Let It Beat: How Lifestyle and Psychosocial Factors Affect the Risk of Sudden Cardiac Death—A 10-Year Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
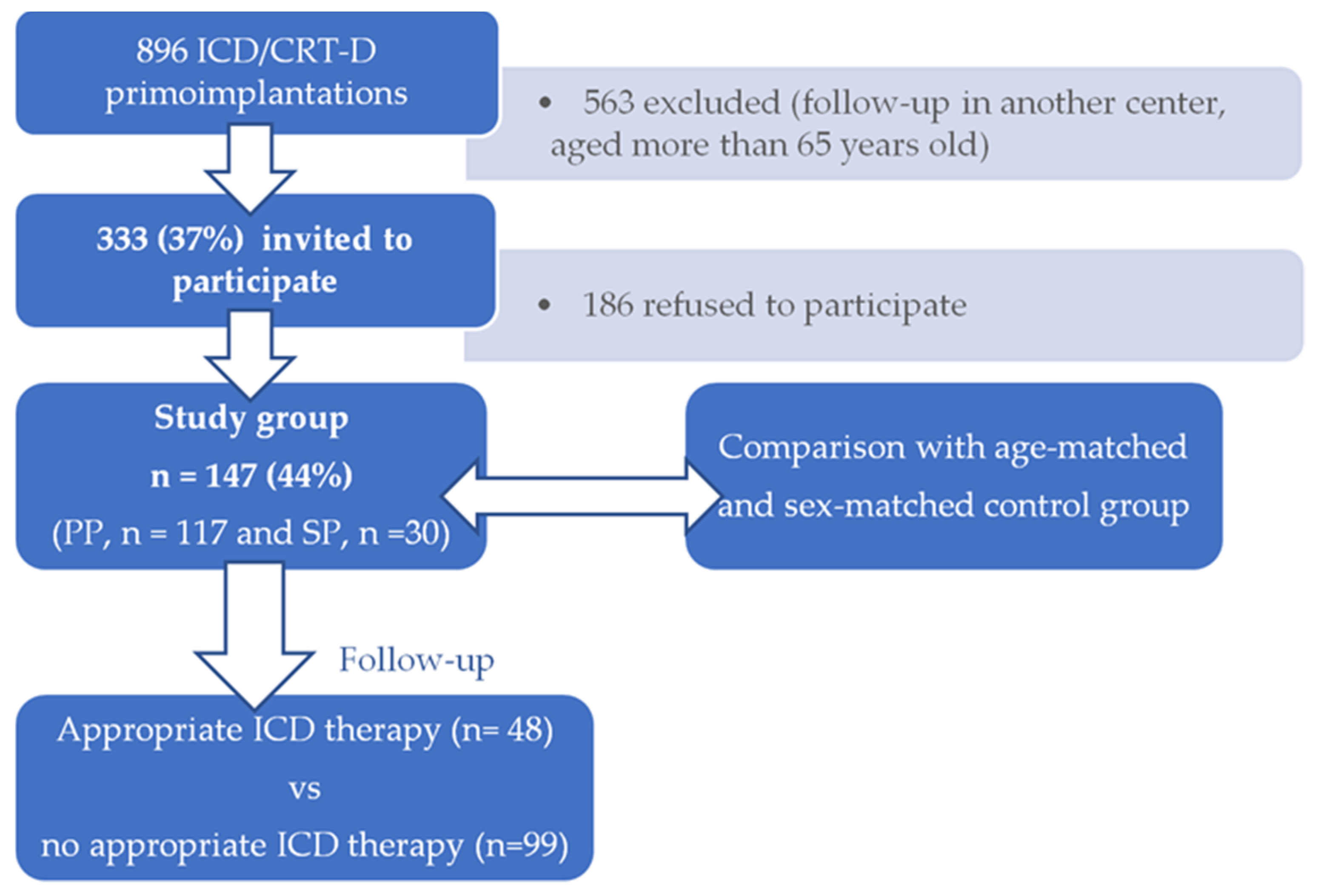
2.1. Participants and Procedure
2.2. Questionnaire
2.3. Statistical Methods
3. Results
3.1. Lifestyle and Psychosocial Factors in Primary Prevention Patients
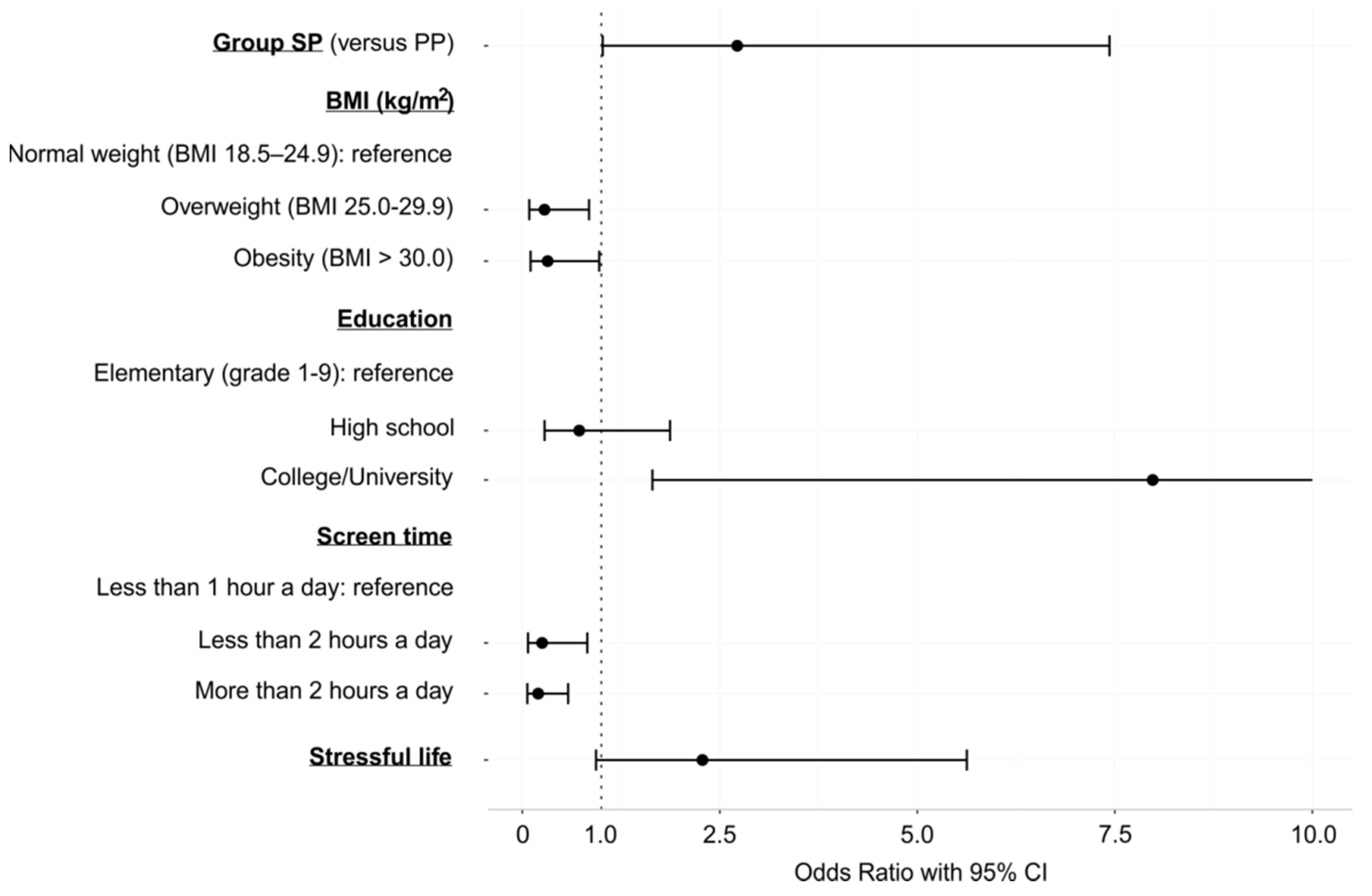
3.2. The Effect of Lifestyle and Psychosocial Factors on the Appropriate ICD Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myerburg, R.J.; Goldberger, J.J. Cardiac Arrest and Sudden Cardiac Death. In Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef] [PubMed]
- Bardy, G.H.; Lee, K.L.; Mark, D.B.; Poole, J.E.; Packer, D.L.; Boineau, R.; Domanski, M.; Troutman, C.; Anderson, J.; Johnson, G.; et al. Amiodarone or an Implantable Cardioverter–Defibrillator for Congestive Heart Failure. N. Engl. J. Med. 2005, 352, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A Comparison of Antiarrhythmic-Drug Therapy with Implantable Defibrillators in Patients Resuscitated from near-Fatal Ventricular Arrhythmias. N. Engl. J. Med. 1997, 337, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Buxton, A.E.; Lee, K.L.; Fisher, J.D.; Josephson, M.E.; Prystowsky, E.N.; Hafley, G. A Randomized Study of the Prevention of Sudden Death in Patients with Coronary Artery Disease. N. Engl. J. Med. 1999, 341, 1882–1890. [Google Scholar] [CrossRef]
- Kuck, K.-H.; Cappato, R.; Siebels, J.; Rüppel, R. Randomized Comparison of Antiarrhythmic Drug Therapy With Implantable Defibrillators in Patients Resuscitated From Cardiac Arrest: The Cardiac Arrest Study Hamburg (CASH). Circulation 2000, 102, 748–754. [Google Scholar] [CrossRef]
- Connolly, S. Meta-Analysis of the Implantable Cardioverter Defibrillator Secondary Prevention Trials. Eur. Heart J. 2000, 21, 2071–2078. [Google Scholar] [CrossRef]
- Moss, A.J.; Zareba, W.; Hall, W.J.; Klein, H.; Wilber, D.J.; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Brown, M.W.; Andrews, M.L. Prophylactic Implantation of a Defibrillator in Patients with Myocardial Infarction and Reduced Ejection Fraction. N. Engl. J. Med. 2002, 346, 877–883. [Google Scholar] [CrossRef]
- Gräsner, J.-T.; Herlitz, J.; Tjelmeland, I.B.M.; Wnent, J.; Masterson, S.; Lilja, G.; Bein, B.; Böttiger, B.W.; Rosell-Ortiz, F.; Nolan, J.P.; et al. European Resuscitation Council Guidelines 2021: Epidemiology of Cardiac Arrest in Europe. Resuscitation 2021, 161, 61–79. [Google Scholar] [CrossRef]
- Wellens, H.J.J.; Schwartz, P.J.; Lindemans, F.W.; Buxton, A.E.; Goldberger, J.J.; Hohnloser, S.H.; Huikuri, H.V.; Kaab, S.; La Rovere, M.T.; Malik, M.; et al. Risk Stratification for Sudden Cardiac Death: Current Status and Challenges for the Future. Eur. Heart J. 2014, 35, 1642–1651. [Google Scholar] [CrossRef]
