Long-Term PM2.5 Exposure Is Associated with Symptoms of Acute Respiratory Infections among Children under Five Years of Age in Kenya, 2014
Abstract
:1. Introduction
2. Materials and Methods
2.1. Individual Data
2.2. Air Pollution Data
2.3. Statistical and Analytic Methods
3. Results
3.1. Sample Characteristics
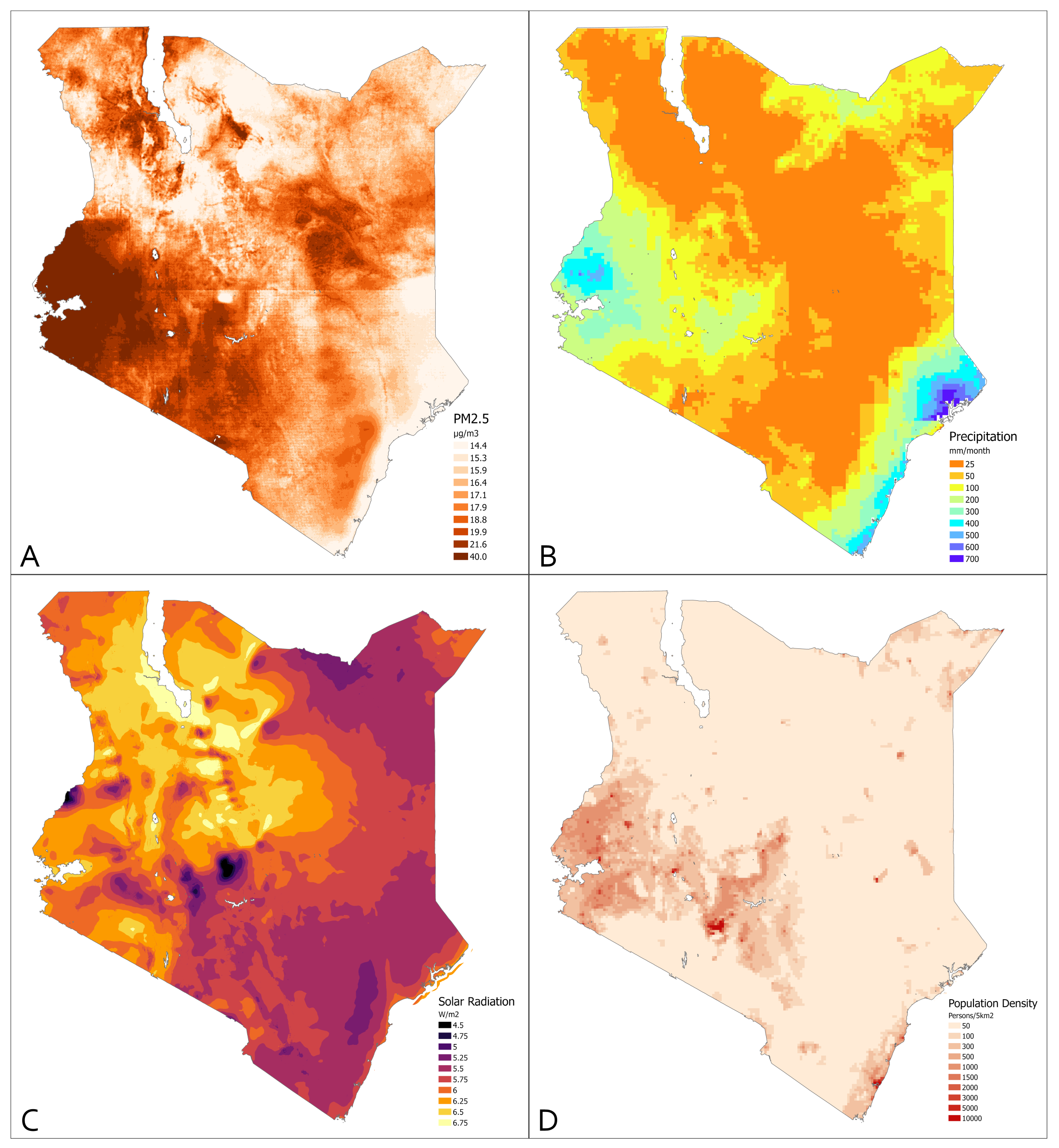
3.2. Environmental Measures
3.3. Univariate Associations of Demographic and Environmental Variables with ARI Symptoms
3.4. Multivariate Model of ARI Symptoms
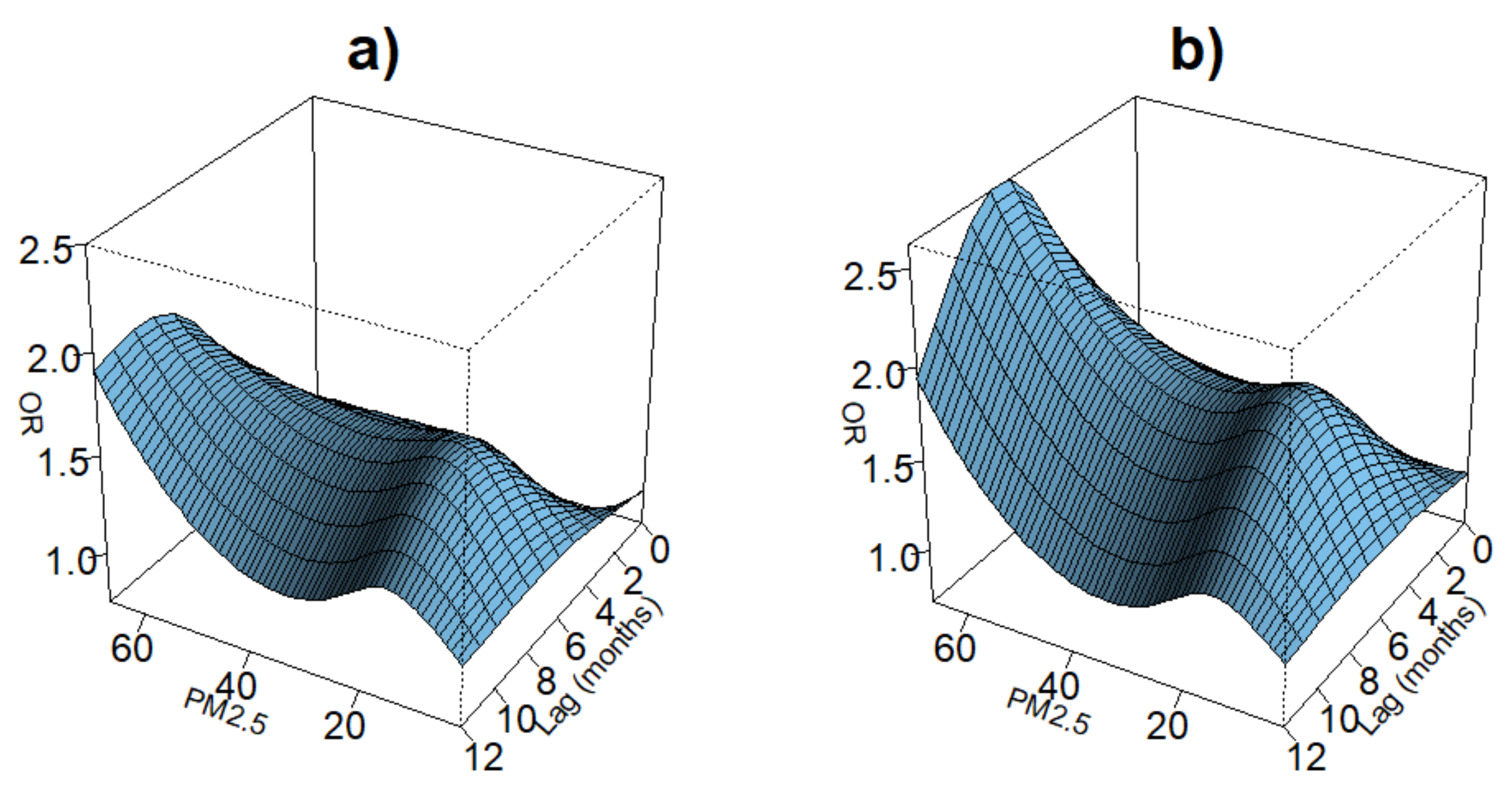
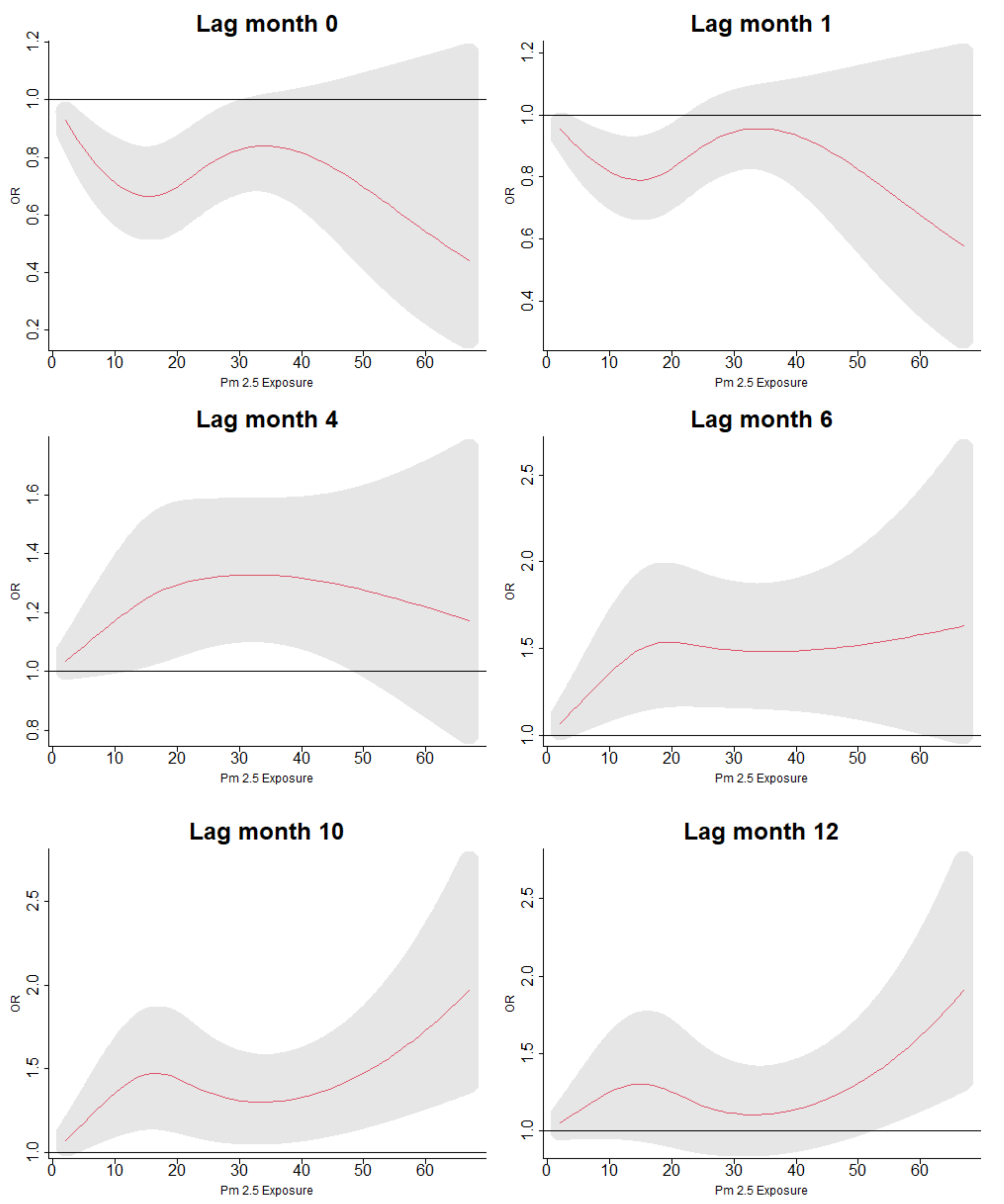
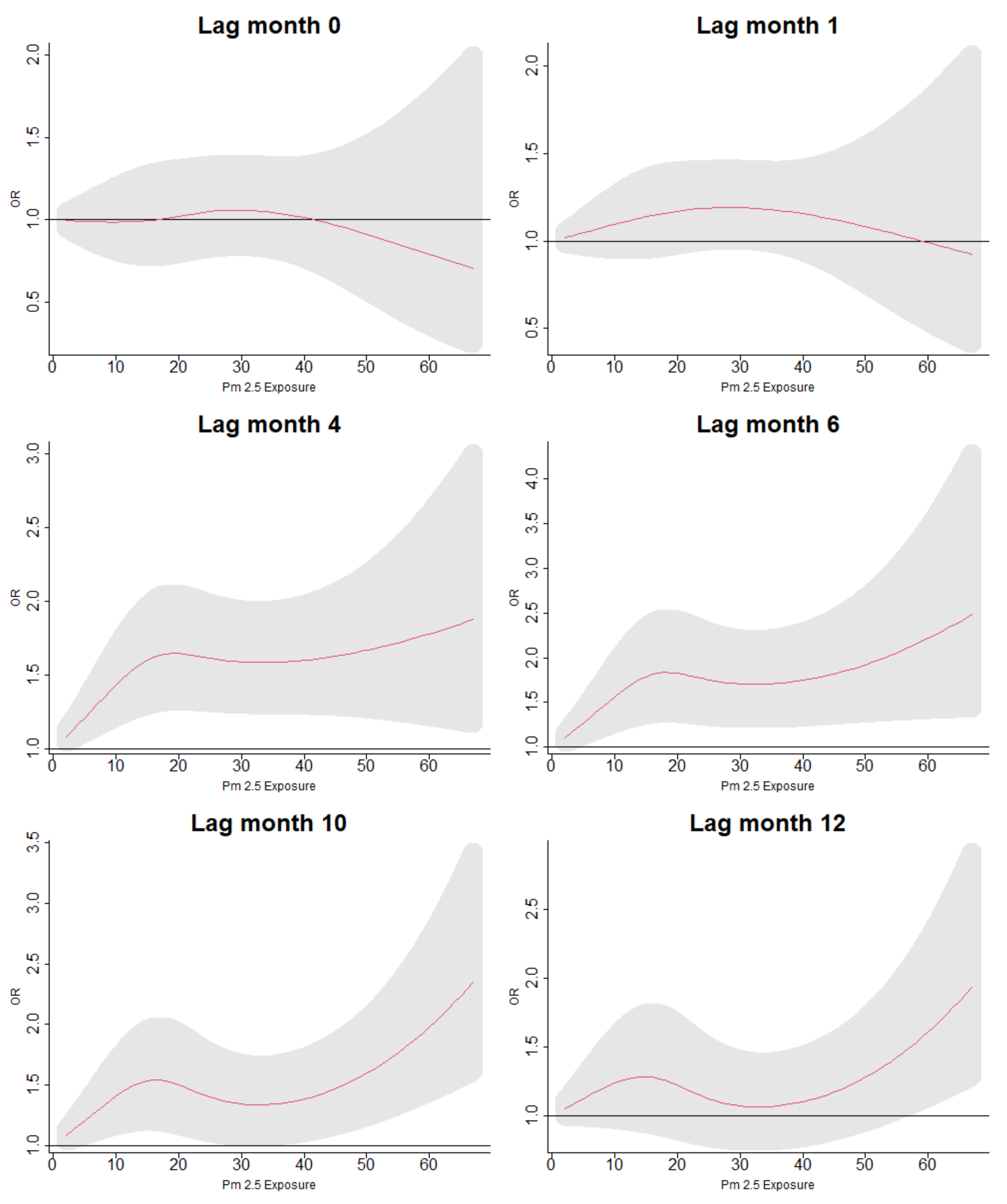
3.5. Lag Associations of PM Exposure and ARI Symptoms
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARI | Acute respiratory infection |
| BC | Black carbon |
| CI | Confidence interval |
| DHS | Demographic health survey |
| DLNM | Distributed lag nonlinear models |
| MODIS | Moderate Resolution Imaging Spectroradiometer |
| MISR | Multiangle Imaging Spectroradiometer |
| NOx | Nitrous oxide |
| OR | Odds ratio |
| RSV | Respiratory syncytial virus |
| SO | Sulfuric oxide |
| O3 | Ozone |
| SSA | Sub-Saharan Africa |
| USAID | United States Agency for International Development |
References
- Abera, A.; Friberg, J.; Isaxon, C.; Jerrett, M.; Malmqvist, E.; Sjöström, C.; Taj, T.; Vargas, A.M. Air quality in Africa: Public health implications. Annu. Rev. Public Health 2021, 42, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Agbo, K.E.; Walgraeve, C.; Eze, J.I.; Ugwoke, P.E.; Ukoha, P.O.; Van Langenhove, H. A review on ambient and indoor air pollution status in Africa. Atmos. Pollut. Res. 2021, 12, 243–260. [Google Scholar] [CrossRef]
- Emmelin, A.; Wall, S. Indoor air pollution: A poverty-related cause of mortality among the children of the world. Chest 2007, 132, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Kurmi, O.P.; Lam, K.B.H.; Ayres, J.G. Indoor air pollution and the lung in low- and medium-income countries. Eur. Respir. J. 2012, 40, 239–254. [Google Scholar] [CrossRef]
- Masekela, R.; Vanker, A. Lung health in children in Sub-Saharan Africa: Addressing the need for cleaner air. Int. J. Environ. Res. Public Health 2020, 17, 6178. [Google Scholar] [CrossRef]
- Jafta, N.; Jeena, P.M.; Barregard, L.; Naidoo, R.N. Association of childhood pulmonary tuberculosis with exposure to indoor air pollution: A case control study. BMC Public Health 2019, 19, 275. [Google Scholar] [CrossRef] [Green Version]
- Pathak, U.; Gupta, N.C.; Suri, J.C. Risk of COPD due to indoor air pollution from biomass cooking fuel: A systematic review and meta-analysis. Int. J. Environ. Health Res. 2020, 30, 75–88. [Google Scholar] [CrossRef]
- Olaniyan, T.; Dalvie, M.A.; Röösli, M.; Naidoo, R.; Künzli, N.; de Hoogh, K.; Parker, B.; Leaner, J.; Jeebhay, M. Asthma-related outcomes associated with indoor air pollutants among schoolchildren from four informal settlements in two municipalities in the Western Cape Province of South Africa. Indoor Air 2019, 29, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Thacher, J.D.; Emmelin, A.; Madaki, A.J.; Thacher, T.D. Biomass fuel use and the risk of asthma in Nigerian children. Respir. Med. 2013, 107, 1845–1851. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.G.; Lee, P.H.; Choi, S.M.; An, M.H.; Jang, A.S. Effects of air pollutants on airway diseases. Int. J. Environ. Res. Public Health 2021, 18, 9905. [Google Scholar] [CrossRef]
- Gehring, U.; Beelen, R.; Eeftens, M.; Hoek, G.; de Hoogh, K.; de Jongste, J.C.; Keuken, M.; Koppelman, G.H.; Meliefste, K.; Oldenwening, M.; et al. Particulate Matter Composition and Respiratory Health: The PIAMA Birth Cohort Study. Epidemiology 2015, 26, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowiecki, P.; Adamkiewicz, T.; Mucha, D.; Czechowski, P.O.; Soliński, M.; Chciałowski, A.; Badyda, A. Impact of air pollution on lung function among preadolescent children in two cities in Poland. J. Clin. Med. 2021, 10, 2375. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Yu, L.Y.; Mu, H.J.; Xing, L.Y.; Li, Y.X.; Pan, G.W. Shape of concentration-response curves between long-term particulate matter exposure and morbidities of chronic bronchitis: A review of epidemiological evidence. J. Thorac. Dis. 2014, 6, S720–S727. [Google Scholar] [CrossRef]
