Prevalence and Risk Factors of Gestational Diabetes Mellitus in Bangladesh: Findings from Demographic Health Survey 2017–2018
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Settings
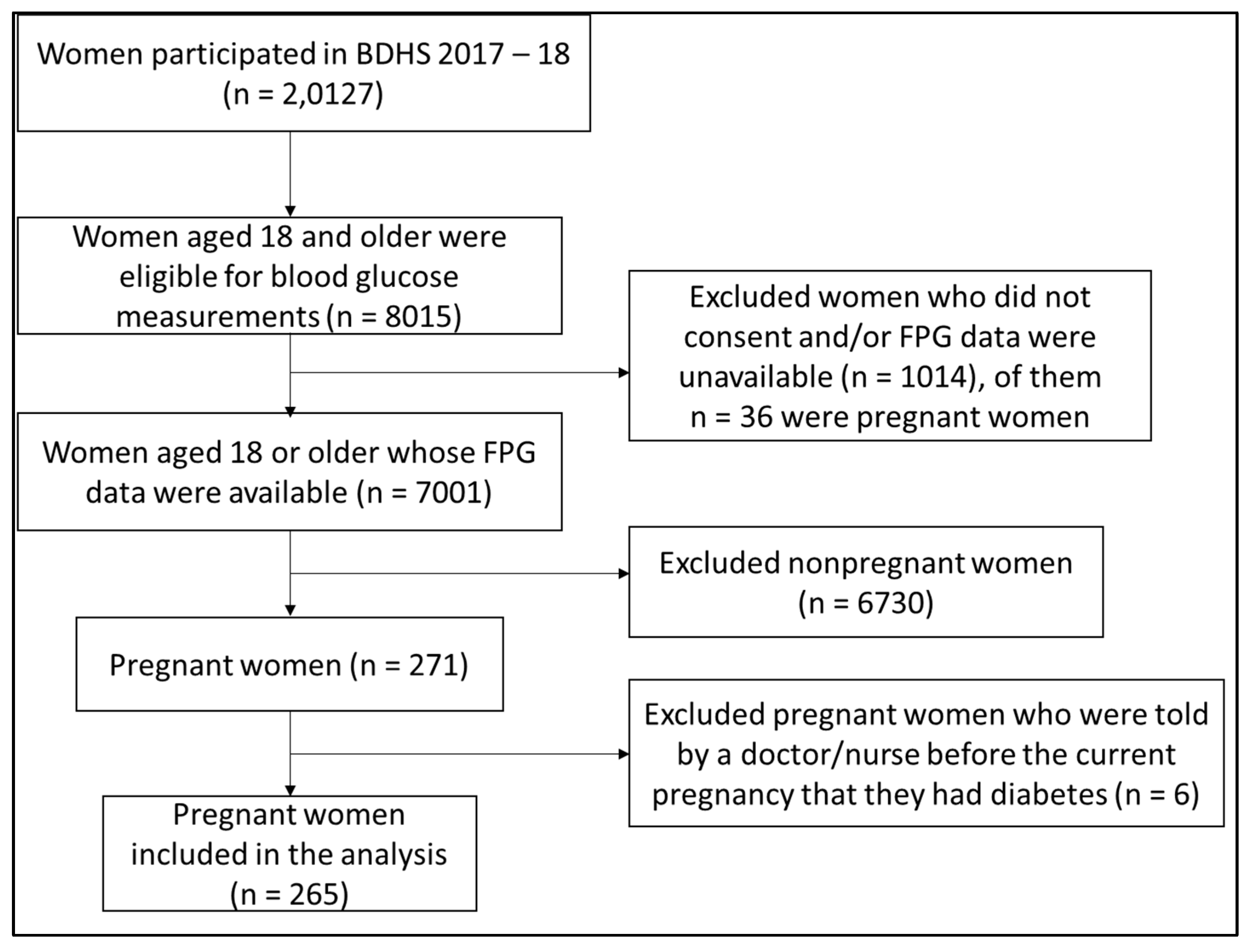
2.2. Study Population, Sample Size, and Sampling
2.3. Data Collection, Outcome, and Exposure Variables
2.4. GDM Diagnosis Criteria Used in This Study
- Fasting plasma glucose: 5.1–6.9 mmol/L or (92–125) mg/dL
- One-hour plasma glucose: ≥10.0 mmol/L or ≥180 mg/dL following a 75 g oral glucose load
- Two-hour plasma glucose: 8.5–11.0 mmol/L or (153–199 mg/dL) following a 75 g oral glucose dose
2.5. Variables of Interest
2.6. Data Analysis
2.7. Ethical Approval and Consent
3. Results
3.1. Prevalence of GDM
3.2. Risk Factors of GDM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANC | Antenatal Care |
| aOR | Adjusted Odds Ratio |
| BDHS | Bangladesh Demographic and Health Survey |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| DHS | Demographic and Health Survey |
| DM | Diabetes Mellitus |
| EAs | Enumeration Areas |
| FPG | Fasting Plasma Glucose |
| GDM | Gestational Diabetes Mellitus |
| HHs | Households |
| IADPSG | International Association of Diabetes and Pregnancy Study Groups |
| NCD | Noncommunicable Disease |
| OR | Odds Ratio |
| PSU | Primary Sampling Unit |
| SD | Standard Deviation |
| SDG | Sustainable Development Goal |
| uOR | Unadjusted Odds Ratio |
| WHO | World Health Organization |
References
- World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization Guideline. Diabetes Res. Clin. Pract. 2014, 103, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Shostrom, D.C.V.; Sun, Y.; Oleson, J.J.; Snetselaar, L.G.; Bao, W. History of Gestational Diabetes Mellitus in Relation to Cardiovascular Disease and Cardiovascular Risk Factors in US Women. Front. Endocrinol. 2017, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.; Davis, B.; Falhammar, H. Pregnancy and neonatal outcomes in Indigenous Australians with diabetes in pregnancy. World J. Diabetes 2015, 6, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Najafi, L.; Abedini, A.; Kadivar, M.; Khajavi, A.; Bordbar, A.; Noohi, A.H.; Mashak, B.; Hashemnejad, M.; Khamseh, M.E.; Malek, M. Gestational diabetes mellitus: The correlation between umbilical coiling index, and intrapartum as well as neonatal outcomes. J. Diabetes Metab. Disord. 2019, 18, 51–57. [Google Scholar] [CrossRef]
- Giannakou, K.; Evangelou, E.; Yiallouros, P.; Christophi, C.A.; Middleton, N.; Papatheodorou, E.; Papatheodorou, S.I. Risk factors for gestational diabetes: An umbrella review of meta-analyses of observational studies. PLoS ONE 2019, 14, e0215372. [Google Scholar] [CrossRef]