- Van Rees, J.B.; Borleffs, C.J.W.; De Bie, M.K.; Stijnen, T.; Van Erven, L.; Bax, J.J.; Schalij, M.J. Inappropriate Implantable Cardioverter-Defibrillator Shocks. J. Am. Coll. Cardiol. 2011, 57, 556–562. [Google Scholar] [CrossRef]
- Daubert, J.P.; Zareba, W.; Cannom, D.S.; McNitt, S.; Rosero, S.Z.; Wang, P.; Schuger, C.; Steinberg, J.S.; Higgins, S.L.; Wilber, D.J.; et al. Inappropriate Implantable Cardioverter-Defibrillator Shocks in MADIT II. J. Am. Coll. Cardiol. 2008, 51, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Persson, R.; Earley, A.; Garlitski, A.C.; Balk, E.M.; Uhlig, K. Adverse Events Following Implantable Cardioverter Defibrillator Implantation: A Systematic Review. J. Interv. Card. Electrophysiol. 2014, 40, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, G.M.; Castelnuovo, G.; Compare, A.; Pagnini, F.; Essebag, V.; Proietti, R. Psychological Effects of Implantable Cardioverter Defibrillator Shocks. A Review of Study Methods. Front. Psychol. 2015, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of Potentially Modifiable Risk Factors Associated with Myocardial Infarction in 52 Countries (the INTERHEART Study): Case-Control Study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Rosengren, A.; Hawken, S.; Ôunpuu, S.; Sliwa, K.; Zubaid, M.; Almahmeed, W.A.; Blackett, K.N.; Sitthi-amorn, C.; Sato, H.; Yusuf, S. Association of Psychosocial Risk Factors with Risk of Acute Myocardial Infarction in 11,119 Cases and 13,648 Controls from 52 Countries (the INTERHEART Study): Case-Control Study. Lancet 2004, 364, 953–962. [Google Scholar] [CrossRef]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable Risk Factors, Cardiovascular Disease, and Mortality in 155 722 Individuals from 21 High-Income, Middle-Income, and Low-Income Countries (PURE): A Prospective Cohort Study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef]
- Dennison, R.A.; Feldman, A.L.; Usher-Smith, J.A.; Griffin, S.J. The Association between Psychosocial Factors and Change in Lifestyle Behaviour Following Lifestyle Advice and Information about Cardiovascular Disease Risk. BMC Public Health 2018, 18, 731. [Google Scholar] [CrossRef]
- Guimarães, P.O.; Granger, C.B.; Stebbins, A.; Chiswell, K.; Held, C.; Hochman, J.S.; Krug-Gourley, S.; Lonn, E.; Lopes, R.D.; Stewart, R.A.H.; et al. Sex Differences in Clinical Characteristics, Psychosocial Factors, and Outcomes Among Patients with Stable Coronary Heart Disease: Insights from the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) Trial. J. Am. Heart Assoc. 2017, 6, e006695. [Google Scholar] [CrossRef]
