Psychometric Properties of Quality of Life Questionnaires for Patients with Breast Cancer-Related Lymphedema: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.5.1. Step 1. COSMIN Risk of Bias Checklist
2.5.2. Step 2. Applying Criteria for Good Measurement Properties
- i.
- Step 2a: Content validity
- ii.
- Step 2b: Remaining measurement properties
2.5.3. Step 3. Summary of Evidence
- i.
- Step 3a. Content validity
- ii.
- Step 3b. Remaining measurement properties
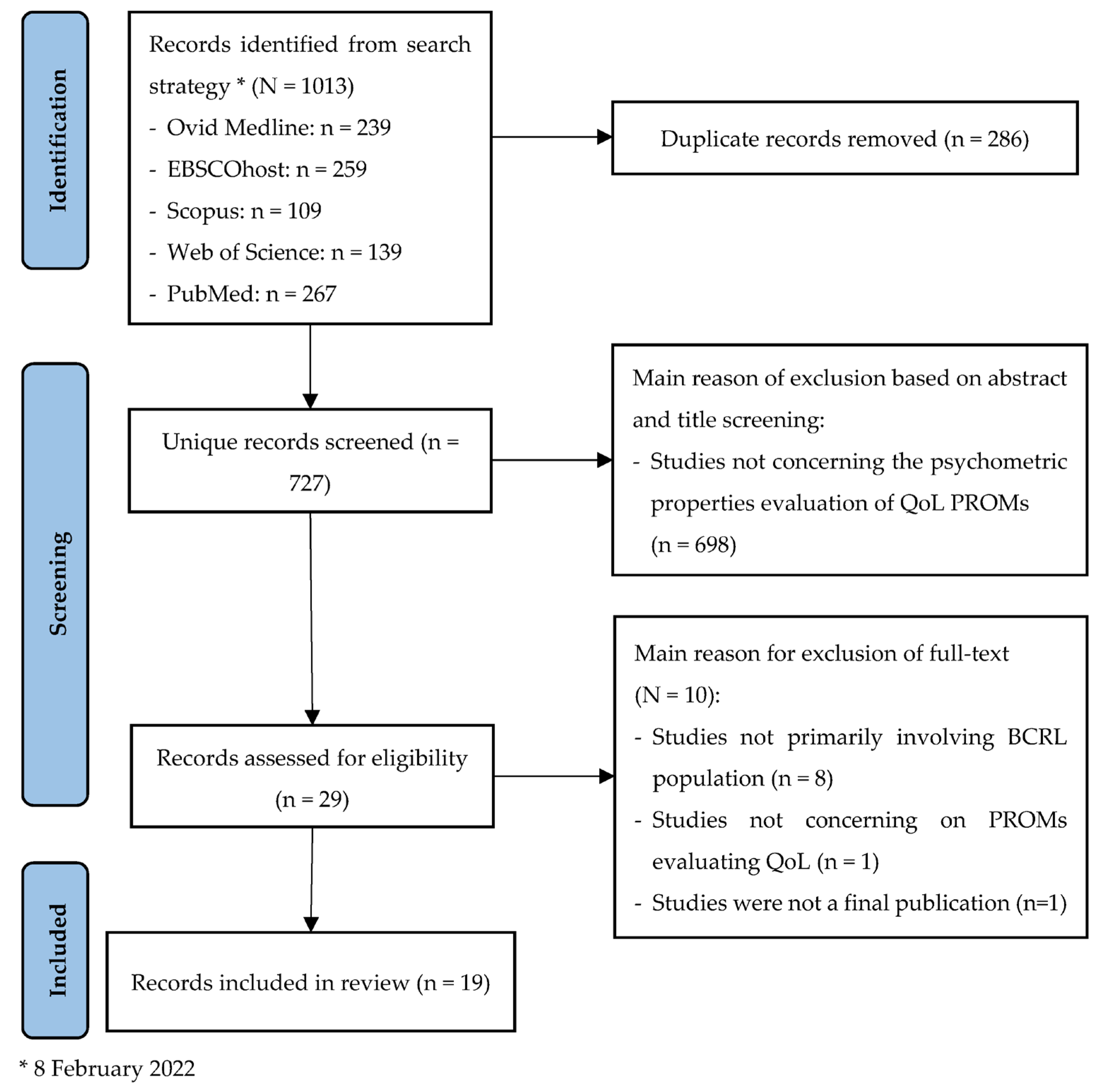
3. Results
3.1. Study Outcomes
3.2. Characteristics of Included Studies
3.3. Characteristics of Included PROMs
3.4. Quality Assessment
3.4.1. Methodological Quality and Rating against Good Measurement Properties for Results of Each Included Studies
3.4.2. Overall Rating and Grading of the Quality of Evidence per Measurement Properties for Each PROM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Cancer Observatory: Cancer Today; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Ganz, P. Quality of Care and Cancer Survivorship: The Challenge of Implementing the Institute of Medicine Recommendation. In Cancer Quality Alliance Proceedings; American Society of Clinical Oncology: Los Angeles, CA, USA, 2009; Volume 5, pp. 101–105. [Google Scholar] [CrossRef] [Green Version]
- Cidón, E.U.; Perea, C.; López-Lara, F. Life after Breast Cancer: Dealing with Lymphoedema. Clin. Med. Insights Oncol. 2011, 5, CMO.S6389. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.Y.; Nadia, A. Methods to Improve Rehabilitation of Patients Following Breast Cancer Surgery: A Review of Systematic Reviews. Breast Cancer Targets Ther. 2015, 7, 81–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodai, B. Breast Cancer Survivorship: A Comprehensive Review of Long-Term Medical Issues and Lifestyle Recommendations. Perm. J. 2019, 19, 48. [Google Scholar] [CrossRef] [Green Version]
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of Unilateral Arm Lymphoedema after Breast Cancer: A Systematic Review and Meta-Analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef]
- Cormier, J.; Askew, R.; Mungovan, K.; Xing, Y.; Ross, M.; Armer, J. Lymphedema beyond Breast Cancer: A Systematic Review and Meta-Analysis of Cancer-Related Secondary Lymphedema. Cancer 2010, 116, 5138–5149. [Google Scholar] [CrossRef]
- Rockson, S.G.; Rivera, K.K. Estimating the Population Burden of Lymphedema. Ann. N. Y. Acad. Sci. 2008, 1131, 147–154. [Google Scholar] [CrossRef]
- Paskett, E.D.; Naughton, M.J.; McCoy, T.P.; Case, L.D.; Abbott, J.M. The Epidemiology of Arm and Hand Swelling in Premenopausal Breast Cancer Survivor. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 775–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garfein, E.; Borud, L.; Warren, A.; Slavin, S. Learning from a Liymphedema Clinic: An Algorithm for the Management of Localized Swelling. Plast. Reconstr. Surg. 2008, 121, 521–528. [Google Scholar] [CrossRef]
- Szuba, A.; Rockson, S.G. Lymphedema: Classification, Diagnosis and Therapy. Vasc. Med. 1998, 3, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Armer, J.; Stewart, B. Post-Breast Cancer Lymphedema: Incidence Increases from 12 to 30 to 60 Months. Lymphology 2010, 43, 118–127. [Google Scholar]
- Didem, K.; Ufuk, Y.S.; Serdar, S.; Zümre, A. The Comparison of Two Different Physiotherapy Methods in Treatment of Lymphedema after Breast Surgery. Breast Cancer Res. Treat. 2005, 93, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Lawenda, B.D.; Mondry, T.E.; Johnstone, P.A.S. Lymphedema: A Primer on the Identification and Management of a Chronic Condition in Oncologic Treatment. CA Cancer J. Clin. 2009, 59, 8–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coen, J.J.; Taghian, A.G.; Kachnic, L.A.; Assaad, S.I.; Powell, S.N. Risk of Lymphedema after Regional Nodal Irradiation with Breast Conservation Surgery. Int. J. Radiant. Oncol. Biol. Phys. 2003, 55, 1209–1215. [Google Scholar] [CrossRef]
- Ozaslan, C.; Kuru, B. Lymphedema after Treatment of Breast Cancer. Am. J. Surg. 2004, 187, 69–72. [Google Scholar] [CrossRef]
