Longitudinal and Long-Term Wastewater Surveillance for COVID-19: Infection Dynamics and Zoning of Urban Community
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Community
2.2. Sampling Details
2.3. Sample Processing
2.4. RNA Extraction and RT-PCR
2.5. Statistical Methods and Data Management
3. Results and Discussion
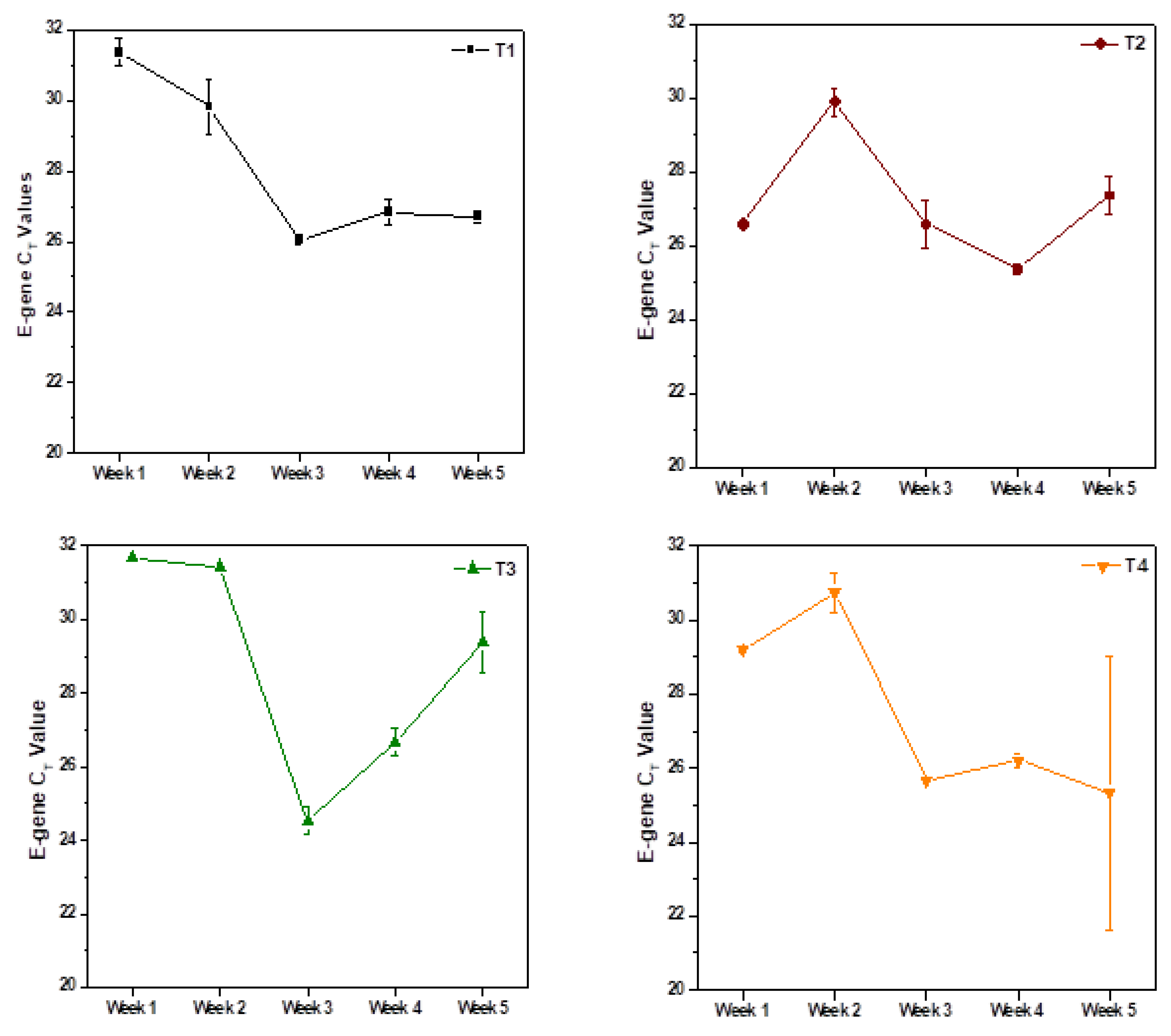
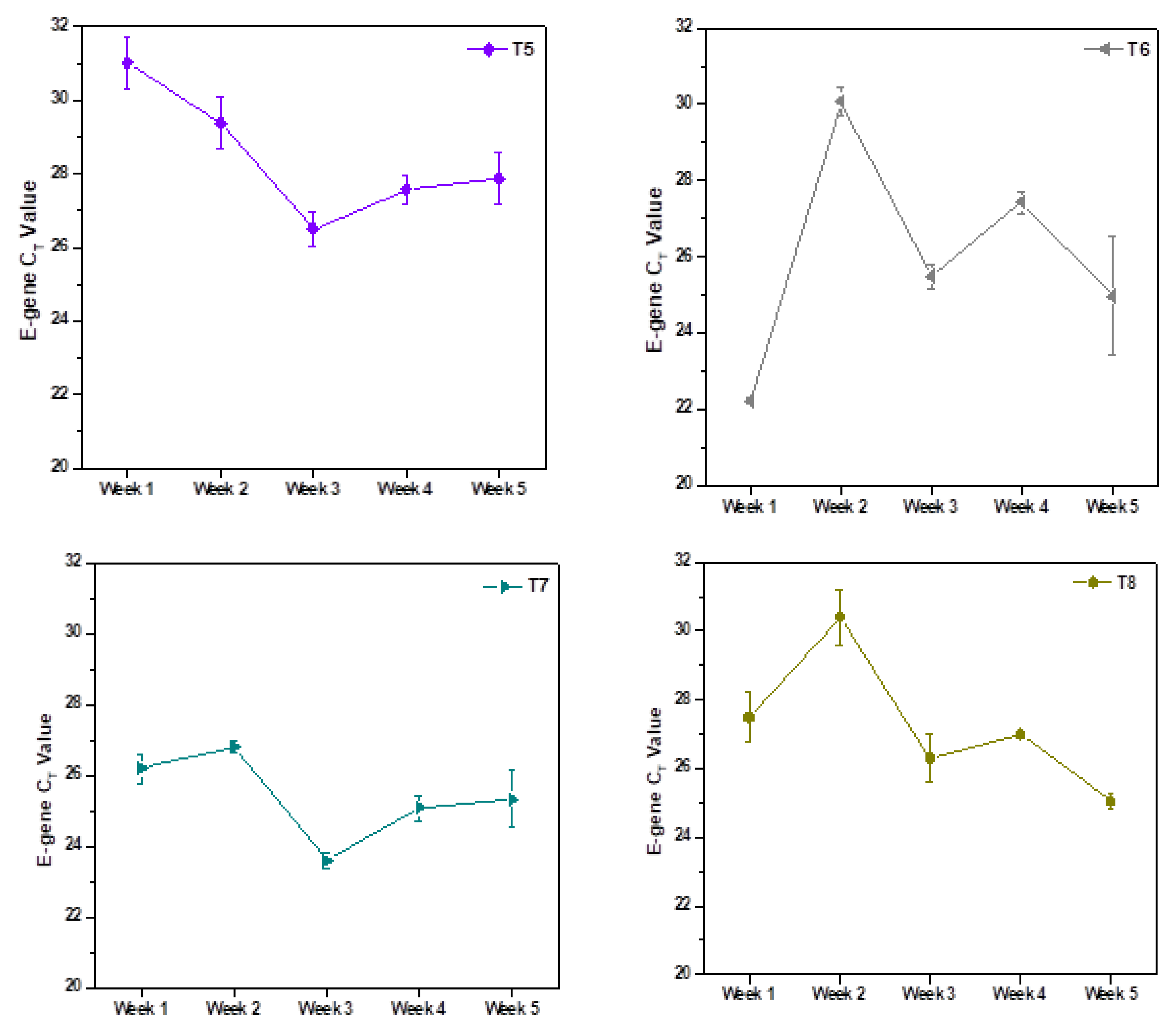
3.1. Weekly Sample Analysis
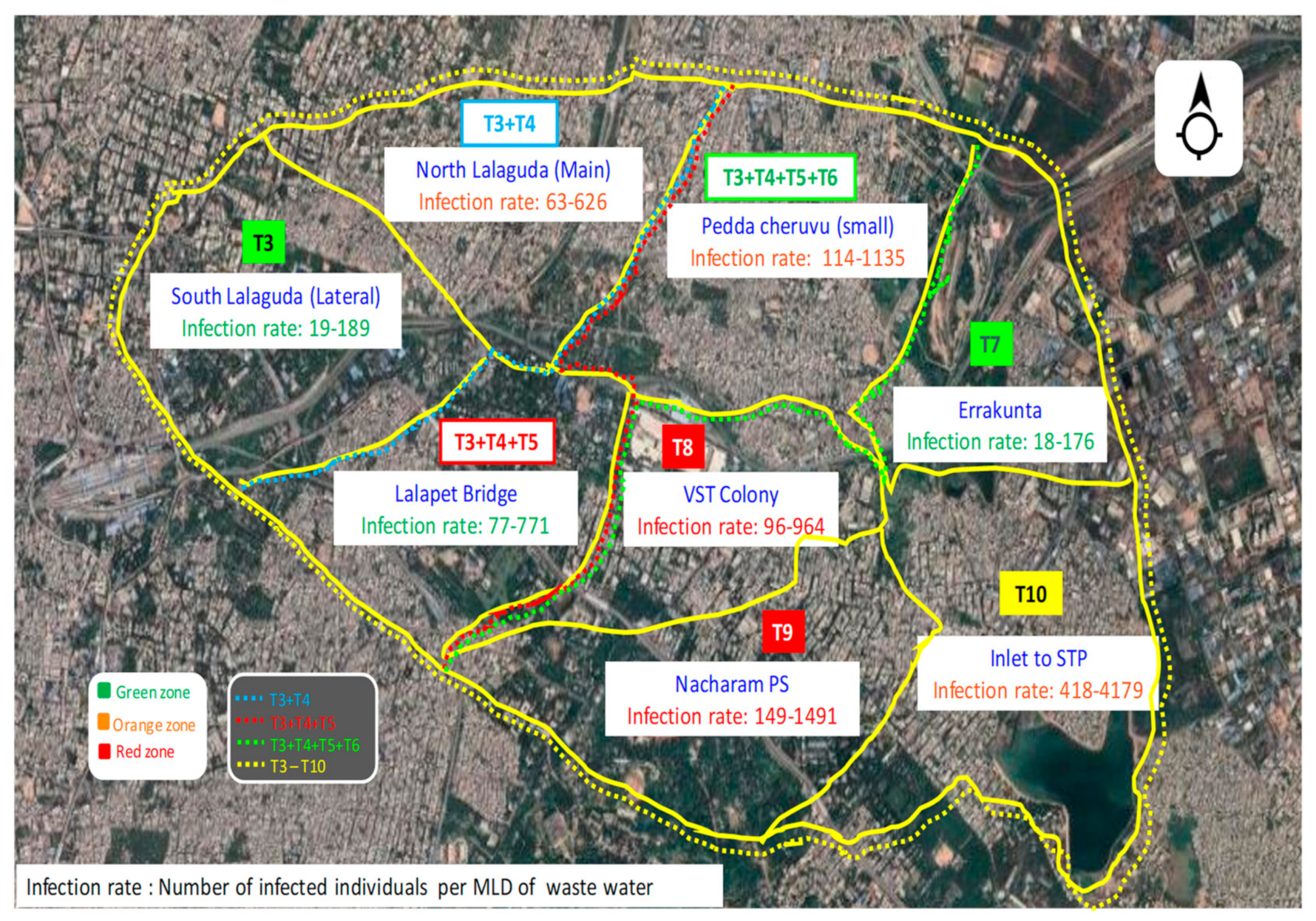
Community Zoning Based on Infection for Sustainable Management
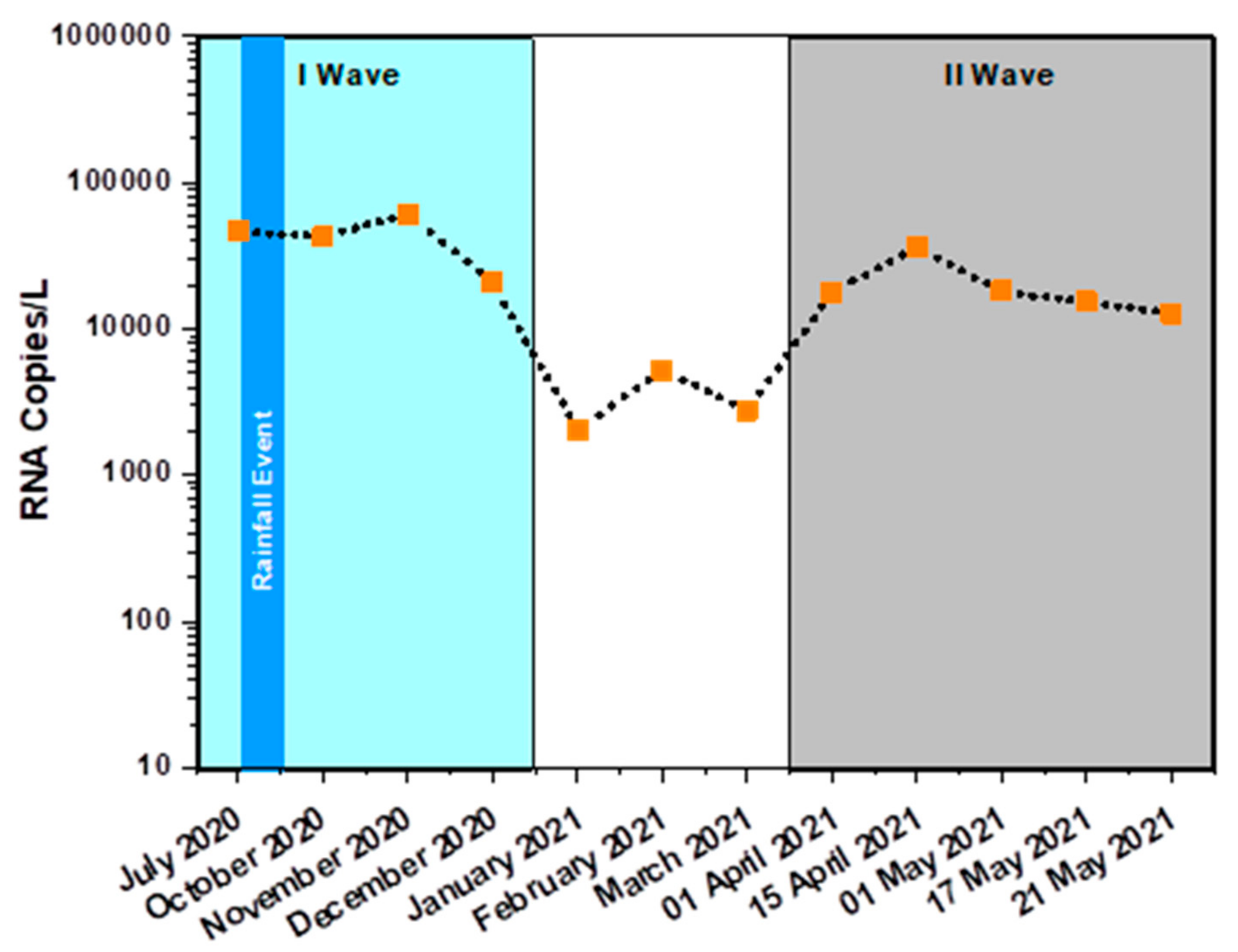
3.2. Monthly Sample Analysis
Infection Surge and Dynamics
3.3. Epidemiological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asghar, H.; Diop, O.M.; Weldegebriel, G.; Malik, F.; Shetty, S.; el Bassioni, L.; Akande, A.O.; al Maamoun, E.; Zaidi, S.; Adeniji, A.J.; et al. Environmental surveillance for polioviruses in the Global Polio Eradication Initiative. J. Infect. Dis. 2014, 210, 294–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Reilly, K.M.; Allen, D.J.; Fine, P.; Asghar, H. The challenges of informative wastewater sampling for SARS-CoV-2 must be met: Lessons from polio eradication. Lancet Microbe 2020, 1, 189–190. [Google Scholar] [CrossRef]
- Ni, G.; Lu, J.; Maulani, N.; Tian, W.; Yang, L.; Harliwong, I.; Wang, Z.; Mueller, J.; Yang, B.; Yuan, Z.; et al. Novel multiplexed amplicon-based sequencing to quantify SARS-CoV-2 RNA from wastewater. Environ. Sci. Technol. Lett. 2021, 8, 683–690. [Google Scholar] [CrossRef]
- Xagoraraki, I.; O’Brien, E. Wastewater-Based Epidemiology for Early Detection of Viral Outbreaks; Springer: Cham, Switzerland, 2020; pp. 75–97. [Google Scholar]
- Shaw, A.G.; Majumdar, M.; Troman, C.; O’Toole, Á.; Benny, B.; Abraham, D.; Praharaj, I.; Kang, G.; Sharif, S.; Alam, M.M.; et al. Rapid and sensitive direct detection and identification of poliovirus from stool and environmental surveillance samples by use of nanopore sequencing. J. Clin. Microbiol. 2020, 58, e00920-20. [Google Scholar] [CrossRef]
- Randazzo, W.; Truchado, P.; Cuevas-Ferrando, E.; Simón, P.; Allende, A.; Sánchez, G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020, 181, 115942. [Google Scholar] [CrossRef]
