33.4%) | 34,001 (22.9%) | <0.0001 |
| Midwest or North Central | 367,812 (12.2%) | 352,235 (12.3%) | 15,577 (10.5%) | <0.0001 |
| South | 1344,453 (44.6%) | 1,265,753 (44.2%) | 78,701 (53%) | <0.0001 |
| West | 307,964 (10.2%) | 287,618 (10%) | 20,346 (13.7%) | <0.0001 |
| Died | 98,361 (3.3%) | 83,666 (2.9%) | 14,695 (9.9%) | <0.0001 |
| Admission day is a weekend | 634,783 (21.1%) | 601,454 (21%) | 33,328 (22.4%) | <0.0001 |
| Disposition of patient (uniform) | ||||
| Routine | 2,108,095 (70%) | 2,008,111 (70.1%) | 99,984 (67.3%) | <0.0001 |
| Short-term hospital | 53,929 (1.8%) | 51,218 (1.8%) | 2712 (1.8%) | <0.0001 |
| Skilled Nursing Facility (SNF) | 332,526 (11%) | 318,668 (11.1%) | 13,858 (9.3%) | <0.0001 |
| Intermediate Care Facility (ICF) | 252,057 (8.4%) | 241,519 (8.4%) | 10,538 (7.1%) | <0.0001 |
| Another type of facility | 165,155 (5.5%) | 158,414 (5.5%) | 6741 (4.5%) | <0.0001 |
| Home Health Care (HHC) | 98,361 (3.3%) | 83,666 (2.9%) | 14,695 (9.9%) | <0.0001 |
| Against medical advice (AMA) | 1600 (0.1%) | 1503 (0.1%) | 97 (0.1%) | <0.0001 |
| Elective admission | 352,729 (11.7%) | 345,138 (12.1%) | 7591 (5.1%) | <0.0001 |
| Primary expected payer (uniform) | ||||
| Medicare | 878,453 (29.2%) | 853,848 (29.9%) | 24,604 (16.7%) | <0.0001 |
| Medicaid | 1,222,318 (40.6%) | 1,164,708 (40.8%) | 57,610 (39%) | <0.0001 |
| Private insurance | 484,582 (16.1%) | 451,652 (15.8%) | 32,930 (22.3%) | <0.0001 |
| Self-pay | 267,607 (8.9%) | 245,845 (8.6%) | 21,761 (14.7%) | <0.0001 |
| No charge | 40,276 (1.3%) | 37,399 (1.3%) | 2877 (1.9%) | <0.0001 |
| Other | 109,624 (3.6%) | 101,666 (3.6%) | 7958 (5.4%) | <0.0001 |
| Median household income quartile for patient’s ZIP Code | ||||
| 0–25th percentile | 1,298,141 (43.1%) | 1,233,011 (49.5%) | 65,130 (48.1%) | <0.0001 |
| 26th to 50th percentile (median) | 603,834 (20%) | 572,324 (23%) | 31,510 (23.3%) | <0.0001 |
| 51st to 75th percentile | 446,650 (14.8%) | 422,667 (17%) | 23,983 (17.7%) | <0.0001 |
| 76th to 100th percentile | 280,062 (9.3%) | 265,201 (10.6%) | 14,861 (11%) | <0.0001 |
| Bed size of hospital | ||||
| Small | 273,420 (9.1%) | 261,546 (9.2%) | 11,873 (8%) | <0.0001 |
| Medium | 748,044 (24.8%) | 710,475 (24.9%) | 37,569 (25.4%) | <0.0001 |
| Large | 1,979,628 (65.7%) | 1,880,911 (65.9%) | 98,718 (66.6%) | <0.0001 |
| Control/ownership of hospital | ||||
| Government or private (collapsed category) | 1,825,278 (60.6%) | 1,732,348 (77.6%) | 92,930 (74.4%) | <0.0001 |