- Pedersen, S.S.; Von Känel, R.; Tully, P.J.; Denollet, J. Psychosocial Perspectives in Cardiovascular Disease. Eur. J. Prev. Cardiol. 2017, 24, 108–115. [Google Scholar] [CrossRef]
- Neylon, A.; Canniffe, C.; Anand, S.; Kreatsoulas, C.; Blake, G.J.; Sugrue, D.; McGorrian, C. A Global Perspective on Psychosocial Risk Factors for Cardiovascular Disease. Prog. Cardiovasc. Dis. 2013, 55, 574–581. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Albus, C.; Waller, C.; Fritzsche, K.; Gunold, H.; Haass, M.; Hamann, B.; Kindermann, I.; Köllner, V.; Leithäuser, B.; Marx, N.; et al. Significance of Psychosocial Factors in Cardiology: Update 2018: Position Paper of the German Cardiac Society. Clin. Res. Cardiol. 2019, 108, 1175–1196. [Google Scholar] [CrossRef] [PubMed]
- Pogosova, N.; Saner, H.; Pedersen, S.S.; Cupples, M.E.; McGee, H.; Höfer, S.; Doyle, F.; Schmid, J.-P.; Von Känel, R. Psychosocial Aspects in Cardiac Rehabilitation: From Theory to Practice. A Position Paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation of the European Society of Cardiology. Eur. J. Prev. Cardiol. 2015, 22, 1290–1306. [Google Scholar] [CrossRef]
- Braunschweig, F.; Boriani, G.; Bauer, A.; Hatala, R.; Herrmann-Lingen, C.; Kautzner, J.; Pedersen, S.S.; Pehrson, S.; Ricci, R.; Schalij, M.J. Management of Patients Receiving Implantable Cardiac Defibrillator Shocks: Recommendations for Acute and Long-Term Patient Management. Europace 2010, 12, 1673–1690. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization. J. Am. Coll. Cardiol. 2021, 79, S0735109721061581. [Google Scholar] [CrossRef]
- Vaccarino, V.; Badimon, L.; Bremner, J.D.; Cenko, E.; Cubedo, J.; Dorobantu, M.; Duncker, D.J.; Koller, A.; Manfrini, O.; Milicic, D.; et al. Depression and Coronary Heart Disease: 2018 Position Paper of the ESC Working Group on Coronary Pathophysiology and Microcirculation. Eur. Heart J. 2020, 41, 1687–1696. [Google Scholar] [CrossRef]
- Steptoe, A.; Kivimäki, M. Stress and Cardiovascular Disease: An Update on Current Knowledge. Annu. Rev. Public Health 2013, 34, 337–354. [Google Scholar] [CrossRef]
- Heikkilä, K.; Pentti, J.; Madsen, I.E.H.; Lallukka, T.; Virtanen, M.; Alfredsson, L.; Bjorner, J.; Borritz, M.; Brunner, E.; Burr, H.; et al. Job Strain as a Risk Factor for Peripheral Artery Disease: A Multi-Cohort Study. J. Am. Heart Assoc. 2020, 9, e013538. [Google Scholar] [CrossRef]
- Glover, L.M.; Cain-Shields, L.R.; Spruill, T.M.; O’Brien, E.C.; Barber, S.; Loehr, L.; Sims, M. Goal-Striving Stress and Incident Cardiovascular Disease in Blacks: The Jackson Heart Study. J. Am. Heart Assoc. 2020, 9, e015707. [Google Scholar] [CrossRef] [PubMed]