- Yusof, K.M.; Avery-Kiejda, K.A.; Ahmad Suhaimi, S.; Ahmad Zamri, N.; Rusli, M.E.F.; Mahmud, R.; Saini, S.M.; Abdul Wahhab Ibraheem, S.; Abdullah, M.; Rosli, R. Assessment of Potential Risk Factors and Skin Ultrasound Presentation Associated with Breast Cancer-Related Lymphedema in Long-Term Breast Cancer Survivors. Diagnostics 2021, 11, 1303. [Google Scholar] [CrossRef]
- Golshan, M.; Smith, B. Prevention and Management of Arm Lymphedema in the Patient with Breast Cancer. J. Support. Oncol. 2006, 4, 381–386. [Google Scholar]
- Passik, S.; Newmann, M.; Brennan, M.; Holland, J. Psychiatric Consultation for Women Undergoing Rehabilitation for Upper-Extremity Lymphedema Following Breast Cancer Treatment. J. Pain. Symptom. Manag. 1993, 8, 226–233. [Google Scholar] [CrossRef]
- Crouch, M.; McKenzie, H. Social Realities of Loss and Suffering Following Mastectomy. Health 2000, 4, 196–215. [Google Scholar] [CrossRef]
- Pusic, A.; Cemal, Y.; Albornos, C.; Klassen, A.; Cano, S.; Sulimanoff, I.; Hernandez, M.; Massey, M.; Cordeiro, P.; Morrow, M.; et al. Quality of Life among Breast Cancer Patients with Lymphedema: A Systematic Review of Patient-Reported Outcome Instruments and Outcomes. J. Cancer Surviv. 2013, 7, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Johansson, K.; Holmstrom, H.; Nilsson, I. Breast Cancer Patients’ Experiences of Lymphoedema. Scand. J. Caring Sci. 2003, 17, 35–42. [Google Scholar] [CrossRef]
- Wilson, R.; Hutson, L.; Vanstry, D. Comparison of 2 Quality-of-Life Questionnaires in Women Treated for Breast Cancer: The RAND 36-Item Health Survey and the Functional Living Index-Cancer. Phys. Ther. 2005, 85, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, G.; Doller, W.; Roth, R. Quality of Life and Body Image Impairments in Patients with Lymphedema. Lymphology 2006, 39, 193–200. [Google Scholar]
- Pyszel, A.; Malyszczak, K.; Pyszel, K. Disability, Psychological Distress, and Quality of Life in Breast Cancer Survivors with Arm Lymphedema. Lymphology 2006, 39, 185–192. [Google Scholar] [PubMed]
- Black, N. Patient Reported Outcome Measures Could Help Transform Healthcare. BMJ 2013, 346, f167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, E.C.; Eftimovska, E.; Lind, C.; Hager, A.; Wasson, J.H.; Lindblad, S. Patient Reported Outcome Measures in Practice. BMJ 2015, 350, g7818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beelen, L.M.; van Dishoeck, A.-M.; Tsangaris, E.; Coriddi, M.; Dayan, J.H.; Pusic, A.L.; Klassen, A.; Vasilic, D. Patient-Reported Outcome Measures in Lymphedema: A Systematic Review and COSMIN Analysis. Ann. Surg. Oncol. 2021, 28, 1656–1668. [Google Scholar] [CrossRef]
- Prinsen, C.A.C.; Mokkink, L.B.; Bouter, L.M.; Alonso, J.; Patrick, D.L.; de Vet, H.C.W. COSMIN Guideline for Systematic Reviews of Patient-Reported Outcome Measures. Qual. Life Res. 2018, 27, 1147–1157. [Google Scholar] [CrossRef] [Green Version]
- Mokkink, L.B.; de Vet, H.C.W.; Prinsen, C.A.C.; Patrick, D.L.; Alonso, J.; Bouter, L.M.; Terwee, C.B. COSMIN Risk of Bias Checklist for Systematic Reviews of Patient-Reported Outcome Measures. Qual. Life Res. 2018, 27, 1171–1179. [Google Scholar] [CrossRef] [Green Version]
- Treanor, C.; Donnelly, M. A Methodological Review of the Short Form Health Survey 36 (SF-36) and Its Derivatives among Breast Cancer Survivors. Qual. Life Res. 2015, 24, 339–362. [Google Scholar] [CrossRef]
- Cornelissen, A.J.M.; Kool, M.; Keuter, X.H.A.; Heuts, E.M.; Piatkowski De Grzymala, A.A.; Van Der Hulst, R.R.W.J.; Qiu, S.S. Quality of Life Questionnaires in Breast Cancer-Related Lymphedema Patients: Review of the Literature. Lymphat. Res. Biol. 2018, 16, 134–139. [Google Scholar] [CrossRef]
- Meilani, E.; Zanudin, A.; Nordin, N.A.M. Psychometric Properties of Quality of Life Questionnaires for Patients with Breast Cancer-Related Lymphedema: A Protocol for a Systematic Review. Medicine 2020, 99. [Google Scholar] [CrossRef] [PubMed]
- Terwee, C.B.; Jansma, E.P.; Riphagen, I.I.; de Vet, H.C.W. Development of a Methodological PubMed Search Filter for Finding Studies on Measurement Properties of Measurement Instrument. Qual. Life Res. 2009, 18, 1115–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]; Higgins, J.; Green, S. (Eds.) The Cochrane Collaboration, 2011; Available online: https://crtha.iums.ac.ir/files/crtha/files/cochrane.pdf (accessed on 10 August 2020).
- Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Reviews; Deeks, J.J.; Bossuyt, P.M.; Gatsonis (Eds.) The Cochrane Collaboration.: London, UK, 2013; pp. 3–15. Available online: https://methods.cochrane.org/sdt/ (accessed on 10 August 2020).
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, A.L. Preferred Reposrting Items for Systematic Review and Meta-Analysis (PRISMA) 2015 Statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eden, J.; Levit, L.; Berg, A.; Morton, S. Finding What Sorts in Health Care: Standards for Systematic Reviews; National Academic Press: Washington, DC, USA, 2011. [Google Scholar]
- GRADE Handbook. Handbook for Grading the Quality of Evidence and the Strength Recommendation Using GRADE Approach; Schunemann, H., Brozek, J., Guyatt, G., Oxman, G., Eds.; 2013; Available online: https://gdt.gradepro.org/app/handbook/handbook.html#h.z014s19g02b2 (accessed on 10 August 2020).
- Terwee, C.B.; Prinsen, C.A.C.; Chiarotto, A.; Westerman, M.; Patrick, D.L.; Alonso, J.; Bouter, L.M.; de Vet, H.C.W.; Mokkink, L.B. COSMIN Methodology for Evaluating the Content Validity of Patient-Reported Outcome Measures: A Delphi Study. Qual. Life Res. 2018, 27, 1159–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakar, Y.; Tugral, A.; Ozdemir, O.; Duygu, E.; Uyeturk, U. Translation and Validation of the Turkish Version of Lymphedema Quality of Life Tool (LYMQOL) in Patients with Breast Cancer Related Lymphedema. Eur. J. Breast Health 2017, 13, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karayurt, Ö.; Deveci, Z.; Eyigör, S.; Özgürnbat, M. Adaptation of Quality of Life Measure for Limb Lymphedema-Arm in Turkish Women with Breast Cancer-Related Lymphedema. Cancer Nurs. 2021, 44, 45–52. [Google Scholar] [CrossRef]
- Borman, P.; Yaman, A.; Denizli, M.; Karahan, S.; Özdemir, O. The Reliability and Validity of Lymphedema Quality of Life Questionnaire-Arm in Turkish Patients with Upper Limb Lymphedema Related with Breast Cancer. Turk. J. Phys. Med. Rehabil. 2018, 64, 205–212. [Google Scholar] [CrossRef]
- Değirmenci, B.; Tüzün, Ş.; Of, N.S.; Oral, A.; Sindel, D. Reliability and Validity of Turkish Version of Lymphedema Life Impact Scale. Turk. J. Phys. Med. Rehabil. 2019, 65, 147–153. [Google Scholar] [CrossRef]
- Haghighat, S.; Montazeri, A.; Zayeri, F.; Ebrahimi, M.; Weiss, J. Psychometric Evaluation of the Persian Version of the Lymphedema Life Impact Scale (LLIS, Version 1) in Breast Cancer Patients. Health Qual. Life Outcomes 2018, 16, 132. [Google Scholar] [CrossRef]
- Orhan, C.; Uzelpasaci, E.; Baran, E.; Nakip, G.; Ozgul, S.; Aksoy, S.; Akbayrak, T. The Reliability and Validity of the Turkish Version of the Lymphedema Life Impact Scale in Patients with Breast Cancer-Related Lymphedema. Cancer Nurs. 2020, 43, 375–383. [Google Scholar] [CrossRef]
- Abu Sharour, L. Psychometric Evaluation of the Arabic Version of the Lymphedema Life Impact Scale in Breast Cancer Patients. Breast J. 2020, 26, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Devoogdt, N.; Van Kampen, M.; Geraerts, I.; Coremans, T.; Christiaens, M.-R. Lymphoedema Functioning, Disability and Health Questionnaire (Lymph-ICF): Reliability and Validity. Phys. Ther. 2011, 91, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Grarup, K.R.; Devoogdt, N.; Strand, L.I. The Danish Version of Lymphoedema Functioning, Disability and Health Questionnaire (Lymph-ICF) for Breast Cancer Survivors: Translation and Cultural Adaptation Followed by Validity and Reliability Testing. Physiother. Theory Pract. 2018, 35, 327–340. [Google Scholar] [CrossRef]
- De Vrieze, T.; Vos, L.; Gebruers, N.; De Groef, A.; Dams, L.; Van Der Gucht, E.; Nevelsteen, I.; Devoogdt, N. Revision of the Lymphedema Functioning, Disability and Health Questionnaire for Upper Limb Lymphedema (Lymph-ICF-UL): Reliability and Validity. Lymphat. Res. Biol. 2019, 17, 347–355. [Google Scholar] [CrossRef] [PubMed]
- de Vrieze, T.; Gebruers, N.; Nevelsteen, I.; Tjalma, W.A.A.; Thomis, S.; de Groef, A.; Dams, L.; Devoogdt, N. Responsiveness of the Lymphedema Functioning, Disability, and Health Questionnaire for Upper Limb Lymphedema in Patients with Breast Cancer-Related Lymphedema. Lymphat. Res. Biol. 2020, 18, 365–373. [Google Scholar] [CrossRef] [PubMed]
- de Vrieze, T.; Frippiat, J.; Deltombe, T.; Gebruers, N.; Tjalma, W.A.A.; Nevelsteen, I.; Thomis, S.; Vandermeeren, L.; Belgrado, J.-P.; de Groef, A.; et al. Cross-Cultural Validation of the French Version of the Lymphedema Functioning, Disability and Health Questionnaire for Upper Limb Lymphedema (Lymph-ICF-UL). Disabil. Rehabil. 2021, 43, 2797–2804. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.H.; Wu, Y.N.; Tao, Y.L.; Zhou, C.L.; de Vrieze, T.; Li, X.J.; Chen, L.L. Psychometric Validation of the Chinese Version of the Lymphedema Functioning, Disability, and Health Questionnaire for Upper Limb Lymphedema in Patients With Breast Cancer-Related Lymphedema. Cancer Nurs. 2022, 45, 70–82. [Google Scholar] [CrossRef]
- Ridner, S.H.; Dietrich, M.S. Development and Validation of the Lymphedema Symptom and Intensity Survey-Arm. Supportive Care Cancer 2015, 23, 3103–3112. [Google Scholar] [CrossRef]
- Deveci, Z.; Karayurt, O.; Çelik, B.; Eyigör, S. Validity and Reliability of the Turkish Version of the Lymphedema Symptom Intensity and Distress Survey. Turk. J. Phys. Med. Rehabil. 2021, 67, 428–438. [Google Scholar] [CrossRef]