| Government, nonfederal (public) | 113,581 (3.8%) | 106,306 (4.8%) | 7275 (5.8%) | <0.0001 |
| Private, not-for-profit (voluntary) | 234,390 (7.8%) | 219,797 (9.8%) | 14,593 (11.7%) | <0.0001 |
| Private, investor-owned (proprietary) | 172,701 (5.7%) | 163,042 (7.3%) | 9658 (7.7%) | <0.0001 |
| Private (collapsed category) | 11,387 (0.4%) | 10,929 (0.5%) | 458 (0.4%) | <0.0001 |
| Location/teaching status of hospital | ||||
| Rural | 103,534 (3.4%) | 98,666 (3.5%) | 4869 (3.3%) | <0.0001 |
| Urban nonteaching | 803,918 (26.7%) | 762,579 (26.7%) | 41,338 (27.9%) | <0.0001 |
| Urban teaching | 2,093,640 (69.5%) | 1,991,687 (69.8%) | 101,953 (68.8%) | <0.0001 |
| Alcohol abuse | 278,873 (9.3%) | 269,281 (9.5%) | 9592 (6.5%) | <0.0001 |
| Deficiency anemias | 670,353 (22.3%) | 622,040 (21.9%) | 48,314 (32.7%) | <0.0001 |
| Rheumatoid arthritis/collagen vascular diseases | 20,064 (0.7%) | 19,553 (0.7%) | 512 (0.3%) | <0.0001 |
| Chronic blood loss anemia | 37,557 (1.2%) | 36,512 (1.3%) | 1045 (0.7%) | <0.0001 |
| Congestive heart failure | 141,072 (4.7%) | 134,102 (4.7%) | 6970 (4.7%) | <0.0001 |
| Chronic pulmonary disease | 536,730 (17.8%) | 508,861 (17.9%) | 27,869 (18.9%) | <0.0001 |
| Coagulopathy | 226,624 (7.5%) | 215,079 (7.6%) | 11,544 (7.8%) | <0.0001 |
| Depression | 323,648 (10.7%) | 311,747 (11%) | 11,901 (8.1%) | <0.0001 |
| Diabetes, uncomplicated | 324,690 (10.8%) | 315,834 (11.1%) | 8855 (6%) | <0.0001 |
| Diabetes with chronic complications | 65,236 (2.2%) | 63,743 (2.2%) | 1493 (1%) | <0.0001 |
| Drug abuse | 640,836 (21.3%) | 615,264 (21.6%) | 25,572 (17.3%) | <0.0001 |
| Hypertension | 935,590 (31.1%) | 909,666 (32%) | 25,924 (17.5%) | <0.0001 |
| Hypothyroidism | 81,825 (2.7%) | 79,423 (2.8%) | 2403 (1.6%) | <0.0001 |
| Liver disease | 351,442 (11.7%) | 339,904 (11.9%) | 11,538 (7.8%) | <0.0001 |
| Lymphoma | 74,646 (2.5%) | 72,198 (2.5%) | 2448 (1.7%) | <0.0001 |
| Fluid and electrolyte disorders | 815,607 (27.1%) | 752,205 (26.4%) | 63,402 (42.9%) | <0.0001 |
| Metastatic cancer | 31,004 (1%) | 30,498 (1.1%) | 506 (0.3%) | <0.0001 |
| Other neurological disorders | 237,746 (7.9%) | 230,051 (8.1%) | 7695 (5.2%) | <0.0001 |
| Obesity | 101,271 (3.4%) | 98,513 (3.5%) | 2758 (1.9%) | <0.0001 |
| Paralysis | 56,265 (1.9%) | 55,135 (1.9%) | 1130 (0.8%) | <0.0001 |
| Peripheral vascular disorders | 47,390 (1.6%) | 46,691 (1.6%) | 699 (0.5%) | <0.0001 |