- Carazo, M.R.; Kolodziej, M.S.; DeWitt, E.S.; Kasparian, N.A.; Newburger, J.W.; Duarte, V.E.; Singh, M.N.; Opotowsky, A.R. Prevalence and Prognostic Association of a Clinical Diagnosis of Depression in Adult Congenital Heart Disease: Results of the Boston Adult Congenital Heart Disease Biobank. J. Am. Heart Assoc. 2020, 9, e014820. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, J.H.; Froelicher, E.S.; Blumenthal, J.A.; Carney, R.M.; Doering, L.V.; Frasure-Smith, N.; Freedland, K.E.; Jaffe, A.S.; Leifheit-Limson, E.C.; Sheps, D.S.; et al. Depression as a Risk Factor for Poor Prognosis Among Patients with Acute Coronary Syndrome: Systematic Review and Recommendations: A Scientific Statement from the American Heart Association. Circulation 2014, 129, 1350–1369. [Google Scholar] [CrossRef] [PubMed]
- De Mestral, C.; Stringhini, S. Socioeconomic Status and Cardiovascular Disease: An Update. Curr. Cardiol. Rep. 2017, 19, 115. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W.M.; Kelli, H.M.; Lisko, J.C.; Varghese, T.; Shen, J.; Sandesara, P.; Quyyumi, A.A.; Taylor, H.A.; Gulati, M.; Harold, J.G.; et al. Socioeconomic Status and Cardiovascular Outcomes: Challenges and Interventions. Circulation 2018, 137, 2166–2178. [Google Scholar] [CrossRef] [PubMed]
- Lagraauw, H.M.; Kuiper, J.; Bot, I. Acute and Chronic Psychological Stress as Risk Factors for Cardiovascular Disease: Insights Gained from Epidemiological, Clinical and Experimental Studies. Brain. Behav. Immun. 2015, 50, 18–30. [Google Scholar] [CrossRef]
- Mostofsky, E.; Penner, E.A.; Mittleman, M.A. Outbursts of Anger as a Trigger of Acute Cardiovascular Events: A Systematic Review and Meta-Analysis. Eur. Heart J. 2014, 35, 1404–1410. [Google Scholar] [CrossRef]
- Crowley, M.J.; Zullig, L.L.; Shah, B.R.; Shaw, R.J.; Lindquist, J.H.; Peterson, E.D.; Bosworth, H.B. Medication Non-Adherence After Myocardial Infarction: An Exploration of Modifying Factors. J. Gen. Intern. Med. 2015, 30, 83–90. [Google Scholar] [CrossRef]
- Leifheit-Limson, E.C.; Kasl, S.V.; Lin, H.; Buchanan, D.M.; Peterson, P.N.; Spertus, J.A.; Lichtman, J.H. Adherence to Risk Factor Management Instructions after Acute Myocardial Infarction: The Role of Emotional Support and Depressive Symptoms. Ann. Behav. Med. 2012, 43, 198–207. [Google Scholar] [CrossRef][Green Version]
- Crawshaw, J.; Auyeung, V.; Norton, S.; Weinman, J. Identifying Psychosocial Predictors of Medication Non-Adherence Following Acute Coronary Syndrome: A Systematic Review and Meta-Analysis. J. Psychosom. Res. 2016, 90, 10–32. [Google Scholar] [CrossRef]
- Lampert, R. Behavioral Influences on Cardiac Arrhythmias. Trends Cardiovasc. Med. 2016, 26, 68–77. [Google Scholar] [CrossRef]