- Saeedi, M.; Cao, Y.; Fadl, H.; Gustafson, H.; Simmons, D. Increasing prevalence of gestational diabetes mellitus when implementing the IADPSG criteria: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2021, 172, 108642. [Google Scholar] [CrossRef]
- Behboudi-Gandevani, S.; Amiri, M.; Bidhendi Yarandi, R.; Ramezani Tehrani, F. The impact of diagnostic criteria for gestational diabetes on its prevalence: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019, 11, 11. [Google Scholar] [CrossRef]
- Begum, I.A. Gestational Diabetes Mellitus in Primi Gravida of Bangladesh in Different Trimesters. Int. J. Biol. 2014, 6, 18. [Google Scholar] [CrossRef][Green Version]
- Jesmin, S.; Akter, S.; Akashi, H.; Al-Mamun, A.; Rahman, M.A.; Islam, M.M.; Sohael, F.; Okazaki, O.; Moroi, M.; Kawano, S.; et al. Screening for gestational diabetes mellitus and its prevalence in Bangladesh. Diabetes Res. Clin. Pract. 2014, 103, 57–62. [Google Scholar] [CrossRef]
- Mannan, M.A.; Rahman, M.H.; Ara, I.; Afroz, H. Prevalence and Pregnancy Outcome of Gestational Diabetes Mellitus among Bangladeshi Urban Pregnant Women. J. Med. 2012, 13, 147–151. [Google Scholar] [CrossRef]
- Sultana, N.; Hasanat, M.A.; Jahan, S.; Hasan, M.; Aktar, Y.; Panthi, S.; Rahman, M.A.; Fariduddin, M. Association of Risk Factors in Gestational Diabetes Mellitus among Pregnant Mothers Attending at a Tertiary Care Hospital in Bangladesh. J. Bioinform. Diabetes 2016, 1, 54–60. [Google Scholar] [CrossRef]
- Sayeed, M.A.; Jahan, S.; Rhaman, M.M.; Chowdhury, M.M.H.; Khanam, P.A.; Begum, T.; Ruman, U.; Banu, A.; Mahtab, H. Prevalence and perinatal outcomes in GDM and non-GDM in a rural pregnancy cohort of Bangladesh. Ibrahim Med. Coll. J. 2013, 7, 21–27. [Google Scholar] [CrossRef]
- World Health Organization; Country Office for Bangladesh. National STEPS Survey for Non-Communicable Diseases Risk Factors in Bangladesh 2018; World Health Organization; Country Office for Bangladesh: Dhaka, Bangladesh, 2018. [Google Scholar]
- Kapur, A. Links between maternal health and NCDs. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 32–42. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Noncommunicable Diseases Country Profiles 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Riaz, B.K.; Islam, M.Z.; Islam, A.N.M.S.; Zaman, M.M.; Hossain, M.A.; Rahman, M.M.; Khanam, F.; Amin, K.M.B.; Noor, I.N. Risk factors for non-communicable diseases in Bangladesh: Findings of the population-based cross-sectional national survey 2018. BMJ Open 2020, 10, e041334. [Google Scholar] [CrossRef] [PubMed]
- United Nations. The 17 Goals. Available online: https://sdgs.un.org/goals (accessed on 28 September 2021).