- Viehoff, P.B.; Van Genderen, F.R.; Wittink, H. Upper Limb Lymphedema 27 (ULL27): Dutch Translation and Validation of an Illness-Specific Health-Related Quality of Life Questionnaire for Patients with Upper Limb Lymphedema. Lymphology 2008, 41, 131–138. [Google Scholar]
- Kayali Vatansever, A.; Yavuzşen, T.; Karadibak, D. The Reliability and Validity of Quality of Life Questionnaire Upper Limb Lymphedema (ULL-27) Turkish Patient With Breast Cancer Related Lymphedema. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.E.; Rapport, F.; Russell, I.T.; Hutchings, H.A. Psychometric Development of the Upper Limb Lymphedema Quality of Life Questionnaire Demonstrated the Patient-Reported Outcome Measure to Be a Robust Measure for Breast Cancer–Related Lymphedema. J. Clin. Epidemiol. 2018, 100, 61–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klassen, A.F.; Tsangaris, E.; Kaur, M.N.; Poulsen, L.; Beelen, L.M.; Jacobsen, A.L.; Jørgensen, M.G.; Sørensen, J.A.; Vasilic, D.; Dayan, J.; et al. Development and Psychometric Validation of a Patient-Reported Outcome Measure for Arm Lymphedema: The LYMPH-Q Upper Extremity Module. Ann. Surg. Oncol. 2021, 28, 5166–5182. [Google Scholar] [CrossRef]
- WHO. International Classification of Functioning, Disability, and Health: ICF; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
| Measurement Properties | Definition * |
|---|---|
| Content validity | The degree to which the content of a PROM is an adequate reflection of the construct to be measured |
| Structural validity | The degree to which the scores of a PROM are an adequate reflection of the dimensionality of the construct to be measured |
| Internal consistency | The degree of the interrelatedness among the items |
| Cross-cultural validity | The degree to which the performance of the items on a translated or culturally adapted PROM is an adequate reflection of the original version of the PROM |
| Reliability | The proportion of the total variance in the measurements which is due to “true” differences between patients |
| Measurement error | The systematic and random error of a patient’s score that is not attributed to true changes in the construct to be measured |
| Criterion validity | The degree to which the scores of a PROM are an adequate reflection of a “gold standard” |
| Hypothesis testing for construct validity | The degree to which the scores of a PROM are consistent with the hypothesis (for instance with regard to internal relationships, relationships to scores of other instruments, or differences between relevant groups) based on the assumption that the PROM validly measures the construct to be measured |
| Responsiveness | The degree to which the scores of a PROM to detect change over time in the construct is to be measured |
| Measurement Properties | Rating | Criteria * |
|---|---|---|
| Structural validity | + | CTT: CFA: CFI or TLI or comparable measure >0.95 OR RMSEA <0.06 OR SRMR <0.082 IRT/Rasch: No violation of unidimensionality: CFI or TLI or comparable measure >0.95 OR RMSEA <0.06 OR SRMR <0.082 AND no violation of monotonicity: adequate looking graphs OR item scalability >0.30 AND adequate model fit: IRT: χ2 > 0.01 Rasch: infit and outfit mean squares ≥0.5 and ≤1.5 OR Z-standardized values >−2 and <2 |
| ? | CTT: Not all information for ‘+’ reported IRT/Rasch: Model fit not reported | |
| − | Criteria for ‘+’ not met | |
| Internal consistency | + | At least low evidence for sufficient structural validity AND Cronbach’s alpha(s) ≥0.70 for each unidimensional scale or subscale |
| ? | Criteria for “At least low evidence for sufficient structural validity” not met | |
| − | At least low evidence for sufficient structural validity AND Cronbach’s alpha(s) < 0.70 for each unidimensional scale or subscale | |
| Reliability | + | ICC or weighted Kappa ≥ 0.70 |
| ? | ICC or weighted Kappa not reported | |
| − | ICC or weighted Kappa < 0.70 | |
| Measurement error | + | SDC or LoA < MIC |
| ? | MIC not defined | |
| − | SDC or LoA > MIC | |
| Hypothesis testing for construct validity | + | The result is in accordance with the hypothesis |
| ? | No hypothesis defined (by the review team) | |
| − | The result is not in accordance with the hypothesis | |
| Cross-cultural validity | + | No important differences found between group factors (such as age, gender, language) in multiple group factor analysis OR no important DIF for group factors (McFadden’s R2 < 0.02) |
| ? | No multiple group factor analysis OR DIF analysis performed | |
| − | Important differences between group factors were found | |
| Criterion validity | + | Correlation with gold standard ≥ 0.70 OR AUC ≥ 0.70 |
| ? | Not all information for ‘+’ reported | |
| − | Correlation with gold standard < 0.70 OR AUC < 0.70 | |
| Responsiveness | + | The result is in accordance with the hypothesis OR AUC ≥ 0.70 |
| ? | No hypothesis defined (by the review team) | |
| − | The result is not in accordance with the hypothesis OR AUC < 0.70 |
| Author (ref) | Country | PROM | Objective of Study | Sample Size | Age Mean ± SD (Range) Year | Gender (% Female) | Lymphedema Characteristics | ||
|---|---|---|---|---|---|---|---|---|---|
| Type | Duration | Severity | |||||||
| Bakar et al. 2017 [41] | Turkey | LYMQoL-Arm A | To translate the English version of LYMQoL to Turkish and to test the reliability and validity of the Turkish version of LYMQoL among patients with BCRL in Turkey | 4 translators 20 patients for pilot study 65 patients for validation studies | 50.6 ± 12.45 (24–75) | 100% | BCRL | 4.32 ± 3.06 (1–18) years | Not specified |