- Dimitrova, R.; Lurponglukana, N.; Fernando, H.J.S.; Runger, G.C.; Hyde, P.; Hedquist, B.C.; Anderson, J.; Bannister, W.; Johnson, W. Relationship between particulate matter and childhood asthma—Basis of a future warning system for central Phoenix. Atmos. Chem. Phys. 2012, 12, 2479–2490. [Google Scholar] [CrossRef] [Green Version]
- Keeler, G.J.; Dvonch, T.; Yip, F.Y.; Parker, E.A.; Isreal, B.A.; Marsik, F.J.; Morishita, M.; Barres, J.A.; Robins, T.G.; Brakefield-Caldwell, W.; et al. Assessment of personal and community-level exposures to particulate matter among children with asthma in Detroit, Michigan, as part of Community Action Against Asthma (CAAA). Environ. Health Perspect. 2002, 110, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.; Chen, Y.; Burnett, R.T.; Villeneuve, P.J.; Krewski, D. The influence of ambient coarse particulate matter on asthma hospitalization in children: Case-crossover and time-series analyses. Environ. Health Perspect. 2002, 110, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.W.; HuangFu, H.; Li, Y.J.; Han, R. Particulate matter pollution and allergic rhinitis. Lin Chuang Er Bi Yan Hou Ke Za Zhi 2019, 33, 285–288. [Google Scholar] [CrossRef]
- Burte, E.; Leynaert, B.; Marcon, A.; Bousquet, J.; Benmerad, M.; Bono, R.; Carsin, A.E.; De Hoogh, K.; Forsberg, B.; Gormand, F.; et al. Long-term air pollution exposure is associated with increased severity of rhinitis in two European cohorts. J. Allergy Clin. Immunol. 2020, 145, 834–842.e6. [Google Scholar] [CrossRef] [Green Version]
- Habre, R.; Moshier, E.; Castro, W.; Nath, A.; Grunin, A.; Rohr, A.; Godbold, J.; Schachter, N.; Kattan, M.; Coull, B.; et al. The effects of PM2.5 and its components from indoor and outdoor sources on cough and wheeze symptoms in asthmatic children. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 380–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, X.; Xu, X.; Zheng, X.; Reponen, T.; Chen, A.; Huo, X. Heavy metals in PM2.5 and in blood, and children’s respiratory symptoms and asthma from an e-waste recycling area. Environ. Pollut. 1987, 210, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Kambayashi, Y.; Ohkura, N.; Fujimura, M.; Nakai, S.; Honda, Y.; Saijoh, K.; Hayakawa, K.; Kobayashi, F.; Michigami, Y.; et al. Effects of Asian dust on daily cough occurrence in patients with chronic cough: A panel study. Atmos. Environ. 2014, 92, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, A.; Badyda, A.; Czechowski, P.O.; Czarnecki, A.; Dubrawski, M.; Feleszko, W. Air pollution increases the incidence of upper respiratory tract symptoms among Polish children. J. Clin. Med. 2021, 10, 2150. [Google Scholar] [CrossRef] [PubMed]
- Spira-Cohen, A.; Chen, L.C.; Kendall, M.; Lall, R.; Thurston, G.D. Personal exposures to traffic-related air pollution and acute respiratory health among Bronx schoolchildren with asthma. Environ. Health Perspect. 2011, 119, 559–565. [Google Scholar] [CrossRef]
- World Bank. World Bank Open Data; World Bank: Washington, DC, USA, 2020. [Google Scholar]
- Ngo, N.S.; Gatari, M.; Yan, B.; Chillrud, S.N.; Bouhamam, K.; Kinney, P.L. Occupational exposure to roadway emissions and inside informal settlements in sub-Saharan Africa: A pilot study in Nairobi, Kenya. Atmos. Environ. 2015, 111, 179–184. [Google Scholar] [CrossRef] [Green Version]
- de Souza, P. Air pollution in Kenya: A review. Air Qual. Atmos. Health 2020, 13, 1487–1495. [Google Scholar] [CrossRef]
- Health Effects Institute; Institute for Health Metrics and Evaluation’s. State of Global Air 2020. 2020. Available online: https://fundacionio.com/wp-content/uploads/2020/10/soga-2020-report.pdf (accessed on 15 December 2021).
- Egondi, T.; Ettarh, R.; Kyobutungi, C.; Ng, N.; Rocklöv, J. Exposure to outdoor particles (PM2.5) and associated child morbidity and mortality in socially deprived neighborhoods of Nairobi, Kenya. Atmosphere 2018, 9, 351. [Google Scholar] [CrossRef] [Green Version]