- National Institute of Population Research and Training (NIPORT). Bangladesh Demographic and Health Survey 2017-18; National Institute of Population Research and Training (NIPORT); ICF International: Dhaka, Bangladesh, 2020. [Google Scholar]
- International Association of Diabetes Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Institute of Medicine. Weight Gain during Pregnancy: Reexamining the Guidelines. In The National Academies Collection: Reports Funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1, Diagnosis and Classification of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Seshiah, V.; Balaji, V.; Balaji, M.S.; Paneerselvam, A.; Arthi, T.; Thamizharasi, M.; Datta, M. Gestational diabetes mellitus manifests in all trimesters of pregnancy. Diabetes Res. Clin. Pract. 2007, 77, 482–484. [Google Scholar] [CrossRef]
- Arora, G.P.; Thaman, R.G.; Prasad, R.B.; Almgren, P.; Brøns, C.; Groop, L.C.; Vaag, A.A. Prevalence and risk factors of gestational diabetes in Punjab, North India: Results from a population screening program. Eur. J. Endocrinol. 2015, 173, 257–267. [Google Scholar] [CrossRef]
- Mohan, M.A.; Chandrakumar, A. Evaluation of prevalence and risk factors of gestational diabetes in a tertiary care hospital in Kerala. Diabetes Metab. Syndr. 2016, 10, 68–71. [Google Scholar] [CrossRef]
- Akhtar, S.; Nasir, J.A.; Sarwar, A.; Nasr, N.; Javed, A.; Majeed, R.; Salam, M.A.; Billah, B. Prevalence of diabetes and pre-diabetes in Bangladesh: A systematic review and meta-analysis. BMJ Open 2020, 10, e036086. [Google Scholar] [CrossRef]
- Saquib, N.; Saquib, J.; Ahmed, T.; Khanam, M.A.; Cullen, M.R. Cardiovascular diseases and Type 2 Diabetes in Bangladesh: A systematic review and meta-analysis of studies between 1995 and 2010. BMC Public Health 2012, 12, 434. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, G.; Swaminathan, A.; Corsi, D.J. Prevalence of Gestational Diabetes in India by Individual Socioeconomic, Demographic, and Clinical Factors. JAMA Netw. Open 2020, 3, e2025074. [Google Scholar] [CrossRef] [PubMed]
- Panaitescu, A.; Peltecu, G. Gestational diabetes. Obstetrical perspective. Acta Endocrinol. (Buchar.) 2016, 12, 331. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, M.; Mahtab, H.; Khanam, P.; Begum, R.; Banu, A.; Azad Khan, A. Diabetes and hypertension in pregnancy in a rural community of Bangladesh: A population-based study. Diabet. Med. 2005, 22, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Raets, L.; Beunen, K.; Benhalima, K. Screening for Gestational Diabetes Mellitus in Early Pregnancy: What Is the Evidence? J. Clin. Med. 2021, 10, 1257. [Google Scholar] [CrossRef]
- Biswas, T.; Pervin, S.; Tanim, M.I.A.; Niessen, L.; Islam, A. Bangladesh policy on prevention and control of non-communicable diseases: A policy analysis. BMC Public Health 2017, 17, 582. [Google Scholar] [CrossRef]
- Lee, K.W.; Ching, S.M.; Ramachandran, V.; Yee, A.; Hoo, F.K.; Chia, Y.C.; Wan Sulaiman, W.A.; Suppiah, S.; Mohamed, M.H.; Veettil, S.K. Prevalence and risk factors of gestational diabetes mellitus in Asia: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2018, 18, 494. [Google Scholar] [CrossRef]
| Variables n (%) | Total (Weighted) Frequency of Pregnant Women n (%) N = 272 | Women with GDM a n (%) n = 95 | Women without GDM a n (%) n = 177 | p-Value |