| Karayurt et al. 2021 [42] | Turkey | LYMQoL-Arm A | To adapt Quality of Life Measure for Limb Lymphedema-Arm (LYMQoL-Arm) into Turkish (TR) and test its validity and reliability | 6 translators 5 experts for content validity 10 patients for pilot study 109 patients for structural validity, construct validity, internal consistency, and reliability analysis | 55.69 ± 9.33 (35–79) | 100% | BCRL | 3.28 ± 2.91 (1–13) years | Mild-severe |
| Borman et al. 2018 [43] | Turkey | LYMQoL-Arm B | To translate and validate the LYMQoL-Arm for Turkish breast cancer patients with lymphedema | 4 experts for the translation process 30 patients for pre-testing 135 patients for validation studies | 51.8 ± 9.8 (31–82) | 100% | BCRL | 21.1 ± 38.7 (0.2–164) months | Stage 1–3 |
| Degirmenci et al. 2019 [44] | Turkey | LLIS ver 1 | To investigate the validity and reliability of the Turkish adaptation of the LLIS in patients with lymphedema | 2 translators 10 patients for cognitive debriefing Patients for validation studies → UL = 79; LL = 27 | 53.6 ± 11.8 (28–83) | 97.5% for UL group 96.3% for LL group | 70.7% BCRL; 0.94% lymphoma; 25.4% LL lymphedema | Median = 24 (1–396) months for UL Median = 54 (1–384) months for LL | Stage 1–2 for UL Stage 1–3 for LL |
| Haghighat et al. 2018 [45] | Iran | LLIS ver 1 | To validate the Persian version of the LLIS questionnaire | 2 translators 10 patients for face validity 9 experts for content validity 203 for construct validity and internal consistency 13 for test-retest reliability 200 LE and 200 non-LE for discriminant validity 46 (LLIS vs. EORTC-QLQ-C30) and 400 (LLIS vs. SF-36) for convergent validity | 53.28 ± 10.95 | 100% | Unilateral BCRL | Not specified | Not specified |
| Orhan et al. 2019 [46] | Turkey | LLIS ver 2 | To translate and culturally adapt the LLIS ver 2 into Turkish and perform a psychometric evaluation of the Turkish LLIS ver 2 in patients with BCRL | 10 experts for the translation process 20 patients for pilot testing 78 patients with LE 35 patients without LE for validation studies | 56.5 ± 10.21 | 100% | 69.02% BCRL; 30.9% non-LE | 0–6 mo: 20.5% 6–12 mo: 21.8% 1–3 yr: 24.4% 3–5 yr: 19.2% 5–10 yr: 11.5% >10 yr: 2.6% | Not specified |
| Sharour 2020 [47] | Jordan | LLIS ver 2 | To translate and validate an Arabic version of the LLIS | 3 experts for the translation process 90 patients for validation studies | 44.1 ± 1.10 | 100% | BCRL | 0–6 mo: 80% 6–12 mo: 17.8% 1–2 yr: 2.2% | Not specified |
| Devoogdt et al. 2011 [48] | Belgium | Lymph-ICF-UL | To investigate the reliability (test-retest, internal consistency, measurement variability) and validity (content and construct) of the newly developed Lymph-ICF in breast cancer patients with lymphedema | 20 patients for phase 1 (generating items) 29 patients for phase 2 (validation of the pilot version) 3 translators for phase 3 (translation from Dutch to English) 60 patients LE and 30 patients non-LE for validation studies | 61.2 ± 10.0 (objective LE); 56.7 ± 9.3 (subjective LE); 58.3 ± 11.9 (non-LE) | 100% | 66% BCRL; 33.3% non-LE | Objective LE = 41 ± 64 months Subjective LE = 19 ± 34 months | Not specified |
| Grarup et al. 2018 [49] | Denmark | Lymph-ICF-UL | To translate and culturally adapt the original Dutch version of Lymph-ICF into Danish and examine its content validity and reliability | 4 experts for the translation process 10 patients for cognitive debriefing 52 patients for validation studies | 61 ± 12.4 (validation studies); 61.5 ± 9.7 (cognitive debriefing) | 100% | BCRL | 15.5 ± 58 months for validation studies 24 ± 31 months for cognitive interview | Mild to severe |
| de Vrieze et al. 2019 [50] | Belgium | Lymph-ICF-UL | To examine the validity and reliability of the Lymph-ICF-UL with NRS in patients with BCRL | 56 patients | 62 ± 10 | 100% | BCRL | 34.5 months | Stage I, IIa, IIb |
| de Vrieze et al. 2020 [51] | Belgium | Lymph-ICF-UL | To examine the internal and external responsiveness of the Lymph-ICF-UL in patients with BCRL | 95 patients | 62 ± 10 | 100% | BCRL | 53 ± 42.5 | Stage I, IIa, IIb |
| de Vrieze et al. 2021 [52] | Belgium | Lymph-ICF-UL | To perform a cross-cultural validation of the Lymph-ICF-UL French version in patients with BCRL of the arm and/or hand | 3 experts and 3 patients for the translation process 50 patients for validation studies | 64 ± 11 | 100% | BCRL | 78 months | Stage I, IIa, IIb |
| Zhao et al. 2022 [53] | China | Lymph-ICF-UL | To translate the Lymph-ICF-UL into a Chinese version and subsequently test its reliability and validity among patients with BCRL in a Chinese context | 5 translators 15 patients for pilot testing 6 experts for content validity 155 patients LE and 90 patients non-LE for validation studies | 26–70 | 100% | 63.2% BCRL; 36.7% non-LE | 2–19 months | Stage 0–3 |
| Ridner and Dietrich 2015 [54] | USA | LSIDS-A | To develop and examine the psychometric properties (validity and reliability) of LSIDS-A in breast cancer patients experiencing upper limb lymphedema | 128 for preliminary testing 236 for validation studies | 58.9 ± 11.0 | 100% | BCRL | Not specified | 84.5% had stage II lymphedema |
| Deveci et al. 2021 [55] | Turkey | LSIDS-A | To adapt LSIDS-A into Turkish and to test its validity and reliability in patients with BCRL | 6 translators 5 experts for content validity 20 patients for pilot testing 186 patients for structural validity, construct validity, and internal consistency | 55.4 ± 10.2 (20–80) | 100% | BCRL | 48.8 ± 49.5 (1–204) months | Not specified |