- Ezzati, M.; Kammen, D.M. Indoor air pollution from biomass combustion and acute respiratory infections in Kenya: An exposure-response study. Lancet 2001, 358, 619–624. [Google Scholar] [CrossRef]
- Agarwal, A.; Kirwa, K.; Eliot, M.N.; Alenezi, F.; Menya, D.; Mitter, S.S.; Velazquez, E.J.; Vedanthan, R.; Wellenius, G.A.; Bloomfield, G.S. Household air pollution is associated with altered cardiac function among women in Kenya. Am. J. Respir. Crit. Care Med. 2018, 197, 958–961. [Google Scholar] [CrossRef]
- Yip, F.; Christensen, B.; Sircar, K.; Naeher, L.; Bruce, N.; Pennise, D.; Lozier, M.; Pilishvili, T.; Farrar, J.L.; Stanistreet, D.; et al. Assessment of traditional and improved stove use on household air pollution and personal exposures in rural western Kenya. Environ. Int. 2017, 99, 185–191. [Google Scholar] [CrossRef]
- Ezzati, M.; Saleh, H.; Kammen, D.M. The contributions of emissions and spatial microenvironments to exposure to indoor air pollution from biomass combustion in Kenya. Environ. Health Perspect. 2000, 108, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Lozier, M.J.; Sircar, K.; Christensen, B.; Pillarisetti, A.; Pennise, D.; Bruce, N.; Stanistreet, D.; Naeher, L.; Pilishvili, T.; Farrar, J.L.; et al. Use of temperature sensors to determine exclusivity of improved stove use and associated household air pollution reductions in Kenya. Environ. Sci. Technol. 2016, 50, 4564–4571. [Google Scholar] [CrossRef]
- Pilishvili, T.; Loo, J.D.; Schrag, S.; Stanistreet, D.; Christensen, B.; Yip, F.; Nyagol, R.; Quick, R.; Sage, M.; Bruce, N. Effectiveness of six improved cookstoves in reducing household air pollution and their acceptability in rural Western Kenya. PLoS ONE 2016, 11, e0165529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulis, G.; Mulumba, J.A.A.; Juma, O.; Kakosova, B. Health status of people of slums in Nairobi, Kenya. Environ. Res. 2004, 96, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Kyobutungi, C.; Ziraba, A.K.; Ezeh, A.; Yé, Y. The burden of disease profile of residents of Nairobi’s slums: Results from a demographic surveillance system. Popul. Health Metrics 2008, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbandi, A.M.; Böhnke, J.R.; Schwela, D.; Vallack, H.; Ashmore, M.R.; Emberson, L. Estimating on-road vehicle fuel economy in Africa: A case study based on an urban transport survey in Nairobi, Kenya. Energies 2019, 12, 1177. [Google Scholar] [CrossRef] [Green Version]
- Esposito, S.; Galeone, C.; Lelii, M.; Longhi, B.; Ascolese, B.; Senatore, L.; Prada, E.; Montinaro, V.; Malerba, S.; Patria, M.F.; et al. Impact of air pollution on respiratory diseases in children with recurrent wheezing or asthma. BMC Pulm. Med. 2014, 14, 130. [Google Scholar] [CrossRef] [Green Version]
- Darrow, L.A.; Klein, M.; Flanders, W.D.; Mulholland, J.A.; Tolbert, P.E.; Strickland, M.J. Air pollution and acute respiratory infections among children 0–4 years of age: An 18-year time-series study. Am. J. Epidemiol. 2014, 180, 968–977. [Google Scholar] [CrossRef] [Green Version]
- Brauer, M.; Hoek, G.; Smit, H.A.; de Jongste, J.C.; Gerritsen, J.; Postma, D.S.; Kerkhof, M.; Brunekreef, B. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur. Respir. J. 2007, 29, 879–888. [Google Scholar] [CrossRef]
- Walker, H.K.; Hall, W.D.; Hurst, J.W. Clinical Methods: The History, Physical, and Laboratory Examinations; Butterworth-Heinemann: Oxford, UK, 1990. [Google Scholar]
- Harerimana, J.M.; Nyirazinyoye, L.; Thomson, D.R.; Ntaganira, J. Social, economic and environmental risk factors for acute lower respiratory infections among children under five years of age in Rwanda. Arch. Public Health 2016, 74, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidu, A.A.; Dickson, K.S.; Ahinkorah, B.O.; Amu, H.; Darteh, E.K.M.; Kumi-Kyereme, A. Prevalence and determinants of acute lower respiratory infections among children under-five years in sub–Saharan Africa: Evidence from demographic and health surveys. SSM-Popul. Health 2019, 8, 100443. [Google Scholar] [CrossRef] [PubMed]