- Hemingway, H. Social and Psychosocial Influences on Sudden Cardiac Death, Ventricular Arrhythmia and Cardiac Autonomic Function. Eur. Heart J. 2001, 22, 1082–1101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peacock, J.; Whang, W. Psychological Distress and Arrhythmia: Risk Prediction and Potential Modifiers. Prog. Cardiovasc. Dis. 2013, 55, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Taborsky, M.; Kautzner, J. Zásady pro Implantace Kardiostimulátorů, Implantabilních Kardioverterů-Defi Brilátorů a Systémů pro Srdeční Resynchronizační Léčbu 2009. Cor Vasa 2009, 51, 602–614. [Google Scholar]
- Hammill, S.C.; Stevenson, L.W.; Kadish, A.H.; Kremers, M.S.; Heidenreich, P.; Lindsay, B.D.; Mirro, M.J.; Radford, M.J.; Wang, Y.; Lang, C.M.; et al. Review of the Registry’s First Year, Data Collected, and Future Plans. Heart Rhythm 2007, 4, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Proclemer, A.; Zecchin, M.; D’onofrio, A.; Boriani, G.; Facchin, D.; Rebellato, L.; Ghidina, M.; Bianco, G.; Bernardelli, E.; Miconi, A.; et al. Registro Italiano Pacemaker e Defibrillatori-Bollettino Periodico 2018. Associazione Italiana di Aritmologia e Cardiostimolazione. G. Ital. Cardiol. 2020, 21, 157–169. [Google Scholar] [CrossRef]
- Fernández Lozano, I.; Osca Asensi, J.; Alzueta Rodríguez, J. Spanish Implantable Cardioverter-Defibrillator Registry. 17th Official Report of the Heart Rhythm Association of the Spanish Society of Cardiology (2020). Rev. Esp. Cardiol. Engl. Ed. 2021, 74, 971–982. [Google Scholar] [CrossRef]
- Vandenberk, B.; Garweg, C.; Voros, G.; Floré, V.; Marynissen, T.; Sticherling, C.; Zabel, M.; Ector, J.; Willems, R. Changes in Implantation Patterns and Therapy Rates of Implantable Cardioverter Defibrillators over Time in Ischemic and Dilated Cardiomyopathy Patients: CLINICAL LESSONS FROM A LONG-TERM ICD EXPERIENCE. Pacing Clin. Electrophysiol. 2016, 39, 848–857. [Google Scholar] [CrossRef]
- OECD. Education at a Glance 2021: OECD Indicators; Education at a Glance; OECD: Paris, France, 2021; ISBN 978-92-64-36077-8. [Google Scholar]
- Khaing, W.; Vallibhakara, S.A.; Attia, J.; McEvoy, M.; Thakkinstian, A. Effects of Education and Income on Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2017, 24, 1032–1042. [Google Scholar] [CrossRef]
- Svane, J.; Pedersen-Bjergaard, U.; Tfelt-Hansen, J. Diabetes and the Risk of Sudden Cardiac Death. Curr. Cardiol. Rep. 2020, 22, 112. [Google Scholar] [CrossRef]
- Jouven, X.; Desnos, M.; Guerot, C.; Ducimetière, P. Predicting Sudden Death in the Population: The Paris Prospective Study I. Circulation 1999, 99, 1978–1983. [Google Scholar] [CrossRef] [PubMed]