| Psychoses | 248,541 (8.3%) | 240,240 (8.4%) | 8301 (5.6%) | <0.0001 |
| Pulmonary circulation disorders | 40,013 (1.3%) | 37,177 (1.3%) | 2835 (1.9%) | <0.0001 |
| Renal failure | 361,463 (12%) | 351,618 (12.4%) | 9845 (6.7%) | <0.0001 |
| Solid tumor without metastasis | 44,766 (1.5%) | 43,738 (1.5%) | 1028 (0.7%) | <0.0001 |
| Peptic ulcer disease excluding bleeding | 2417 (0.1%) | 2301 (0.1%) | 116 (0.1%) | <0.0001 |
| Valvular disease | 48,602 (1.6%) | 46,589 (1.6%) | 2013 (1.4%) | <0.0001 |
| Weight loss | 218,633 (7.3%) | 194,137 (6.8%) | 24,496 (16.6%) | <0.0001 |
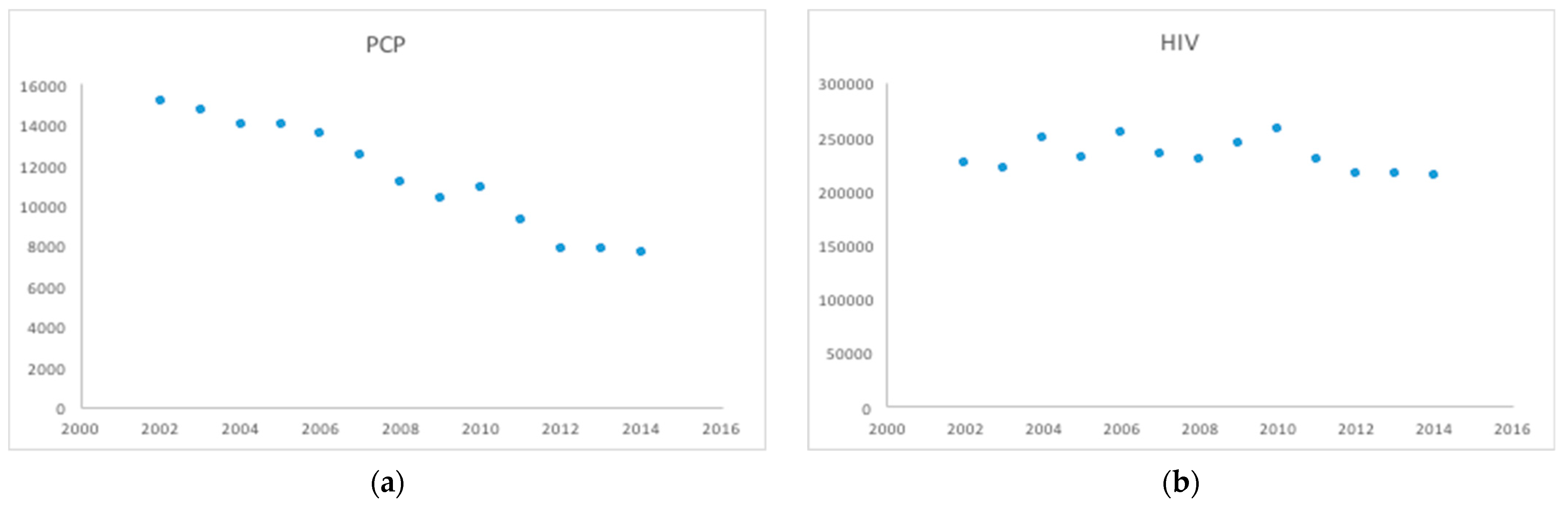
| Calendar Year | Total Number of HIV Hospitalizations | Total Number of HIV Hospitalizations with PCP |
|---|---|---|
| 2002 | 225,202 | 15,144 |
| 2003 | 220,627 | 14,682 |
| 2004 | 248,090 | 14,006 |
| 2005 | 229,448 | 13,982 |
| 2006 | 253,354 | 13,535 |
| 2007 | 233,297 | 12,489 |
| 2008 | 228,136 | 11,138 |
| 2009 | 243,649 | 10,342 |
| 2010 | 257,093 | 10,820 |
| 2011 | 229,071 | 9240 |
| 2012 | 216,110 | 7800 |
| 2013 | 214,685 | 7780 |
| 2014 | 212,960 | 7665 |
| HIV Patients with Comorbidities | Odds Ratio for Mortality | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Pneumocystis pneumonia | 3.082 | 3.007 | 3.159 | <0.0001 |
| Alcohol abuse | 0.799 | 0.773 | 0.827 | <0.0001 |
| Deficiency anemias | 0.715 | 0.701 | 0.73 | <0.0001 |
| Rheumatoid arthritis/collagen vascular diseases | 0.86 | 0.759 | 0.975 | <0.0001 |
| Chronic blood loss anemia | 1.054 | 0.984 | 1.129 | <0.0001 |
| Congestive heart failure | 1.834 | 1.778 | 1.892 | <0.0001 |
| Chronic pulmonary disease | 0.789 | 0.77 | 0.809 | <0.0001 |
| Coagulopathy | 3.54 | 3.467 | 3.615 | <0.0001 |
| Depression | 0.551 | 0.531 | 0.572 | <0.0001 |
| Diabetes, uncomplicated | 0.897 | 0.871 | 0.924 | <0.0001 |
| Diabetes with chronic complications | 0.786 | 0.74 | 0.835 | <0.0001 |
| Drug abuse | 0.679 | 0.663 | 0.696 | <0.0001 |
| Hypertension | 0.675 | 0.66 | 0.69 | <0.0001 |
| Hypothyroidism | 0.97 | 0.919 | 1.023 | <0.0001 |
| Liver disease | 1.388 | 1.356 | 1.421 | <0.0001 |
| Lymphoma | 2.574 | 2.485 | 2.667 | <0.0001 |
| Fluid and electrolyte disorders | 2.904 | 2.854 | 2.955 | <0.0001 |
| Metastatic cancer | 3.787 | 3.601 | 3.983 | <0.0001 |
| Other neurological disorders | 1.845 | 1.799 | 1.893 | <0.0001 |
| Obesity | 0.651 | 0.604 | 0.702 | <0.0001 |
| Paralysis | 1.357 | 1.284 | 1.434 | <0.0001 |