- Mohan, S.V.; Hemalatha, M.; Kopperi, H.; Ranjith, I.; Kumar, A.K. SARS-CoV-2 in environmental perspective: Occurrence, persistence, surveillance, inactivation and challenges. Chem. Eng. J. 2021, 405, 126893. [Google Scholar] [CrossRef]
- Ali, H.A.; Yaniv, K.; Bar-Zeev, E.; Chaudhury, S.; Shagan, M.; Lakkakula, S.; Ronen, Z.; Kushmaro, A.; Nir, O. Tracking SARS-CoV-2 RNA through the wastewater treatment process. ACS EST Water 2021, 1, 1161–1167. [Google Scholar] [CrossRef]
- Casanova, L.M.; Weaver, S.R. Inactivation of an enveloped surrogate virus in human sewage. Environ. Sci. Technol. Lett. 2015, 2, 76–78. [Google Scholar] [CrossRef]
- Brainard, J.; Pond, K.; Hunter, P.R. Censored regression modeling to predict virus inactivation in wastewaters. Environ. Sci. Technol. 2017, 51, 1795–1801. [Google Scholar] [CrossRef] [Green Version]
- Torrey, J.; von Gunten, U.; Kohn, T. Differences in viral disinfection mechanisms as revealed by quantitative transfection of echovirus 11 genomes. Appl. Environ. Microb. 2019, 85, e00961-19. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, W.; Bivins, A.; Bertsch, P.M.; Bibby, K.; Choi, P.M.; Farkas, K.; Gyawali, P.; Hamilton, K.A.; Haramoto, E.; Kitajima, M. Surveillance of SARS-CoV-2 RNA in wastewater: Methods optimization and quality control are crucial for generating reliable public health information. Cur. Opin. Environ. Sci. Health 2020, 17, 82–93. [Google Scholar] [CrossRef]
- Ahmed, W.; Angel, N.; Edson, J.; Bibby, K.; Bivins, A.; O’Brien, J.W.; Choi, P.M.; Kitajima, M.; Simpson, S.L.; Li, J. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020, 728, 138764. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, J.; Xiao, A.; Gu, X.; Lee, W.L.; Armas, F.; Kauffman, K.; Hanage, W.; Matus, M.; Ghaeli, N.; et al. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. Msystems 2020, 5, e00614-20. [Google Scholar] [CrossRef] [PubMed]
- Wurtzer, S.; Marechal, V.; Mouchel, J.M.; Maday, Y.; Teyssou, R.; Richard, E.; Almayrac, J.L.; Moulin, L. Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases. MedRxiv 2020. [Google Scholar] [CrossRef]
- La Rosa, G.; Iaconelli, M.; Mancini, P.; Ferraro, G.B.; Veneri, C.; Bonadonna, L.; Lucentini, L.; Suffredini, E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020, 736, 139652. [Google Scholar] [CrossRef] [PubMed]