- Mulatya, D.M.; Mutuku, F.W. Assessing Comorbidity of Diarrhea and Acute Respiratory Infections in Children Under 5 Years: Evidence From Kenya’s Demographic Health Survey 2014. J. Prim. Care Community Health 2020, 11, 2150132720925190. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, N.L.; Merialdi, M.; van Donkelaar, A.; Vadillo-Ortega, F.; Martin, R.V.; Betran, A.P.; Souza, J.P.; Neill, M.S.O. Outdoor air pollution, preterm birth, and low birth weight: Analysis of the world health organization global survey on maternal and perinatal health. Environ. Health Perspect. 2014, 122, 425–430. [Google Scholar] [CrossRef]
- Bowe, B.; Gibson, A.K.; Xie, Y.; Yan, Y.; Donkelaar, A.v.; Martin, R.V.; Al-Aly, Z. Ambient fine particulate matter air pollution and risk of weight gain and obesity in United States veterans: An observational cohort study. Environ. Health Perspect. 2021, 129, 047003. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, R.; Chen, Y.; Dong, X.; Zhu, J.; Liu, C.; van Donkelaar, A.; Martin, R.V.; Li, H.; Kan, H.; et al. The prospective effects of long-term exposure to ambient PM2.5 and constituents on mortality in rural East China. Chemosphere 2021, 280, 130740. [Google Scholar] [CrossRef]
- Shin, S.; Bai, L.; Burnett, R.T.; Kwong, J.C.; Hystad, P.; van Donkelaar, A.; Lavigne, E.; Weichenthal, S.; Copes, R.; Martin, R.V.; et al. Air pollution as a risk factor for incident chronic obstructive pulmonary disease and asthma. A 15-year population-based cohort study. Am. J. Respir. Crit. Care Med. 2021, 203, 1138–1148. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, S.; Zhang, Y.; Chang, H.; Martin, R.V.; Van Donkelaar, A.; Gaskins, A.; Liu, Y.; Liu, P.; Shi, L. Long-term exposure to PM2.5 major components and mortality in the southeastern United States. Environ. Int. 2022, 158, 106969. [Google Scholar] [CrossRef]
- Kenya National Bureau of Statistics; Ministry of Health; National AIDS Control Council; Kenya Medical Research Institute; National Council for Population and Development; The DHS Program, ICF International. Kenya Demographic and Health Survey 2014; Report. 2015. Available online: https://dhsprogram.com/publications/publication-fr308-dhs-final-reports.cfm (accessed on 15 December 2021).
- GPS Data Collection. Available online: https://dhsprogram.com/Methodology/GPS-Data-Collection.cfm (accessed on 12 December 2021).
- van Donkelaar, A.; Hammer, M.; Bindle, L.; Brauer, M.; Brook, J.; Garay, M.; Hsu, N.; Kalashnikova, O.; Kahn, R.; Lee, C.; et al. Monthly global estimates of fine particulate matter and their uncertainty. Environ. Sci. Technol. 2021, 55, 15287–15300. [Google Scholar] [CrossRef]
- Van Donkelaar, A.; Martin, R.V.; Brauer, M.; Kahn, R.; Levy, R.; Verduzco, C.; Villeneuve, P.J. Global estimates of ambient fine particulate matter concentrations from satellite-based aerosol optical depth: Development and application. Env. Health Perspect 2010, 118, 847–855. [Google Scholar] [CrossRef] [Green Version]
- Ikeuchi, H.; Murakami, M.; Watanabe, S. Scavenging of PM2.5 by precipitation and the effects of precipitation pattern changes on health risks related to PM2.5 in Tokyo, Japan. Water Sci. Technol. 2015, 73, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Fu, J.; Jiang, D.; Wang, J.; Wang, Q.; Dong, D. Spatial variation of the relationship between PM2.5 concentrations and meteorological parameters in China. BioMed Res. Int. 2015, 2015, 684618. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.; Peterson, P.; Landsfeld, M.; Pedreros, D.; Verdin, J.; Shukla, S.; Husak, G.; Rowland, J.; Harrison, L.; Hoell, A.; et al. The climate hazards infrared precipitation with stations—A new environmental record for monitoring extremes. Sci. Data 2015, 2, 150066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zilli Vieira, C.L.; Koutrakis, P. The impact of solar activity on ambient ultrafine particle concentrations: An analysis based on 19-year measurements in Boston, USA. Environ. Res. 2021, 201, 111532. [Google Scholar] [CrossRef]
- Global Solar Atlas 2.0. Available online: https://documents1.worldbank.org/curated/en/529431592893043403/pdf/Global-Solar-Atlas-2-0-Technical-Report.pdf (accessed on 15 December 2021).