| Viehoff and Wittink 2008 [56] | Netherland | ULL-27 | To translate the ULL27 into Dutch and to assess its internal consistency and validity for Dutch patients with upper limb lymphedema | 3 translators 5 patients for cognitive interview 84 patients LE and 61 patients non-LE for validation studies | 59 ± 11.79 (34–80) | 100% | BCRL | 35.51 ± 45.14 (0.5–276) months | Not specified |
| Vatansever et al. 2020 [57] | Turkey | ULL-27 | To perform translation, cultural adaptation, and validation of ULL-27 in Turkish-speaking population of BCRL; To assess QoL of Turkish BCRL patients | 4 translators 15 patients for cognitive interview 81 patients for validation studies | 54.96 ± 11.35 | 100% | BCRL | 23.12 ± 30.88 months | Mild to severe |
| Williams et al. 2018 [58] | Australia | ULL-QoL | To develop PROM specific to the assessment of HRQoL associated with upper limb lymphedema and assess its psychometric properties | 24 patients for PROM development 5 patients and 16 therapists for content validity 103 patients for reliability, construct validity, and responsiveness | 60.3 ± 13.0 (23–86) | 97% | 99% BCRL, 1% Non-Hodgkin’s lymphoma | Not specified | Not specified |
| Klassen et al. 2021 [59] | Canada | LYMPH-Q Upper Extremity | To describe the development and psychometric validation of the LYMPH-Q Upper Extremity Module | 15 patients for qualitative interviews 16 patients for content validity 3222 patients for structural validity, construct validity, internal consistency, and reliability | 40–70 | 100% | BCRL | ≤4 yrs: 31% 5–9 yrs: 36.7% ≥10 yrs: 32.3% | Mild to severe |
| PROM | Ref | Country (Language in which the PROM was Evaluated) | No of Items | Subscales | Recall Period | Response Option | Scoring | Original Language | Available Translation |
|---|---|---|---|---|---|---|---|---|---|
| LYMQOL-Arm A (Lymphedema Quality of Life Tool-Arm A) | Bakar et al. 2017 [41] | Turkey | 21 | 4 domains: function, appearance, symptoms, mood | Not specified | Domains: 4-point Likert scale (1–4); overall QoL: 0–10 scale | Total score of all domains and overall QoL score | English | Turkish |
| Karayurt et al. 2021 [42] | Turkey | 21 | 4 subscales: symptom, body image/appearance, function, mood | Not specified | Domains: 4-point Likert scale (1–4); overall QoL: 0–10 scale | Total score of all domains and overall QoL score | English | Turkish | |
| LYMQoL-Arm B (Lymphedema Quality of Life Tool-Arm B) | Borman et al. 2018 [43] | Turkey | 28 (adding 7 sub-questions) | 4 domains: function, appearance, symptoms, mood | Not specified | Domains: 4-point Likert scale (1–4); overall QoL: 0–10 scale | Total score of all domains and overall QoL score | English | Turkish |
| LLIS 1 (Lymphedema Life Impact Scale version 1) | Degirmenci et al. 2019 [44] | Turkey | 18 | 3 subscales: physical, psychosocial, functional | Not specified | 5-point Likert scale (1–5) | Total score, subscale score | English | Turkish, Persian |
| Haghighat et al. 2018 [45] | Iran | 18 | 3 subscales: physical, psychosocial, functional | Not specified | 5-point Likert scale (1–5) | Total score, subscale score | English | Turkish, Persian | |
| LLIS 2 (Lymphedema Life Impact Scale version 2) | Orhan et al. 2019 [46] | Turkey | 18 | 3 subscales: physical, psychosocial, functional | Not specified | 5-point Likert scale (0–4) | Total score, subscale score | English | Turkish, Arabic |
| Sharour 2020 [47] | Jordan | 18 | 3 subscales: physical, psychosocial, functional | Not specified | 5-point Likert scale (0–4) | Total score, subscale score | English | Turkish, Arabic | |
| Lymph-ICF-UL (Lymphedema Functioning, Disability, and Health Questionnaire for Upper Limb) | Devoogdt et al. 2011 [48] | Belgium | 29 | 5 domains: physical, mental, household, mobility, life, and social activities | Complaints during the last 2 weeks | Visual Analog Scale (VAS) 0–100 mm | Total score, domain score | Dutch | English, Danish, French, Chinese |
| Grarup et al. 2018 [49] | Denmark | 29 | 5 domains: physical, mental, household, mobility, life, and social activities | Complaints during the last 2 weeks | Visual Analog Scale (VAS) 0–100 mm | Total score, domain score | Dutch | English, Danish, French, Chinese | |
| de Vrieze et al. 2019 [50] | Belgium | 29 | 5 domains: physical, mental, household, mobility, life, and social activities | Complaints during the last 2 weeks | 11-point Likert scale (0–10) | Total score, domain score | Dutch | English, Danish, French, Chinese | |
| de Vrieze et al. 2020 [51] | Belgium | 29 | 5 domains: physical, mental, household, mobility, life, and social activities | Complaints during the last 2 weeks | 11-point Likert scale (0–10) | Total score, domain score | Dutch | English, Danish, French, Chinese | |
| de Vrieze et al. 2021 [52] | Belgium | 29 | 5 domains: physical, mental, household, mobility, life, and social activities | Complaints during the last 2 weeks | 11-point Likert scale (0–10) | Total score, domain score | Dutch | English, Danish, French, Chinese | |
| Zhao et al. 2022 [53] | China | 29 | 5 domains: physical, mental, household, mobility, life, and social activities | Complaints during the last 2 weeks | 11-point Likert scale (0–10) | Total score, domain score | Dutch | English, Danish, French, Chinese | |