- Medema, G.; Heijnen, L.; Elsinga, G.; Italiaander, R.; Brouwer, A. Presence of SARS-Coronavirus-2 RNA in Sewage and Correlation with Reported COVID-19 Prevalence in the Early Stage of the Epidemic in The Netherlands. MedRxiv 2020. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Hanna, K. Existence of SARS-CoV-2 in wastewater: Implications for its environmental transmission in developing communities. Environ. Sci. Technol. 2020, 54, 7758–7759. [Google Scholar] [CrossRef] [PubMed]
- Weidhaas, J.; Aanderud, Z.; Roper, D.; VanDerslice, J.; Gaddis, E.; Ostermiller, J.; Hoffman, K.; Jamal, R.; Heck, P.; Zhang, Y.; et al. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci. Total Environ. 2021, 775, 145790. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Hamasaki, M.; Yoshitomi, H.; Ishibashi, T.; Yoshiyama, C.; Maeda, E.; Sera, N.; Yoshida, H. Environmental surveillance of poliovirus in sewage water around the introduction period for inactivated polio vaccine in Japan. Appl. Environ. Microbiol. 2015, 81, 1859–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venugopal, A.; Ganesan, H.; Raja, S.S.; Govindasamy, V.; Arunachalam, M.; Narayanasamy, A.; Sivaprakash, P.; Rahman, P.K.; Gopalakrishnan, A.V.; Siama, Z.; et al. Novel wastewater surveillance strategy for early detection of coronavirus disease 2019 hotspots. Curr. Opin. Environ. Sci. Health 2020, 17, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Kopperi, H.; Tharak, A.; Hemalatha, M.; Kiran, U.; Gokulan, C.G.; Mishra, R.K.; Mohan, S.V. Defining the methodological approach for wastewater-based epidemiological studies—Surveillance of SARS-CoV-2. Environ. Technol. Innov. 2021, 23, 101696. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.G. Monitoring wastewater for assessing community health: Sewage Chemical-Information Mining (SCIM). Sci. Total Environ. 2018, 619–620, 748–764. [Google Scholar] [CrossRef] [PubMed]
- Lednicky, J.A.; Shankar, S.N.; Elbadry, M.A.; Gibson, J.C.; Alam, M.M.; Stephenson, C.J.; Eiguren-Fernandez, A.; Morris, J.G.; Mavian, C.N.; Salemi, M.; et al. Collection of SARS-CoV-2 Virus from the Air of a Clinic within a University Student Health Care Center and Analyses of the Viral Genomic Sequence. Aerosol Air Qual. Res. 2020, 20, 1167. [Google Scholar] [CrossRef] [PubMed]