- EPA. Altitude as a Factor in Air Pollution; EPA: Washington, DC, USA, 1978.
- Hijmans, R.J.; Guarino, L.; Cruz, M.; Rojas, E. Computer tools for spatial analysis of plant genetic resources data: 1. DIVA-GIS. Plant Genet. Resour. Newsl. 2001, 127, 15–19. [Google Scholar]
- Gasparrini, A. Modeling exposure–lag–response associations with distributed lag non-linear models. Stat. Med. 2014, 33, 881–899. [Google Scholar] [CrossRef] [Green Version]
- Entezari, A.; Mayvaneh, F. Applying the distributed lag non-linear model (DLNM) in epidemiology: Temperature and mortality in Mashhad. Iran. J. Public Health 2019, 48, 2108–2111. [Google Scholar] [CrossRef]
- Guo, C.Y.; Huang, X.Y.; Kuo, P.C.; Chen, Y.H. Extensions of the distributed lag non-linear model (DLNM) to account for cumulative mortality. Environ. Sci. Pollut. Res. Int. 2021, 28, 38679–38688. [Google Scholar] [CrossRef]
- Qiu, C.; Ji, J.S.; Bell, M.L. Effect modification of greenness on temperature-mortality relationship among older adults: A case-crossover study in China. Environ. Res. 2021, 197, 111112. [Google Scholar] [CrossRef]
- Bhopdhornangkul, B.; Meeyai, A.C.; Wongwit, W.; Limpanont, Y.; Iamsirithaworn, S.; Iaosiritaworn, Y.; Tantrakarnapa, K. Non-linear effect of different humidity types on scrub typhus occurrence in endemic provinces, Thailand. Heliyon 2021, 7, e06095. [Google Scholar] [CrossRef]
- Qi, H.; Chen, Y.; Xu, D.; Su, H.; Zhan, L.; Xu, Z.; Huang, Y.; He, Q.; Hu, Y.; Lynn, H.; et al. Impact of meteorological factors on the incidence of childhood hand, foot, and mouth disease (HFMD) analyzed by DLNMs-based time series approach. Infect. Dis. Poverty 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushita, N.; Kim, Y.; Ng, C.F.S.; Moriyama, M.; Igarashi, T.; Yamamoto, K.; Otieno, W.; Minakawa, N.; Hashizume, M. Differences of rainfall-malaria associations in lowland and highland in Western Kenya. Int. J. Environ. Res. Public Health 2019, 16, 3693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, G.; Yin, P.; Wang, L.; Zhou, M. Associations between ambient high temperatures and suicide mortality: A multi-city time-series study in China. Environ. Sci. Pollut. Res. Int. 2019, 26, 20377–20385. [Google Scholar] [CrossRef] [PubMed]
- Gronlund, C.J.; Zanobetti, A.; Schwartz, J.D.; Wellenius, G.A.; O’Neill, M.S. Heat, heat waves, and hospital admissions among the elderly in the United States, 1992–2006. Environ. Health Perspect. 2014, 122, 1187–1192. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, R.; Robinson, R.; Mortimer, K. The epidemiology of noncommunicable respiratory disease in sub-Saharan Africa, the Middle East, and North Africa. Malawi Med. J. J. Med. Assoc. Malawi 2017, 29, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Magati, P.; Drope, J.; Mureithi, L.; Lencucha, R. Socio-economic and demographic determinants of tobacco use in Kenya: Findings from the Kenya Demographic and Health Survey 2014. Pan Afr. Med. J. 2018, 30, 166. [Google Scholar] [CrossRef]
- Yang, L.; Li, C.; Tang, X. The impact of PM2.5 on the host defense of respiratory system. Front. Cell Dev. Biol. 2020, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.W.; Lee, T.L.; Chen, Y.C.; Liang, C.J.; Wang, S.H.; Lue, J.H.; Tsai, J.S.; Lee, S.W.; Chen, S.H.; Yang, Y.F.; et al. PM2.5-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the IL-6/AKT/STAT3/NF-κB-dependent pathway. Part. Fibre Toxicol. 2018, 15, 4. [Google Scholar] [CrossRef]
- Akata, K.; Yatera, K.; Yamasaki, K.; Kawanami, T.; Naito, K.; Noguchi, S.; Fukuda, K.; Ishimoto, H.; Taniguchi, H.; Mukae, H. The significance of oral streptococci in patients with pneumonia with risk factors for aspiration: The bacterial floral analysis of 16S ribosomal RNA gene using bronchoalveolar lavage fluid. BMC Pulm. Med. 2016, 16, 79. [Google Scholar] [CrossRef] [Green Version]