| LSIDS-A (Lymphedema Symptom Intensity and Distress Survey-Arm) | Ridner and Dietrich 2015 [54] | USA | 36 | 7 clusters: soft tissue sensation, neurological sensation, function, biobehavioral, resource, sexuality, activity | Reflective period of 1 week | Yes/no response, if ‘yes’ then 1–10 rating was solicited | Overall score, cluster score, intensity, and distress score | English | Turkish |
| Deveci et al. 2021 [55] | Turkey | 36 | 7 clusters: soft tissue sensation, neurological sensation, function, biobehavioral, resource, sexuality, activity | Reflective period of 1 week | Yes/no response, if ‘yes’ then 1–10 rating was solicited | Overall score, cluster score, intensity, and distress score | English | Turkish | |
| ULL27 (Upper Limb Lymphedema 27) | Viehoff and Wittink 2008 [56] | Netherlands | 27 | 3 domains: physical, psychological, social | Not specified | 5-point Likert scale | Total score, domain score | French | Dutch, Turkish, English |
| Vatansever et al. 2020 [57] | Turkey | 27 | 3 domains: physical, psychological, social | Not specified | 5-point Likert scale | Total score, domain score | French | Dutch, Turkish, English | |
| ULL-QoL (Upper Limb Lymphedema Quality of Life Questionnaire) | Williams et al. 2018 [58] | Australia | 14 | 2 dimensions: physical well-being, emotional well-being | Over the previous 2 weeks | 5-point Likert scale | Total score, dimension score | English | None |
| LYMPH-Q Upper Extremity | Klassen et al. 2021 [59] | Canada | 68 | 6 scales:
| Now (appearance); past week (function, psychological, symptoms); N/A (information); most recent (arm sleeve) | 4 response options for each scale:
| Scale score | English | None |
| (a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| COSMIN Measurement Properties | LYMQoL-Arm A [41,42] | LYMQoL-Arm B [43] | LLIS ver 1 [44,45] | ||||||
| Studies (Meth Qual Rating) | Results (Rating) | Summary of Results (Overall Rating) | Studies (Meth Qual Rating) | Results (Rating) | Summary of Results (Overall Rating) | Studies (Meth Qual Rating) | Results (Rating) | Summary of Results (Overall Rating) | |
| V/A/D/I * | +/−/? ** | +/−/±/? ** | V/A/D/I * | +/−/? ** | +/−/±/? ** | V/A/D/I * | +/−/? ** | +/−/±/? ** | |
| Content validity | Bakar 2017 (D) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | Content validity: (+) | Borman 2018 (D) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | Content validity: (+) | Degirmenci 2019 (D) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | Content validity: (+) |
| Karayurt 2021 (D) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | Haghighat 2018 (D) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | ||||||
| Structural validity | Bakar 2017 (I) | EFA → factor 1 = 0.624–0.912; factor 2 = 0.587–0.876; factor 3 = 0.376–0.866; factor 4 = 0.788–0.861 (+) | 4 factors with acceptable factor loadings (+) | Borman 2018 (I) | CFA → CMIN/df: 1.733, RMSEA: 0.074, GFI: 0.782, IFI: 0.904, CFI: 0.902, TLI: 0.888 (−) | Criteria for model fit were not met (−) | Degirmenci 2019 (I) | EFA → factor 1 = 0.214–0.770; factor 2 = 0.571–0.818; factor 3 = 0.309–0.748 (+) | 3 factors with acceptable factor loadings (+) |
| Karayurt 2021 (A) | CFA → CMIN/df: 1.86, RMSEA: 0.089, SRMR: 0.09, CFI: 0.81, GFI: 0.74, AGFI: 0.68 (−) | Haghighat 2018 (V) | CFA → NFI: 0.856, NNFI: 0.894, CFI: 0.908, MFI: 0.909, RMSEA: 0.087; EFA → factor 1 = 0.621–0.884; factor 2 = 0.651–0.821; factor 3 = 0.443–0.631 (+) | ||||||
| Internal consistency | Bakar 2017 (V) | Cronbach’s α (total) = 0.91; Cronbach’s α (domains) = 0.70–0.94 (+) | Cronbach’s α = 0.70–0.94 (+) | Borman 2018 (V) | Cronbach’s α = 0.85–0.90 (?) | (?) | Degirmenci 2019 (V) | Cronbach’s α (subscales) = 0.771–0.865; Cronbach’s α (total) = 0.916 (+) | Cronbach’s α = 0.771–0.879 (+) |
| Karayurt 2021 (V) | Cronbach’s α (total) = 0.90; Cronbach’s α (domains) = 0.78–0.86 (+) | Haghighat 2018 (V) | Cronbach’s α = 0.853–0.879 (+) | ||||||
| Cross-cultural validity/measurement invariance | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Reliability | Bakar 2017 (A) | Test-retest: ICC (total) = 0.99; ICC (domains) = 0.98–0.99 (+) | Test-retest ICC = 0.92–0.99 (+) | Borman 2018 (V) | Test-retest: ICC (total) = 0.627; ICC (domains) = 0.451–0.714 (−) | (−) | Degirmenci 2019 (V) | Test-retest: ICC (subscales) = 0.963–0.985; ICC (total) = 0.991 (+) | Test-retest ICC = 0.855–0.991 (+) |
| Haghighat 2018 (A) | Test retest: ICC (subscales) = 0.855–0.977; ICC (total) = 0.962 (+) | ||||||||
| Measurement error | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Criterion validity | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Hypothesis testing (for construct validity) | Bakar 2017 (V) | LYMQoL-Arm A and NHP r = 0.539–0.643, p < 0.05; LYMQoL-Arm A and Overall QoL r = −0.535 to −0.707, p < 0.05 (2+) | Result in line with 6 hypotheses, but not with 1 hypothesis (+) | Borman 2018 (V) | Convergent validity → LYMQoL-Arm B and EORTC-BR23 (body image, future, systemic complications, breast symptoms, arm symptoms) r = 0.203 to 0.637, p < 0.05; LYMQoL-Arm B and FACT-B4 r = −0.100 to −0.530, p < 0.05; Divergent validity → LYMQoL-Arm B and EORTC-BR23 (sexuality, hair loss) r = −0.017 to 0.214, p < 0.05 (3+) | Result in line with 3 hypotheses (+) | Degirmenci 2019 (V) | LLIS 1 and SF-12 rs = −0.453 to −0.703, p < 0.01; LLIS 1 and EORTC QLQ-C30 rs = 0.496–0.723, p < 0.01; LLIS 1 and DASH rs = 0.580–0.785, p < 0.01 (3+) | Result in line with 5 hypotheses, but not with 1 hypothesis (+) |