- Quilliam, R.S.; Weidmann, M.; Moresco, V.; Purshouse, H.; O’Hara, Z.; Oliver, D.M. COVID-19: The environmental implications of shedding SARS-CoV-2 in human faeces. Environ. Int. 2020, 140, 105790. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Zhang, H.; Yang, Z. Can a paper-based device trace COVID-19 sources with wastewater-based epidemiology? Environ. Sci. Technol. 2020, 54, 3733–3735. [Google Scholar] [CrossRef]
- Hart, O.E.; Halden, R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: Feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020, 730, 138875. [Google Scholar] [CrossRef]
- Hemalatha, M.; Kiran, U.; Kuncha, S.K.; Kopperi, H.; Gokulan, C.G.; Mohan, S.V.; Mishra, R.K. Surveillance of SARS-CoV-2 spread using wastewater-based epidemiology: Comprehensive study. Sci. Total Environ. 2021, 768, 144704. [Google Scholar] [CrossRef]
- Hellmér, M.; Paxéus, N.; Magnius, L.; Enache, L.; Arnholm, B.; Johansson, A.; Bergström, T.; Norder, H. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014, 80, 6771–6781. [Google Scholar] [CrossRef] [Green Version]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The characterization of feces and urine: A review of the literature to inform advanced treatment technology. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1827–1879. [Google Scholar] [CrossRef] [Green Version]
- Foladori, P.; Cutrupi, F.; Segata, N.; Manara, S.; Pinto, F.; Malpei, F.; Bruni, L.; la Rosa, G. SARS-CoV-2 from faeces to wastewater treatment: What do we know? A review. Sci. Total Environ. 2020, 743, 140444. [Google Scholar] [CrossRef]
- Bivins, A.; North, D.; Ahmad, A.; Ahmed, W.; Alm, E. Wastewater-based epidemiology: Global collaborative to maximize contributions in the fight against COVID-19. Environ. Sci. Technol. 2020, 54, 7754–7757. [Google Scholar] [CrossRef]
- Yonar, T.; Sivrioğlu, Ö.; Özengin, N. Physico-chemical treatment of dairy industry wastewaters: A review. Technological Approaches for Novel Applications in Dairy Processing; 2018; p. 179. Available online: https://www.intechopen.com/chapters/61457 (accessed on 21 January 2022).