- Hookana, E.; Junttila, M.J.; Kaikkonen, K.S.; Ukkola, O.; Kesäniemi, Y.A.; Kortelainen, M.-L.; Huikuri, H.V. Comparison of Family History of Sudden Cardiac Death in Nonischemic and Ischemic Heart Disease. Circ. Arrhythm. Electrophysiol. 2012, 5, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Kaikkonen, K.S.; Kortelainen, M.-L.; Linna, E.; Huikuri, H.V. Family History and the Risk of Sudden Cardiac Death as a Manifestation of an Acute Coronary Event. Circulation 2006, 114, 1462–1467. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018, 138, e272–e391. [Google Scholar] [CrossRef]
- Stiles, M.K.; Wilde, A.A.M.; Abrams, D.J.; Ackerman, M.J.; Albert, C.M.; Behr, E.R.; Chugh, S.S.; Cornel, M.C.; Gardner, K.; Ingles, J.; et al. 2020 APHRS/HRS Expert Consensus Statement on the Investigation of Decedents with Sudden Unexplained Death and Patients with Sudden Cardiac Arrest, and of Their Families. Heart Rhythm 2021, 18, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Paratz, E.D.; Rowsell, L.; Zentner, D.; Parsons, S.; Morgan, N.; Thompson, T.; James, P.; Pflaumer, A.; Semsarian, C.; Smith, K.; et al. Cardiac Arrest and Sudden Cardiac Death Registries: A Systematic Review of Global Coverage. Open Heart 2020, 7, e001195. [Google Scholar] [CrossRef]
- Shi, S.; Liu, T.; Liang, J.; Hu, D.; Yang, B. Depression and Risk of Sudden Cardiac Death and Arrhythmias: A Meta-Analysis. Psychosom. Med. 2017, 79, 153–161. [Google Scholar] [CrossRef]
- Silverman, A.L.; Herzog, A.A.; Silverman, D.I. Hearts and Minds: Stress, Anxiety, and Depression: Unsung Risk Factors for Cardiovascular Disease. Cardiol. Rev. 2019, 27, 202–207. [Google Scholar] [CrossRef]
- Sharma, A.; Lavie, C.J.; Borer, J.S.; Vallakati, A.; Goel, S.; Lopez-Jimenez, F.; Arbab-Zadeh, A.; Mukherjee, D.; Lazar, J.M. Meta-Analysis of the Relation of Body Mass Index to All-Cause and Cardiovascular Mortality and Hospitalization in Patients With Chronic Heart Failure. Am. J. Cardiol. 2015, 115, 1428–1434. [Google Scholar] [CrossRef]
- Stein, K.M.; Mittal, S.; Gilliam, F.R.; Gilligan, D.M.; Zhong, Q.; Kraus, S.M.; Meyer, T.E. Predictors of Early Mortality in Implantable Cardioverter-Defibrillator Recipients. Europace 2009, 11, 734–740. [Google Scholar] [CrossRef]
- Jahangir, A.; Mirza, M.; Shahreyar, M.; Mengesha, T.; Shearer, R.; Sultan, S.; Jahangir, A.; Choudhuri, I.; Nangia, V.; Dhala, A.; et al. Presence of Obesity Is Associated with Lower Mortality in Elderly Patients with Implantable Cardioverter Defibrillator. Int. J. Obes. 2018, 42, 169–174. [Google Scholar] [CrossRef] [PubMed]