|---|---|---|---|---|
| Age group | 0.145 | |||
| <25 | 153 (56.1) | 47 (30.7) | 106 (69.3) | |
| ≥25 | 119 (43.9) | 48 (40.5) | 71 (59.5) | |
| Education | 0.350 | |||
| Primary or below | 105 (38.6) | 31 (29.2) | 74 (70.8) | |
| Secondary | 128 (47.3) | 50 (39.0) | 78 (61.0) | |
| Higher | 38 (14.1) | 14 (37.3) | 24 (62.7) | |
| Occupation | 0.585 | |||
| Employed | 100 (36.7) | 33 (32.6) | 67 (67.4) | |
| Unemployed | 172 (63.3) | 63 (36.4) | 110 (63.6) | |
| Place of residence | <0.001 | |||
| Rural | 196 (72.0) | 54 (27.4) | 142 (72.6) | |
| Urban | 76 (28.0) | 41 (54.5) | 35 (45.5)) | |
| Wealth index | 0.025 | |||
| Lowest | 93 (34.4) | 27 (28.8) | 66 (71.2) | |
| Middle | 93 (34.2) | 27 (29.3) | 66 (70.7) | |
| Highest | 86 (31.5) | 41 (48.0) | 45 (52.0) | |
| Birth order | 0.452 | |||
| First | 92 (34.0) | 27 (29.3) | 65 (70.7) | |
| Second | 88 (32.6) | 34 (38.7) | 54 (61.3) | |
| Third | 91 (33.4) | 34 (37.1) | 57 (62.9) | |
| Duration of pregnancy in weeks | <0.001 | |||
| Mean (SD b) | 21.7 (9.7) | 17.8 (8.9) | 23.8 (9.5) | |
| Pregnancy trimesters c | <0.001 | |||
| First | 49 (18.6) | 33 (67.2) | 16 (32.8) | |
| Second | 100 (37.7) | 36 (36.5) | 63 (63.5) | |
| Third | 115 (43.6) | 23 (20.3) | 92 (79.7) | |
| BMI d | 0.072 | |||
| Mean (SD b) | 19.6 (4.1) | 20.2 (4.0) | 19.4 (4.2) | |
| Hypertension | 0.310 | |||
| Yes | 249 (91.5) | 84 (33.9) | 164 (66.1) | |
| No | 23 (8.5) | 11 (46.3) | 12 (53.6) |
| Characteristics | uOR a (95% CI) | aOR b (95% CI) |
|---|---|---|
| Age (group) | ||
| <25 | 1.00 | 1.00 |
| ≥25 | 1.53 (0.93–2.54) | 2.02 (1.13–3.62) |
| Place of residence | ||
| Rural | 1.00 | 1.00 |
| Urban | 3.18 (1.83–5.51) | 2.74 (1.43–5.28) |
| Wealth quintile | ||
| Lowest | 1.00 | 1.00 |
| Middle | 1.02 (0.54–1.93) | 0.84 (0.41–1.70) |
| Highest | 2.28 (1.23–4.23) | 1.22 (0.58–2.59) |
| Duration of pregnancy (week) | 0.93 (0.91–0.96) | 0.93 (0.90–0.96) |
| BMI c | 1.05 (0.98–1.11) | 0.99 (0.92–1.96) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazumder, T.; Akter, E.; Rahman, S.M.; Islam, M.T.; Talukder, M.R. Prevalence and Risk Factors of Gestational Diabetes Mellitus in Bangladesh: Findings from Demographic Health Survey 2017–2018. Int. J. Environ. Res. Public Health 2022, 19, 2583. https://doi.org/10.3390/ijerph19052583
Mazumder T, Akter E, Rahman SM, Islam MT, Talukder MR. Prevalence and Risk Factors of Gestational Diabetes Mellitus in Bangladesh: Findings from Demographic Health Survey 2017–2018. International Journal of Environmental Research and Public Health. 2022; 19(5):2583. https://doi.org/10.3390/ijerph19052583
Chicago/Turabian StyleMazumder, Tapas, Ema Akter, Syed Moshfiqur Rahman, Md. Tauhidul Islam, and Mohammad Radwanur Talukder. 2022. "Prevalence and Risk Factors of Gestational Diabetes Mellitus in Bangladesh: Findings from Demographic Health Survey 2017–2018" International Journal of Environmental Research and Public Health 19, no. 5: 2583. https://doi.org/10.3390/ijerph19052583
APA StyleMazumder, T., Akter, E., Rahman, S. M., Islam, M. T., & Talukder, M. R. (2022). Prevalence and Risk Factors of Gestational Diabetes Mellitus in Bangladesh: Findings from Demographic Health Survey 2017–2018. International Journal of Environmental Research and Public Health, 19(5), 2583. https://doi.org/10.3390/ijerph19052583