| Peripheral vascular disorders | 1.256 | 1.177 | 1.342 | <0.0001 |
| Psychoses | 0.653 | 0.627 | 0.68 | <0.0001 |
| Pulmonary circulation disorders | 1.811 | 1.711 | 1.916 | <0.0001 |
| Renal failure | 1.811 | 1.766 | 1.856 | <0.0001 |
| Solid tumor without metastasis | 1.657 | 1.567 | 1.752 | <0.0001 |
| Peptic ulcer disease excluding bleeding | 0.828 | 0.648 | 1.058 | <0.0001 |
| Valvular disease | 1.027 | 0.971 | 1.086 | <0.0001 |
| Weight loss | 1.873 | 1.829 | 1.918 | <0.0001 |
| Calendar Year | Mortality in PCP | Mortality in PCP (%) |
|---|---|---|
| 2002 | 1736 | 11.5 |
| 2003 | 1572 | 10.7 |
| 2004 | 1590 | 11.4 |
| 2005 | 1394 | 10 |
| 2006 | 1393 | 10.3 |
| 2007 | 1126 | 9 |
| 2008 | 1153 | 10.4 |
| 2009 | 1010 | 9.8 |
| 2010 | 945 | 8.7 |
| 2011 | 685 | 7.4 |
| 2012 | 685 | 8.8 |
| 2013 | 760 | 9.8 |
| 2014 | 645 | 8.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elango, K.; Mudgal, M.; Murthi, S.; Yella, P.R.; Nagrecha, S.; Srinivasan, V.; Sekar, V.; Koshy, M.; Ramalingam, S.; Gunasekaran, K. Trends in the Epidemiology and Outcomes of Pneumocystis Pneumonia among Human Immunodeficiency Virus (HIV) Hospitalizations. Int. J. Environ. Res. Public Health 2022, 19, 2768. https://doi.org/10.3390/ijerph19052768
Elango K, Mudgal M, Murthi S, Yella PR, Nagrecha S, Srinivasan V, Sekar V, Koshy M, Ramalingam S, Gunasekaran K. Trends in the Epidemiology and Outcomes of Pneumocystis Pneumonia among Human Immunodeficiency Virus (HIV) Hospitalizations. International Journal of Environmental Research and Public Health. 2022; 19(5):2768. https://doi.org/10.3390/ijerph19052768
Chicago/Turabian StyleElango, Kalaimani, Mayuri Mudgal, Swetha Murthi, Prashanth Reddy Yella, Savan Nagrecha, Vedhapriya Srinivasan, Vijaykumar Sekar, Maria Koshy, Sathishkumar Ramalingam, and Kulothungan Gunasekaran. 2022. "Trends in the Epidemiology and Outcomes of Pneumocystis Pneumonia among Human Immunodeficiency Virus (HIV) Hospitalizations" International Journal of Environmental Research and Public Health 19, no. 5: 2768. https://doi.org/10.3390/ijerph19052768
APA StyleElango, K., Mudgal, M., Murthi, S., Yella, P. R., Nagrecha, S., Srinivasan, V., Sekar, V., Koshy, M., Ramalingam, S., & Gunasekaran, K. (2022). Trends in the Epidemiology and Outcomes of Pneumocystis Pneumonia among Human Immunodeficiency Virus (HIV) Hospitalizations. International Journal of Environmental Research and Public Health, 19(5), 2768. https://doi.org/10.3390/ijerph19052768