- González-Cambeiro, M.C.; Rodríguez-Mañero, M.; Abellas-Sequeiros, A.; Moreno-Arribas, J.; Filgueira-Rama, D.; González-Juanatey, J.R. Prognostic Effect of Body Mass Index in Patients with an Implantable Cardioverter-Defibrillator for Primary Prevention of Sudden Death. Rev. Esp. Cardiol. Engl. Ed. 2016, 69, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Pietrasik, G.; Goldenberg, I.; McNITT, S.; Moss, A.J.; Zareba, W. Obesity As a Risk Factor for Sustained Ventricular Tachyarrhythmias in MADIT II Patients. J. Cardiovasc. Electrophysiol. 2007, 18, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhao, S.; Tang, M.; Chen, K.; Hua, W.; Su, Y.; Chen, S.; Liang, Z.; Xu, W.; Li, X.; et al. Overweight and Obesity as Protective Factors against Mortality in Nonischemic Cardiomyopathy Patients with an Implantable Cardioverter Defibrillator. Clin. Cardiol. 2020, 43, 1435–1442. [Google Scholar] [CrossRef]
- Varghese, S.; Geller, J.C.; Ohlow, M.-A. Decision Regret in Implantable Cardioverter-Defibrillator Recipients: A Cross-Sectional Analysis on Patients That Regret Their Decision after ICD Implantation. Herzschrittmachertherapie Elektrophysiologie 2020, 31, 77–83. [Google Scholar] [CrossRef]
| Characteristics | Primary Prevention Patients (PP) | Secondary Prevention Patients (SP) | PP vs. SP | Control Group (CG) | CG vs. PP | CG vs. SP |
|---|---|---|---|---|---|---|
| n = 117 | n = 30 | p-Value | n = 205 | p-Value | p-Value | |
| Age: Mean (SD) | n.s. | n.s. | n.s. | |||
| 55.9 (9.7) | 55.2 (8.8) | 53.0 (13.4) | ||||
| Gender: n (%) | n.s. | 0.001 | n.s. | |||
| Male | 95 (81.2) | 23 (76.7) | 132 (64.4) | |||
| Female | 22 (18.8) | 7 (23.3) | 73 (35.6) | |||
| BMI: n (%) | n.s. | 0.021 | n.s. | |||
| Underweight (BMI < 18.4 kg/m2) | 1 (0.9) | 1 (3.3) | 1 (0.5) | |||
| Normal weight (BMI 18.5–24.9 kg/m2) | 16 (13.7) | 6 (20.0) | 51 (24.9) | |||
| Overweight (BMI 25.0–29.9 kg/m2) | 41 (35.0) | 15 (50.0) | 77 (37.6) | |||
| Obesity (BMI > 30.0 kg/m2) | 58 (49.6) | 8 (26.7) | 68 (33.2) | |||
| Education: n (%) | n.s. | 0.003 | 0.037 | |||
| Elementary (grade 1–9) | 28 (23.9) | 7 (23.3) | 23 (11.2) | |||
| High school | 75 (64.1) | 21 (70.0) | 142 (69.3) | |||
| College/University | 9 (7.7) | 1 (3.3) | 33 (16.1) | |||
| Screen time a: n (%) | n.s. | 0.005 | 0.007 | |||
| Not at all | 0 (0.0) | 0 (0.0) | 10 (4.9) | |||
| Less than 1 h a day | 19 (16.2) | 3 (10.0) | 44 (21.5) | |||
| Less than 2 h a day | 38 (32.5) | 7 (23.3) | 80 (39.0) | |||
| More than 2 h a day | 60 (51.3) | 20 (66.7) | 70 (34.2) | |||
| Sport b: n (%) | n.s. | 0.042 | n.s. | |||
| Less than once a week | 92 (78.6) | 25 (83.3) | 139 (67.8) | |||
| Once or twice a week | 19 (16.2) | 3 (10.0) | 41 (20.0) | |||
| At least 3x per week | 5 (4.3) | 2 (6.7) | 24 (11.7) | |||
| Health status c: n (%) | ||||||
| Smoking | 52 (44.4) | 12 (40.0) | n.s. | 70 (34.1) | n.s. | n.s. |
| Stressful life | 28 (23.9) | 11 (36.7) | n.s. | 59 (28.8) | n.s. | n.s. |
| Major life event during past year | 45 (38.5) | 14 (46.7) | n.s. | 106 (51.7) | n.s. | n.s. |
| SCD in family | 21 (18.0) | 6 (20.0) | n.s. | 14 (6.8) | 0.002 | 0.016 |