- Barcelo, D. An environmental and health perspective for COVID-19 outbreak: Meteorology and air quality influence, sewage epidemiology indicator, hospitals disinfection, drug therapies and recommendations. J. Environ. Chem. Eng. 2020, 8, 104006. [Google Scholar] [CrossRef] [PubMed]
- Westhaus, S.; Weber, F.A.; Schiwy, S.; Linnemann, V.; Brinkmann, M.; Widera, M.; Greve, C.; Janke, A.; Hollert, H.; Wintgens, T.; et al. Detection of SARS-CoV-2 in raw and treated wastewater in Germany–suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2021, 751, 141750. [Google Scholar] [CrossRef] [PubMed]
- Peccia, J.; Zulli, A.; Brackney, D.E.; Grubaugh, N.D.; Kaplan, E.H.; Casanovas-Massana, A.I.; Ko, A.A.; Malik, D.; Wang, M.; Warren, J.L. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020, 38, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Understanding SARS-CoV-2 Infection and Dynamics with Long Term Wastewater Based Epidemiological Surveillance. Available online: https://timesofindia.indiatimes.com/city/hyderabad/master-plan-report-on-sewageby-dec (accessed on 15 September 2019).
- 54% of Hyderabad’s Population Has Antibodies against COVID-19: Study, ‘Number of People in Hyderabad with Covid Antibodies Could Be Much More than Sero-Survey Results,’ Says CCMB Director. Available online: https://www.thehindu.com/news/cities/Hyderabad/half-of-citys-population-have-antibodies-saysstudy/article33991478.eceutm_source=hyderabad&utm_medium=sticky_footer (accessed on 4 March 2021).
- Alygizakis, N.; Markou, A.N.; Rousis, N.I.; Galani, A.; Avgeris, M.; Adamopoulos, P.G.; Scorilas, A.; Lianidou, E.S.; Paraskevis, D.; Tsiodras, S.; et al. Analytical methodologies for the detection of SARS-CoV-2 in wastewater: Protocols and future perspectives. TrAC Trend Anal. Chem. 2020, 134, 16125. [Google Scholar] [CrossRef]
- Dhama, K.; Patel, S.K.; Yatoo, M.I.; Tiwari, R.; Sharun, K.; Dhama, J.; Natesan, S.; Malik, Y.S.; Singh, K.P.; Harapan, H. SARS-CoV-2 existence in sewage and wastewater: A global public health concern? J. Environ. Manag. 2021, 280, 111825. [Google Scholar] [CrossRef]
- Elsamadony, M.; Fujii, M.; Miura, T.; Watanabe, T. Possible transmission of viruses from contaminated human feces and sewage: Implications for SARS-CoV-2. Sci. Total Environ. 2021, 755, 142575. [Google Scholar] [CrossRef]
- Gundy, P.M.; Gerba, C.P.; Pepper, I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009, 1, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Black, J.; Aung, P.; Nolan, M.; Roney, E.; Poon, R.; Hennessy, D.; Crosbie, N.D.; Deere, D.; Jex, A.R.; John, N.; et al. Epidemiological evaluation of sewage surveillance as a tool to detect the presence of COVID-19 cases in a low case load setting. Sci. Total Environ. 2021, 786, 147469. [Google Scholar] [CrossRef]
- Rooney, C.M.; Moura, I.B.; Wilcox, M.H. Tracking COVID-19 via sewage. Curr. Opin. Gastroenterol. 2021, 37, 4–8. [Google Scholar] [CrossRef]
- Panchal, D.; Prakash, O.; Bobde, P.; Pal, S. SARS-CoV-2: Sewage surveillance as an early warning system and challenges in developing countries. Environ. Sci. Pollut. Res. 2021, 28, 22221–22240. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Li, J.S.; Guo, T.K.; Zhen, B.; Kong, Q.X.; Yi, B.; Li, Z.; Song, N.; Jin, M.; Xiao, W.J.; et al. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital. J. Virol. Methods 2005, 128, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Alexyuk, M.S.; Turmagambetova, A.S.; Alexyuk, P.G.; Bogoyavlenskiy, A.P.; Berezin, V.E. Comparative study of viromes from freshwater samples of the Ile-Balkhash region of Kazakhstan captured through metagenomic analysis. Virus Dis. 2017, 28, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Patel, A.K.; Shah, A.V.; Raval, J.; Rajpara, N.; Joshi, M.; Joshi, C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020, 746, 141326. [Google Scholar] [CrossRef]
- Prado, T.; Fumian, T.M.; Mannarino, C.F.; Maranhao, A.G.; Siqueira, M.M.; Miagostovich, M.P. Preliminary results of SARS-CoV-2 detection in sewerage system in Niterói municipality, Rio de Janeiro, Brazil. Memórias Inst. Oswaldo Cruz 2020, 115, e200196. [Google Scholar] [CrossRef]
- Wang, J.; Feng, H.; Zhang, S.; Ni, Z.; Ni, L.; Chena, Y.; Zhoud, L.; Zhong, Z. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020, 94, 103–106. [Google Scholar] [CrossRef]
- Mlejnkova, H.; Sovova, K.; Vasickova, P.; Ocenaskova, V.; Jasikova, L.; Juranova, E. Preliminary study of Sars-Cov-2 occurrence in wastewater in the Czech Republic. Int. J. Environ. Res. Public Health 2020, 17, 5508. [Google Scholar] [CrossRef]