- Marsh, R.; Kaestli, M.; Chang, A.; Binks, M.; Pope, C.; Hoffman, L.; Smith-Vaughan, H. The microbiota in bronchoalveolar lavage from young children with chronic lung disease includes taxa present in both the oropharynx and nasopharynx. Microbiome 2016, 4, 37. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Cheng, H.; Wang, D.; Zhao, B.; Zhang, J.; Cheng, L.; Yao, P.; Di Narzo, A.; Shen, Y.; Yu, J.; et al. Airway microbiome is associated with respiratory functions and responses to ambient particulate matter exposure. Ecotoxicol. Environ. Saf. 2019, 167, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Li, S.; Zhou, W.; Shang, D.; Zhao, S.; Zhu, X.; Chen, K.; Wang, R. The effect of air pollutants on the microecology of the respiratory tract of rats. Environ. Toxicol. Pharmacol. 2013, 36, 588–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, S.; Shin, H.; Burnett, R.; North, T.; Cohen, A.J. Ambient particulate air pollution and acute lower respiratory infections: A systematic review and implications for estimating the global burden of disease. Air Qual. Atmos. Health 2013, 6, 69–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertz-Picciotto, I.; Baker, R.J.; Yap, P.S.; Dostál, M.; Joad, J.P.; Lipsett, M.; Greenfield, T.; Herr, C.E.; Beneš, I.; Shumway, R.H.; et al. Early childhood lower respiratory illness and air pollution. Environ. Health Perspect. 2007, 115, 1510–1518. [Google Scholar] [CrossRef] [Green Version]
- Karr, C.; Lumley, T.; Schreuder, A.; Davis, R.; Larson, T.; Ritz, B.; Kaufman, J. Effects of subchronic and chronic exposure to ambient air pollutants on infant bronchiolitis. Am. J. Epidemiol. 2007, 165, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Brauer, M.; Hoek, G.; Van Vliet, P.; Meliefste, K.; Fischer, P.H.; Wijga, A.; Koopman, L.P.; Neijens, H.J.; Gerritsen, J.; Kerkhof, M.; et al. Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am. J. Respir. Crit. Care Med. 2002, 166, 1092–1098. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.J.; Hu, W.; Wei, F.; Wu, G.; Korn, L.R.; Chapman, R.S. Children’s respiratory morbidity prevalence in relation to air pollution in four Chinese cities. Environ. Health Perspect. 2002, 110, 961–967. [Google Scholar] [CrossRef]
- Wrotek, A.; Badyda, A.; Czechowski, P.O.; Owczarek, T.; Dąbrowiecki, P.; Jackowska, T. Air pollutants’ concentrations are associated with increased number of RSV hospitalizations in polish children. J. Clin. Med. 2021, 10, 3224. [Google Scholar] [CrossRef]
- Ye, Q.; Fu, J.f.; Mao, J.h.; Shang, S.Q. Haze is a risk factor contributing to the rapid spread of respiratory syncytial virus in children. Environ. Sci. Pollut. Res. 2016, 23, 20178–20185. [Google Scholar] [CrossRef]
- Mikeš, O.; Vrbová, M.; Klánová, J.; Čupr, P.; Švancara, J.; Pikhart, H. Early-life exposure to household chemicals and wheezing in children. Sci. Total Environ. 2019, 663, 418–425. [Google Scholar] [CrossRef] [Green Version]
- Duijts, L.; Granell, R.; Sterne, J.A.; Henderson, A.J. Childhood wheezing phenotypes influence asthma, lung function and exhaled nitric oxide fraction in adolescence. Eur. Respir. J. 2016, 47, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Hodgekiss, C.; Arshad, S.H.; Roberts, G.; Jenkins, E.; Kurukulaaratchy, R. Characterisation of lung function at 18 by early life wheeze phenotype. Eur. Respir. J. 2016, 48, PA4595. [Google Scholar]
- Holst, G.J.; Pedersen, C.B.; Thygesen, M.; Brandt, J.; Geels, C.; Bønløkke, J.H.; Sigsgaard, T. Air pollution and family related determinants of asthma onset and persistent wheezing in children: Nationwide case-control study. BMJ 2020, 370, m2791. [Google Scholar] [CrossRef] [PubMed]
- Brunst, K.J.; Ryan, P.H.; Brokamp, C.; Bernstein, D.; Reponen, T.; Lockey, J.; Khurana Hershey, G.K.; Levin, L.; Grinshpun, S.A.; LeMasters, G. Timing and duration of traffic-related air pollution exposure and the risk for childhood wheeze and asthma. Am. J. Respir. Crit. Care Med. 2015, 192, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.S.; Gibson, H.; Ramakrishnan, R.; Mamouei, M.; Rahimi, K. Ambient air pollution and respiratory health in sub-Saharan African children: A cross-sectional analysis. Int. J. Environ. Res. Public Health 2021, 18, 9729. [Google Scholar] [CrossRef]
- Open AQ. 2021. Available online: https://openaq.org/#/ (accessed on 15 December 2021).