| Coronary artery disease | 53 (45.3) | 14 (46.7) | n.s. | 15 (7.3) | <0.001 | <0.001 |
| Diabetes mellitus | 41 (35.0) | 5 (16.7) | n.s. | 19 (9.3) | <0.001 | n.s. |
| Dyslipidemia | 59 (50.4) | 12 (40.0) | n.s. | 28 (13.7) | <0.001 | 0.001 |
| Hypertension | 74 (63.2) | 19 (63.3) | n.s. | 81 (39.5) | <0.001 | 0.015 |
| Depression | 14 (12.0) | 3 (10.0) | n.s. | 4 (2.0) | <0.001 | 0.015 |
| Other diseases | 63 (54.9) | 12 (40.0) | n.s. | 81 (39.5) | 0.013 | n.s. |
| Predictor | OR | 95% CI | p-Value |
|---|---|---|---|
| SCD in family | 2.89 | 1.06, 8.46 | 0.043 |
| Coronary artery disease | 9.30 | 4.23, 22.83 | <0.001 |
| Diabetes mellitus | 2.53 | 1.15, 5.70 | 0.022 |
| Depression | 7.12 | 1.69, 48.78 | 0.016 |
| Predictor | OR | 95% CI | p-Value |
|---|---|---|---|
| Group SP (versus PP) | 2.72 | 1.02, 7.43 | 0.047 |
| BMI | |||
| Underweight (BMI < 18.4 kg/m2) | - | - | - |
| Normal weight (BMI 18.5–24.9 kg/m2) | |||
| Overweight (BMI 25.0–29.9 kg/m2) | 0.28 | 0.09, 0.84 | 0.026 |
| Obesity (BMI > 30.0 kg/m2) | 0.32 | 0.10, 0.97 | 0.046 |
| Education | |||
| Elementary (grade 1–9) | |||
| High school | 0.72 | 0.28, 1.87 | 0.489 |
| College/University | 7.98 | 1.65, 47.71 | 0.014 |
| Screen time a | |||
| Not at all | - | - | - |
| Less than 1 h a day | |||
| Less than 2 h a day | 0.25 | 0.07, 0.82 | 0.025 |
| More than 2 h a day | 0.20 | 0.06, 0.58 | 0.004 |
| Stressful life | 2.28 | 0.93, 5.63 | 0.071 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obrova, J.; Sovova, E.; Ivanova, K.; Furstova, J.; Taborsky, M. Let It Beat: How Lifestyle and Psychosocial Factors Affect the Risk of Sudden Cardiac Death—A 10-Year Follow-Up Study. Int. J. Environ. Res. Public Health 2022, 19, 2627. https://doi.org/10.3390/ijerph19052627
Obrova J, Sovova E, Ivanova K, Furstova J, Taborsky M. Let It Beat: How Lifestyle and Psychosocial Factors Affect the Risk of Sudden Cardiac Death—A 10-Year Follow-Up Study. International Journal of Environmental Research and Public Health. 2022; 19(5):2627. https://doi.org/10.3390/ijerph19052627
Chicago/Turabian StyleObrova, Jana, Eliska Sovova, Katerina Ivanova, Jana Furstova, and Milos Taborsky. 2022. "Let It Beat: How Lifestyle and Psychosocial Factors Affect the Risk of Sudden Cardiac Death—A 10-Year Follow-Up Study" International Journal of Environmental Research and Public Health 19, no. 5: 2627. https://doi.org/10.3390/ijerph19052627
APA StyleObrova, J., Sovova, E., Ivanova, K., Furstova, J., & Taborsky, M. (2022). Let It Beat: How Lifestyle and Psychosocial Factors Affect the Risk of Sudden Cardiac Death—A 10-Year Follow-Up Study. International Journal of Environmental Research and Public Health, 19(5), 2627. https://doi.org/10.3390/ijerph19052627