- Lodder, W.; de Roda, A.M. SARS-CoV-2 in wastewater: Potential health risk, but also data source. Lancet Gastroenterol. Hepatol. 2020, 5, 533–534. [Google Scholar] [CrossRef]
- Haramoto, E.; Malla, B.; Thakali, O.; Kitajima, M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020, 731, 140405. [Google Scholar] [CrossRef]
- Sherchan, S.P.; Shahin, S.; Ward, L.M.; Tandukar, S.; Aw, T.G.; Schmiz, B.; Ahmed, W.; Kitajima, M. First detection of SARS-CoV-2 RNA in wastewater in North America: A study in Louisiana, USA. Sci. Total Environ. 2020, 743, 140621. [Google Scholar] [CrossRef]
- Gonzalez, R.; Curtis, K.; Bivins, A.; Bibby, K.; Weir, M.H.; Yetka, K.; Thompson, H.; Keeling, D.; Mitchell, J.; Gonzalez, D. COVID-19 surveillance in Southeastern Virginia using wastewater-based epidemiology. Water Res. 2020, 186, 116296. [Google Scholar] [CrossRef] [PubMed]
- Miyani, B.; Fonoll, X.; Norton, J.; Mohrotra, A.; Xagoraraki, I. SARS-CoV-2 in Detroit wastewater. J. Environ. Eng. 2020, 11, 06020004. [Google Scholar] [CrossRef]
- Wannigama, D.L.; Amarasiri, M.; Hurst, C.; Phattharapornjaroen, P.; Abe, S.; Hongsing, P.; Rad, S.A.H.; Pearson, L.; Saethang, T.; Luk-In, S.; et al. Tracking COVID-19 with wastewater to understand asymptomatic transmission. Int. J. Infect. Dis. 2021, 108, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Hayes, E.K.; Sweeney, C.L.; Anderson, L.E.; Li, B.; Erjavec, G.B.; Gouthro, M.T.; Krkosek, W.H.; Stoddart, A.K.; Gagnon, G.A. A novel passive sampling approach for SARS-CoV-2 in wastewater in a Canadian province with low prevalence of COVID-19. Environ. Sci. Water Res. Technol. 2021, 7, 1576–1586. [Google Scholar] [CrossRef]
- Sanjuán, R.; Domingo-Calap, P. Reliability of wastewater analysis for monitoring COVID-19 incidence revealed by a long-term follow-up study. Front. Virol. 2021. [Google Scholar] [CrossRef]
- Sharif, S.; Ikram, A.; Khurshid, A.; Salman, M.; Mehmood, N.; Arshad, Y.; Ahmed, J.; Safdar, R.M.; Rehman, L.; Mujtaba, G.; et al. Detection of SARs-CoV-2 in wastewater using the existing environmental surveillance network: A potential supplementary system for monitoring COVID-19 transmission. PLoS ONE 2021, 16, e0249568. [Google Scholar] [CrossRef]
- Pino, N.J.; Rodriguez, D.C.; Cano, L.C.; Rodriguez, A. Detection of SARS-CoV-2 in wastewater is influenced by sampling time, concentration method, and target analyzed. J. Water Health 2021, 19, 775–784. [Google Scholar] [CrossRef]
- McMahan, C.S.; Self, S.; Rennert, L.; Kalbaugh, C.; Kriebel, D.; Graves, D.; Colby, C.; Deaver, J.A.; Popat, S.C.; Karanfil, T.; et al. COVID-19 wastewater epidemiology: A model to estimate infected populations. Lancet Planet. Health 2021, 5, e874–e881. [Google Scholar] [CrossRef]
- Agrawal, S.; Orschler, L.; Lackner, S. Long-term monitoring of SARS-CoV-2 RNA in wastewater of the Frankfurt metropolitan area in Southern Germany. Sci. Rep. 2021, 11, 5372. [Google Scholar] [CrossRef]
- Hemalatha, M.; Tharak, A.; Kopperi, H.; Kiran, U.; Gokulan, C.G.; Mishra, R.K.; Mohan, S.V. Comprehensive and temporal surveillance of SARS-CoV-2 in urban water bodies: Early signal of second wave onset. MedRxiv 2021. [Google Scholar] [CrossRef]


| Weekly Monitoring | |||||
|---|---|---|---|---|---|
| S. No. | Sampling Point | Location | Drain | Sample Code | Sampling Date and Time |
| 1 | South Lalaguda (Point–3) | South Lalaguda | Lateral Drain | T1 | 7 October 2020 28 October 2020 4 November 2020 11 November 2020 18 November 2020 8:00 to 8:30 am |
| 2 | North Lalaguda (Point–4) | North Lalaguda | Main Drain | T2 | |
| 3 | Lalapet | Lalapet Bridge | Main Drain | T3 | |
| 4 | Tarnaka (Drain–1) | Near PeddaCheruvu (Small) | Main Drain | T4 | |
| 5 | Tarnaka (Drain–2 | Errakunta | Lateral Drain | T5 | |
| 6 | Tarnaka (Drain–3) | VST Colony | Lateral Drain | T6 | |
| 7 | Tarnaka (Drain–4) | Behind Nacharam PS | Lateral Drain | T7 | |
| 8 | Nacharam (Drain–1) | Inlet to STP | Main Drain | T8 | |
| Monthly Monitoring | |||||
| Location; Point of Drain | Sampling date (8:00 to 8:30 am) | ||||
| 1 | Nacharam | T8 | 22 July 2020 | ||
| 2 | All (eight) sampling points | T1 to T8 | 7 October 2020 4 November 2020 | ||
| Nacharam | T8 | 11 December 2020 | |||
| 3 | Five sampling points | T4–T8 | 20 January 2021 13 February 2021 2 March 2021 | ||
| 4 | Three sampling points | T4, T7 and T8 | 1 April 2021 15 April 2021 | ||
| 5 | Three sampling points | T4, T7 and T8 | 1 May 2021 17 May 2021 21 May 2021 | ||
| 6 | Three sampling points | T4, T7 and T8 | 4 June 2021 18 June 2021 24 June 2021 | ||