- Hunter, D.J. Environmental pollution and human health: Estimation and action, from local to global and back. J. Health Pollut. 2011, 1, 2–4. [Google Scholar] [CrossRef]
- Amoabeng Nti, A.A.; Arko-Mensah, J.; Botwe, P.K.; Dwomoh, D.; Kwarteng, L.; Takyi, S.A.; Acquah, A.A.; Tettey, P.; Basu, N.; Batterman, S.; et al. Effect of particulate matter exposure on respiratory health of e-waste workers at Agbogbloshie, Accra, Ghana. Int. J. Environ. Res. Public Health 2020, 17, 3042. [Google Scholar] [CrossRef]
- Rylance, S.; Jewell, C.; Naunje, A.; Mbalume, F.; Chetwood, J.D.; Nightingale, R.; Zurba, L.; Flitz, G.; Gordon, S.B.; Lesosky, M.; et al. Non-communicable respiratory disease and air pollution exposure in Malawi: A prospective cohort study. Thorax 2020, 75, 220–226. [Google Scholar] [CrossRef] [Green Version]

| [ALL] | N | |
|---|---|---|
| N = 7036 | ||
| Wheezing: | 7036 | |
| No wheezing | 3744 (53.2%) | |
| Wheezing | 3292 (46.8%) | |
| Sex: | 7036 | |
| Female | 3510 (49.9%) | |
| Male | 3526 (50.1%) | |
| Current age of child (mean, std. dev.) | 1.97 (1.37) | 7036 |
| Wealth index (1 = low SES, 5 = high SES): | 7036 | |
| 1 | 2174 (30.9%) | |
| 2 | 1624 (23.1%) | |
| 3 | 1272 (18.1%) | |
| 4 | 1085 (15.4%) | |
| 5 | 881 (12.5%) | |
| Someone ever smokes in home: | 3350 | |
| No | 2874 (85.8%) | |
| Yes | 476 (14.2%) | |
| Type of cooking fuel used: | 7035 | |
| Solid fuel | 6547 (93.1%) | |
| Gas | 470 (6.68%) | |
| Electricity | 11 (0.16%) | |
| No food cooked in house | 3 (0.04%) | |
| Other | 4 (0.06%) | |
| Urban vs. Rural cluster: | 7036 | |
| Rural | 4800 (68.2%) | |
| Urban | 2236 (31.8%) |
| Variable | N | Mean | Std. Dev. | Min | Pctl. 25 | Pctl. 75 | Max |
|---|---|---|---|---|---|---|---|
| PM (µg/m) (exposure in month of survey) | 6994 | 18.31 | 9.47 | 2 | 10.7 | 24.3 | 46.8 |
| PM (µg/m) (12 months previous) | 6994 | 22.94 | 12.94 | 2.3 | 13.4 | 28.4 | 66.8 |
| PM (µg/m) (one year average) | 6994 | 22.1 | 4.84 | 10.75 | 18.37 | 25.93 | 34.49 |
| Population (ppl within 5 km) | 6933 | 1238.5 | 3270.49 | 0.15 | 155.48 | 843.01 | 30,644.39 |
| Distance to road (km) | 6994 | 3.24 | 3.99 | 0.01 | 0.71 | 4.26 | 41.99 |
| Distance to river (km) | 6994 | 3.62 | 3.5 | 0 | 1.11 | 4.79 | 26.97 |
| Elevation (meters) | 6972 | 1371.96 | 653.29 | 3 | 1121.25 | 1843 | 3248 |
| Global horizontal irradiance (yearly average) | 6994 | 5.8 | 0.31 | 4.68 | 5.54 | 6.02 | 6.67 |
| Precipitation (mm) (month of survey) | 6874 | 96.76 | 83.98 | 0 | 23.91 | 149.35 | 422.5 |
| Precipitation (mm) (12 months previous) | 6941 | 85.98 | 69.17 | 0 | 23.74 | 138.34 | 334.07 |
| Precipitation (mm) (one year average) | 6941 | 93.05 | 41.75 | 1.35 | 58.42 | 123.43 | 194.07 |
| PM (month of survey) | 1 | ||||||||||
| PM (12 months previous) | 0.62 | 1 | |||||||||
| PM (one year average) | 0.77 | 0.69 | 1 | ||||||||
| Population (1 km) | 0.10 | 0.05 | 0.15 | 1 | |||||||
| Distance to road (km) | −0.12 | −0.14 | −0.18 | −0.14 | 1 | ||||||
| Distance to river (km) | −0.09 | −0.07 | −0.12 | 0.01 | 0.08 | 1 | |||||
| Elevation (meters) | 0.40 | 0.39 | 0.50 | 0.07 | −0.07 | −0.14 | 1 | ||||
| GHI (yearly average) | 0.28 | 0.28 | 0.29 | −0.15 | 0.01 | −0.05 | 0.08 | 1 | |||
| Precipitation (month of survey) | 0.53 | 0.39 | 0.57 | 0.07 | −0.15 | −0.09 | 0.26 | 0.27 | 1 | ||
| Precipitation (12 months previous) | 0.61 | 0.36 | 0.49 | 0.03 | −0.13 | −0.11 | 0.40 | 0.31 | 0.68 | 1 | |
| Precipitation (one year average) | 0.70 | 0.55 | 0.72 | 0.12 | −0.18 | −0.11 | 0.39 | 0.25 | 0.79 | 0.78 | 1 |
| PM (month of survey) | PM (12 months previous) | PM (one year average) | Population (1 km) | Distance to road (km) | Distance to river (km) | Elevation (meters) | GHI (yearly average) | Precipitation (month of survey) | Precipitation (12 months previous) | Precipitation (one year average) |
| No ARI | ARI | No Random Effect | Random Effect | |||
|---|---|---|---|---|---|---|
| N = 3744 | N = 3292 | OR [95% CI] | p | OR [95% CI] | ||
| PM (month of survey) | 17.80 (9.21) | 18.89 (9.72) | 1.012 [1.007, 1.017] | <0.001 | 1.013 [1.006, 1.020] | <0.001 |
| PM (12 months previous) | 22.63 (12.60) | 23.28 (13.30) | 1.004 [1.000, 1.008] | 0.036 | 1.004 [0.999, 1.009] | 0.16 |
| PM (one year average) | 21.83 (4.72) | 22.41 (4.95) | 1.025 [1.015, 1.035] | <0.001 | 1.026 [1.012, 1.040] | <0.001 |
| Sex: | ||||||
| Female | 1923 (51.36%) | 1587 (48.21%) | Ref. | Ref. | ||
| Male | 1821 (48.64%) | 1705 (51.79%) | 1.135 [1.033, 1.246] | 0.008 | 1.153 [1.040, 1.278] | 0.007 |
| Current age of child | 2.03 (1.37) | 1.91 (1.37) | 0.940 [0.909, 0.973] | <0.001 | 0.931 [0.897, 0.967] | <0.001 |
| Wealth index: | ||||||
| 1 (low SES) | 1150 (30.72%) | 1024 (31.11%) | ||||
| 2 | 836 (22.33%) | 788 (23.94%) | 1.059 [0.931, 1.204] | 0.386 | 1.015 [0.872, 1.181] | 0.849 |
| 3 | 650 (17.36%) | 622 (18.89%) | 1.075 [0.936, 1.234] | 0.308 | 1.006 [0.853, 1.187] | 0.94 |
| 4 | 589 (15.73%) | 496 (15.07%) | 0.946 [0.817, 1.095] | 0.454 | 0.934 [0.784, 1.112] | 0.444 |
| 5 (high SES) | 519 (13.86%) | 362 (11.00%) | 0.783 [0.668, 0.918] | 0.002 | 0.776 [0.640, 0.942] | 0.01 |
| Someone smokes in home: | ||||||
| No smoke | 1529 (85.75%) | 1345 (85.83%) | ||||
| Smoke | 254 (14.25%) | 222 (14.17%) | 0.994 [0.818, 1.207] | 0.948 | 0.999 [0.801, 1.246] | 0.993 |
| Type of cooking fuel used: | ||||||
| Solid fuel | 3442 (91.96%) | 3105 (94.32%) | ||||
| Gas | 289 (7.72%) | 181 (5.50%) | 0.694 [0.573, 0.841] | <0.001 | 0.702 [0.559, 0.882] | 0.002 |
| Electricity | 8 (0.21%) | 3 (0.09%) | 0.416 [0.110, 1.568] | 0.195 | 0.405 [0.094, 1.751] | 0.226 |
| No food cooked in house | 1 (0.03%) | 2 (0.06%) | 2.217 [0.201, 24.463] | 0.516 | 2.906 [0.199, 42.484] | 0.436 |
| Other | 3 (0.08%) | 1 (0.03%) | 0.370 [0.038, 3.554] | 0.389 | 0.312 [0.026, 3.679] | 0.355 |
| Urban vs. Rural cluster: | ||||||
| Rural | 2501 (66.80%) | 2299 (69.84%) | ||||
| Urban | 1243 (33.20%) | 993 (30.16%) | 0.869 [0.786, 0.961] | 0.006 | 0.866 [0.755, 0.993] | 0.039 |
| Population (1 km) | 1004.97 (3211.75) | 915.98 (2745.62) | 1.000 [1.000, 1.000] | 0.217 | 1.000 [1.000, 1.000] | 0.33 |
| Distance to road (km) | 3.32 (4.04) | 3.15 (3.92) | 0.989 [0.978, 1.001] | 0.078 | 0.992 [0.976, 1.008] | 0.329 |
| Distance to river (km) | 3.63 (3.49) | 3.61 (3.51) | 0.998 [0.985, 1.012] | 0.817 | 1.001 [0.982, 1.020] | 0.935 |
| Elevation (meters) | 1389.66 (655.78) | 1351.89 (649.97) | 1.000 [1.000, 1.000] | 0.016 | 1.000 [1.000, 1.000] | 0.064 |
| GHI (yearly average) | 5.79 (0.33) | 5.81 (0.30) | 1.212 [1.042, 1.408] | 0.012 | 1.282 [1.043, 1.577] | 0.018 |
| Precip (month of survey) | 90.80 (80.19) | 103.51 (87.61) | 1.002 [1.001, 1.002] | <0.001 | 1.002 [1.001, 1.003] | <0.001 |
| Precip (12 months previous) | 81.45 (66.95) | 91.09 (71.26) | 1.002 [1.001, 1.003] | <0.001 | 1.002 [1.001, 1.003] | <0.001 |
| Precip (one year average) | 89.63 (39.61) | 96.91 (43.72) | 1.004 [1.003, 1.005] | <0.001 | 1.004 [1.003, 1.006] | <0.001 |
| Dependent Variable | ||
|---|---|---|
| ARI | ||
| Full Model | Best Model (AIC) | |
| PM (exposure in month of survey) | 1.002 *** (0.993, 1.011) | |
| PM (12 months previous) | 0.994 *** (0.989, 1.000) | 0.995 *** (0.990, 1.000) |
| PM (one year average) | 1.023 *** (1.002, 1.043) | 1.024 *** (1.006, 1.041) |
| Sex: | ||
| Female | Ref. | Ref. |
| Male | 1.131 *** (1.036, 1.226) | 1.131 *** (1.036, 1.226) |
| Current age of child | 0.942 *** (0.907, 0.977) | 0.942 *** (0.907, 0.976) |
| Wealth index (1 = low SES, 5 = high SES): | ||
| 1 | Ref. | |
| 2 | 0.989 (0.848, 1.130) | |
| 3 | 1.019 (0.866, 1.172) | |
| 4 | 0.963 (0.798, 1.128) | |
| 5 | 0.916 (0.711, 1.122) | |
| Type of cooking fuel used: | ||
| Solid fuel (biomass) | ||
| Gas | 0.840 *** (0.594, 1.085) | 0.809 *** (0.612, 1.006) |
| Other | 0.587 (0.000, 1.598) | 0.572 (0.000, 1.579) |
| Urban vs. Rural cluster: | ||
| Rural | Ref. | |
| Urban | 0.933 *** (0.807, 1.059) | |
| Population (1 km) | 1.000 *** (1.000, 1.000) | |
| Distance to road (km) | 0.993 *** (0.980, 1.007) | |
| Distance to lake (km) | 1.003 *** (1.001, 1.004) | 1.003 *** (1.001, 1.004) |
| Distance to river (km) | 1.004 *** (0.990, 1.018) | |
| Elevation (meters) | 1.000 *** (1.000, 1.000) | 1.000 *** (1.000, 1.000) |
| Global horizontal irradiance (yearly average) | 1.010 (0.837, 1.183) | |
| Precipitation (month of survey) | 1.000 *** (0.999, 1.001) | |
| Precipitation (12 months previous) | 1.000 *** (0.999, 1.001) | |
| Precipitation (one year average) | 1.004 *** (1.002, 1.007) | 1.005 *** (1.003, 1.006) |
| Constant | 0.533 (0.000, 1.528) | 0.539 *** (0.260, 0.818) |
| Observations | 6940 | 6940 |
| Log Likelihood | −4726.647 | −4729.214 |
| Akaike Inf. Crit. | 9497.294 | 9478.428 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larson, P.S.; Espira, L.; Glenn, B.E.; Larson, M.C.; Crowe, C.S.; Jang, S.; O’Neill, M.S. Long-Term PM2.5 Exposure Is Associated with Symptoms of Acute Respiratory Infections among Children under Five Years of Age in Kenya, 2014. Int. J. Environ. Res. Public Health 2022, 19, 2525. https://doi.org/10.3390/ijerph19052525
Larson PS, Espira L, Glenn BE, Larson MC, Crowe CS, Jang S, O’Neill MS. Long-Term PM2.5 Exposure Is Associated with Symptoms of Acute Respiratory Infections among Children under Five Years of Age in Kenya, 2014. International Journal of Environmental Research and Public Health. 2022; 19(5):2525. https://doi.org/10.3390/ijerph19052525
Chicago/Turabian StyleLarson, Peter S., Leon Espira, Bailey E. Glenn, Miles C. Larson, Christopher S. Crowe, Seoyeon Jang, and Marie S. O’Neill. 2022. "Long-Term PM2.5 Exposure Is Associated with Symptoms of Acute Respiratory Infections among Children under Five Years of Age in Kenya, 2014" International Journal of Environmental Research and Public Health 19, no. 5: 2525. https://doi.org/10.3390/ijerph19052525
APA StyleLarson, P. S., Espira, L., Glenn, B. E., Larson, M. C., Crowe, C. S., Jang, S., & O’Neill, M. S. (2022). Long-Term PM2.5 Exposure Is Associated with Symptoms of Acute Respiratory Infections among Children under Five Years of Age in Kenya, 2014. International Journal of Environmental Research and Public Health, 19(5), 2525. https://doi.org/10.3390/ijerph19052525