| 7 | Three sampling points | T4, T7 and T8 | 4 July 2021 10 July 2021 14 July 2021 27 July 2021 | ||
| 8 | Three sampling points | T4, T7 and T8 | 4 July 2021 | ||
| Zoning of Study Area | |||||
| Zones | Covering Areas | Infection Rate | |||
| 1 | Zone I | T1 | 19–189 | ||
| (i) Extended Zone I | T1+T2 | 63–626 | |||
| (ii) Second Extended Zone I | T1+T2+T3 | 77–771 | |||
| (iii) Third Extended Zone I | T1+T2+T3+T4 | 114–1135 | |||
| 2 | Zone II | T5 | 18–176 | ||
| 3 | Zone III | T6 | 96–964 | ||
| 4 | Zone IV | T7 | 149–1491 | ||
| 5 | Cumulative Major Zone | T1 to T8 (Complete study area) | 418–4179 | ||
| Sample Code | E-Gene * (CT) | N-Gene * (CT) | ORF1ab * (CT) | RNA Copies/L ** | |||
|---|---|---|---|---|---|---|---|
| T8 | Average of Longitudinal Sampling | T8 | Average of Longitudinal Sampling | T8 | Average of Longitudinal Sampling | ||
| July 2020 | 27.38 ± 0.36% | SC | 26.12 ± 1.38% | 28.02 ± 1.92% | 46,527 | ||
| October 2020 | 27.5 ± 2.65% | 28.34 ± 1.25 | 26.82 ± 0.63% | 27.24 ± 1.25 | 27.34 ± 4.10% | 27.92 ± 2.09 | 42,772 |
| November 2020 | 26.99 ± 0.09% | 26.49 ± 0.96 | 25.99 ± 0.35% | 25.19 ± 1.65 | 26 ± 0.41% | 25.37 ± 3.23 | 61,160 |
| December 2020 | 28.5 ± 0.21% | SC | 26.84 ± 0.73% | SC | 27.19 ± 0.49% | SC | 20,624 |
| January 2021 | 31.84 ± 0.83% | 30.44 ± 0.84% | 29.49 ± 4.26% | 29.48 ± 4.81% | 28.89 ± 2.70% | 28.99 ± 2.15% | 2036 |
| February 2021 | 30.50 ± 1.30% | 31.10 ± 1.98% | 30.45 ± 2.22% | 31.23 ± 2.95% | 28.30 ± 5.48% | 28.77 ± 2.33% | 5228 |
| March 2021 | 31.39 ± 2.86% | 31.38 ± 1.88% | 29.38 ± 1.30% | 29.53 ± 1.11% | 27.63 ± 3.38% | 27.12 ± 3.35% | 2781 |
| 1 April 2021 | 29.28 ± 2.27% | 30.08 ± 1.99% | 28.09 ± 2.20% | 26.12 ± 0.93% | 26.71 ± 2.61% | 27.43 ± 2.91% | 17,775 |
| 15 April 2021 | 27.73 ± 0.52% | 27.54 ± 1.04% | 26.12 ± 0.93% | 25.91 ± 1.57% | 27.59 ± 0.98% | 27.21 ± 1.89% | 36,399 |
| 1 May 2021 | 28.73 ± 0.63% | 28.63 ± 1.12% | 27.46 ± 0.56% | 27.24 ± 1.23% | 28.06 ± 0.26% | 27.23 ± 3.21% | 18,055 |
| 17 May 2021 | 28.97 ± 0.18% | 29.01 ± 0.67% | 26.03 ± 0.48% | 28.97 ± 0.18% | 26.77 ± 0.15% | 26.56 ± 0.56% | 15,301 |
| 21 May 2021 | 29.23 ± 1.44% | 28.78 ± 1.04% | 26.27 ± 1.57% | 25.51 ± 2.60% | 25.01 ± 1.36% | 25.42 ± 2.24% | 12,672 |
| 4 June 2021 | Below the detectable limits | ||||||
| 18 June 2021 | |||||||
| 24 June 2021 | |||||||
| 4 July 2021 | |||||||
| 10 July 2021 | |||||||
| 14 July 2021 | |||||||
| 27 July 2021 | |||||||
| 8 August 2021 | |||||||
| Sample | Capacity of the STP (in MLD) | RNA Copies/Person Contribution to STP (107 Copies/mL Faeces) | Method 1 | Method 2 | RNA Copies/Person Contribution to STP (106 Copies/mL Faeces) | Method 1 | Method 2 |
|---|---|---|---|---|---|---|---|
| T1 | 30 | 40 | 550 | 587 | 4 | 5501 | 5868 |
| T2 | 1269 | 1353 | 12,688 | 13,534 | |||
| T3 | 421 | 449 | 4208 | 4489 | |||
| T4 | 1056 | 1126 | 10,559 | 11,263 | |||
| T5 | 510 | 544 | 5099 | 5439 | |||
| T6 | 2798 | 2985 | 27,982 | 29,848 | |||
| T7 | 4328 | 4617 | 43281 | 46,166 | |||
| T8 | 1200 | 1280 | 11,996 | 12,796 | |||
| Infected individuals (for study area with 30 MLD; ~2.5 Lakh) | 1516 | 1618 | 15,164 | 16,175 | |||
| Average estimate of infected individuals (for study area with 30 MLD; ~2.5 Lakh) | 1567 | 15,670 | |||||
| Estimate of the population in active phase of the infection during the window period of 77 days (for study area with 30 MLD; ~2.5 Lakh) | 285 | 2849 | |||||
| Infected individuals for 1800 MLD (on total sewage generation of Hyderabad city) | 90,985 | 97,051 | 909,849 | 970,506 | |||
| Average estimate of infected individuals for 1800 MLD (on total sewage generation of Hyderabad city) | 94,018 | 940,177 | |||||
| Estimate of the population in active phase of the infection during the window period of 77 days for 1800 MLD (on total sewage generation of Hyderabad city) | 17,094 | 170,941 | |||||
| Sampling Month | Average Estimate of Infected Individuals (in Study Area of 30 MLD) | Estimate of the Individuals in Active Phase of the Infection during the Window Period (29 Days) | Estimate of Infected Individuals of Hyderabad City (1800 MLD) | Estimate of the Individuals in Active Phase of the Infection during the Window Period (29 Days) | Infection Rate (Person/MLD of Sewage) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 107 Copies/mL Feces | 106 Copies/mL Feces | 107 Copies/mL Feces | 106 Copies/mL Feces | 107 Copies/mL Feces | 106 Copies/mL Feces | 107 Copies/mL Feces | 106 Copies/mL Feces | 107 Copies/mL Feces | 106 Copies/mL Feces | |
| July 2020 | 1127 | 11,268 | 537 | 5366 | 67,610 | 676,095 | 32,195 | 32,1950 | 38 | 376 |
| October 2020 | 1036 | 10,359 | 493 | 4933 | 62,153 | 621,531 | 29,597 | 295,967 | 35 | 345 |
| November 2020 | 1481 | 14,812 | 705 | 7053 | 88,873 | 888,731 | 42,321 | 423,205 | 49 | 494 |
| December 2020 | 499 | 4995 | 238 | 2379 | 29,969 | 299,693 | 14,271 | 142,711 | 17 | 166 |
| January 2021 | 49 | 493 | 23 | 235 | 2959 | 29,586 | 1409 | 14,088 | 2 | 16 |
| February 2021 | 127 | 1266 | 60 | 603 | 7597 | 75,969 | 3618 | 36,176 | 4 | 42 |
| March 2021 | 67 | 674 | 32 | 321 | 4041 | 40,411 | 1924 | 19,244 | 2 | 22 |
| 1 April 2021 | 430 | 4305 | 205 | 2050 | 25,829 | 258,293 | 12,300 | 122,997 | 14 | 143 |
| 15 April 2021 | 882 | 8815 | 420 | 4198 | 52,892 | 528,923 | 25,187 | 251,868 | 29 | 294 |
| 1 May 2021 | 437 | 4373 | 208 | 2082 | 26,236 | 262,362 | 12,493 | 124,934 | 15 | 146 |
| 17 May 2021 | 371 | 3706 | 176 | 1765 | 22,234 | 222,343 | 10,588 | 105,877 | 12 | 123 |
| 21 May 2021 | 307 | 3069 | 146 | 1461 | 18,414 | 184,140 | 8769 | 87,686 | 10 | 102 |
| 4 June 2021 | Not calculated because of low detection limits | |||||||||
| 18 June 2021 | ||||||||||
| 24 June 2021 | ||||||||||
| 4 July 2021 | ||||||||||
| Virus | Sampling | Detection by RT-PCR Assay/Target Gene Used | Reference |
|---|---|---|---|
| SARS-CoV-1 | Grab sampling: Sewage wastewater from two SARS patients | RT-qPCR/Three sets of primers to detect the SARS-CoV RNA: Cor-p-F2, Cor-p-F3 and Cor-p-R1 | [46] |
| Coronaviridae (Virome); Alphacoronavirus; Betacoronavirus | Grab sampling; Water body | Nuclic acid library preparation for sequencing to compare viromes | [47] |
| SARS-CoV-2 | Grab and composite sampling; Sewage wastewater | ORF1ab, E, N and S | [48] |
| Grab and composite sampling; Untreated doemsic wastewater | RT-qPCR;N_Sarbeco and NIID_2019-nCOV_N | [12,13] | |
| Composite sampling; Untreated sewage wastewater. | CDC N2 | [49] | |
| Grab Sampling; Inlets and outlet of WWTP/STP and septic tank influent of hospital | CCDC-ORF1 andCCDC-N | [50] | |
| Composite sampling; Untreated wastewater | NA | [51] | |
| Composite sampling; Untreated and treated wastewater | M and RdRP | [35] | |
| Grab and composite sampling; Untreated wastewater | ORF1ab, N and E | [52] | |
| Grab sampling; Influent and secondary treated wastewater | N_Sarbeco NIID_2019-nCOV_N CDC N1, N2 and N3 | [6,53] | |
| Grab samples, Influent; Secondary and tertiary effluents | CDC N1, N2, N3 | [6] | |
| Grabs and composite sampling; Secondary treated effluent, untreated and treated wastewater | CDC N1, CDC N2 and CDC N3 | [54,55,56] | |
| Grab samples; inlet and outlet of STP/WWTP | E, N and ORF1ab | [28] | |
| Grab and composite sampling; Drains | E, N and ORF1ab | [22] | |
| Grab sampling; Drains | RT-PCR; N1, N2 and N3 | [57] | |
| Passive sampling device: Sewage wastewater | N2 | [58] | |
| Grab and composite sampling; WWTP | N1 and N2 | [59] | |
| Grabsampling; Drains | ORF1ab | [60] | |
| Composite sampling (Auto-sampler); Inlet of WWTP | E, RdRp and N | [61] | |
| Composite sampling; WWTP/STP | N | [62] | |
| Composite sampling; WWTP | N, S and ORF1ab | [63] | |
| Grab Sampling; Water bodies | E, N and ORF1ab | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tharak, A.; Kopperi, H.; Hemalatha, M.; Kiran, U.; C. G., G.; Moharir, S.; Mishra, R.K.; Mohan, S.V. Longitudinal and Long-Term Wastewater Surveillance for COVID-19: Infection Dynamics and Zoning of Urban Community. Int. J. Environ. Res. Public Health 2022, 19, 2697. https://doi.org/10.3390/ijerph19052697
Tharak A, Kopperi H, Hemalatha M, Kiran U, C. G. G, Moharir S, Mishra RK, Mohan SV. Longitudinal and Long-Term Wastewater Surveillance for COVID-19: Infection Dynamics and Zoning of Urban Community. International Journal of Environmental Research and Public Health. 2022; 19(5):2697. https://doi.org/10.3390/ijerph19052697
Chicago/Turabian StyleTharak, Athmakuri, Harishankar Kopperi, Manupati Hemalatha, Uday Kiran, Gokulan C. G., Shivranjani Moharir, Rakesh K. Mishra, and S. Venkata Mohan. 2022. "Longitudinal and Long-Term Wastewater Surveillance for COVID-19: Infection Dynamics and Zoning of Urban Community" International Journal of Environmental Research and Public Health 19, no. 5: 2697. https://doi.org/10.3390/ijerph19052697
APA StyleTharak, A., Kopperi, H., Hemalatha, M., Kiran, U., C. G., G., Moharir, S., Mishra, R. K., & Mohan, S. V. (2022). Longitudinal and Long-Term Wastewater Surveillance for COVID-19: Infection Dynamics and Zoning of Urban Community. International Journal of Environmental Research and Public Health, 19(5), 2697. https://doi.org/10.3390/ijerph19052697