| Karayurt 2021 (V) | Known groups validity → the mean scores of LYMQoL-Arm A total (t = −4.628, p = 0.001), subscales symptom (t = −2.113, p = 0.038), body image/appearance (t = −5.247, p = 0.001), and function (t = −5.874, p = 0.001) in patients with severe LE were significantly higher than patients with mild LE, but no significant different in both groups’ mean scores for subscale mood (t = −0.776, p = 0.446) (4+, 1-) | Haghighat 2018 (V) | Discriminant validity → patients with LE showed higher impairments in all three subscales compared to those without LE, p < 0.01 for physical and functional subscales; Convergent validity → LLIS 1 and SF-36 rs = −0.344 to −0.497, p < 0.01; LLIS 1 and EORTC QLQ-C30 rs ≤ −0.388 to −0.723, p < 0.01 (2+, 1-) | ||||||
| Responsiveness | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| (b) | |||||||||
| COSMIN Measurement Properties | LLIS ver 2 [46,47] | Lymph-ICF-UL [48,49,50,51,52,53] | LSIDS-A [54,55] | ||||||
| Studies (Meth Qual Rating) | Results (Rating) | Summary of Results (Overall Rating) | Studies (Meth Qual Rating) | Results (Rating) | Summary of Results (Overall Rating) | Studies (Meth Qual Rating) | Results (Rating) | Summary of Results (Overall Rating) | |
| V/A/D/I * | +/−/? ** | +/−/±/? ** | V/A/D/I * | +/−/? ** | +/−/±/? ** | V/A/D/I * | +/−/? ** | +/−/±/? ** | |
| Content validity | Orhan 2019 (D) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | Content validity: (+) | Devoogdt 2011 (D) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | Content validity: (+) | Ridner 2015 (D) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | Content validity: (+) |
| Sharour 2020 (D) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | Grarup 2018 (A) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | Deveci 2021 (A) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | ||||
| De Vrieze 2019 (D) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | ||||||||
| De Vrieze 2021 (D) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | ||||||||
| Zhao 2022 (A) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | ||||||||
| Structural validity | Orhan 2019 (A) | EFA → factor 1 = 0.502–0.751; factor 2 = 0.401–0.787; factor 3 = 0.426–0.844 (+) | 3 factors with acceptable factor loadings (+) | Zhao 2022 (A) | EFA → factor 1 = 0.648–0.784; factor 2 = 0.754–0.798; factor 3 = 0.419–0.802; factor 4 = 0.808–0.881; factor 5 = 0.457–0.739 (+) | 5 factors with acceptable factor loadings (+) | Deveci 2021 (A) | CFA → for intensity scale: CMIN/df: 1.52, RMSEA: 0.056, SRMR: 0.19, CFI: 0.91, GFI: 0.83, IFI: 0.91, TLI: 0.90; for distress scale: CMIN/df: 1.55, RMSEA: 0.055, SRMR: 0.27, CFI: 0.90, GFI: 0.84, IFI: 0.90, TLI: 0.893 (+) | Model fit was acceptable (+) |
| Sharour 2020 (D) | EFA → factor 1 = 0.65–0.76; factor 2 = 0.61–0.88; factor 3 = 0.60–0.72 (+) | ||||||||
| Internal consistency | Orhan 2019 (V) | Cronbach’s α (subscales) = 0.76–0.78; Cronbach’s α (total) = 0.89 (+) | Cronbach’s α = 0.76–0.923 (+) | Devoogdt 2011 (V) | Cronbach’s α (domains) = 0.72–0.92; Cronbach’s α (total) = 0.92 (+) | Cronbach’s α = 0.72–0.98 (+) | Ridner 2015 (V) | KR-20 (symptoms occurrence) = 0.88; Cronbach’s α (intensity score) = 0.93; Cronbach’s α (distress score) = 0.94 (+) | KR-20 = 0.83–0.88; Cronbach’s α = 0.68–0.94 (+) |
| Sharour 2020 (V) | Cronbach’s α (subscales) = 0.861–0.901; Cronbach’s α (total) = 0.923 (+) | Grarup 2018 (V) | Cronbach’s α (domains) = 0.92–0.97; Cronbach’s α (total) = 0.98 (+) | Deveci 2021 (V) | KR-20 (symptoms occurrence) = 0.83; Cronbach’s α (intensity score) = 0.76–0.86; Cronbach’s α (distress score) = 0.68–0.86 (+) | ||||
| De Vrieze 2019 (V) | Cronbach’s α (domains) = 0.89–0.98; Cronbach’s α (total) = 0.98 (+) | ||||||||
| De Vrieze 2021 (V) | Cronbach’s α (domains) = 0.77–0.89; Cronbach’s α (total) = 0.95 (+) | ||||||||
| Zhao 2022 (V) | Cronbach’s α (domains) = 0.789–0.910; Cronbach’s α (total) = 0.918 (+) | ||||||||
| Cross-cultural validity/measurement invariance | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Reliability | Orhan 2019 (A) | Test-retest: ICC (subscales) = 0.88–0.93; ICC (total) = 0.91 (+) | Test-retest ICC = 0.88–0.93 (+) | Devoogdt 2011 (I) | Test-retest: ICC (domains) = 0.65–0.91; ICC (total) = 0.93 (+) | Test-retest ICC = 0.65–0.95 (+) | Ridner 2015 (D) | Test-retest: ICC (clusters) = 0.67–0.97; ICC (intensity) = 0.93; ICC (distress) = 0.92 (+) | Test-retest ICC = 0.67–0.93 (+) |
| Grarup 2018 (D) | Test-retest: ICC (domains) = 0.88–0.94; ICC (total) = 0.95 (+) | ||||||||
| De Vrieze 2019 (I) | Test-retest: ICC (domains) = 0.79–0.93; ICC (total) = 0.95 (+) | ||||||||
| De Vrieze 2021 (I) | Test-retest: ICC (domains) = 0.66–0.95; ICC (total) = 0.91 (+) | ||||||||
| Zhao 2022 (V) | Test-retest: ICC (domains) = 0.801–0.834; ICC (total) = 0.828 (+) | ||||||||
| Measurement error | N/A | N/A | N/A | Devoogdt 2011 (I) | Variability → SEM (total) = 4.8; SEM (domains) = 7.0–12.5; Clinically Important Changes → SDC (total) = 13.4; SDC (domains) = 19.4–34.6 (+) | SEM = 4.51–12.6; SDC = 12.5–34.91 (+) | N/A | N/A | N/A |
| Grarup 2018 (D) | Variability → SEM (total) = 4.51; SEM (domains) = 5.69–10.21; Clinically Important Changes → SDC (total) = 12.5; SDC (domains) = 15.8–28.3 (+) | ||||||||
| De Vrieze 2019 (I) | Variability → SEM (total) = 4.89; SEM (domains) = 6.31–12.31; Clinically Important Changes → SDC (total) = 13.56; SDC (domains) = 17.49–34.13 (+) | ||||||||
| De Vrieze 2021 (I) | Variability → SEM (total) = 5.54; SEM (domains) = 6.28–12.6; Clinically Important Changes → SDC (total) = 15.35; SDC (domains) = 17.4–34.91 (+) | ||||||||
| Criterion validity | Orhan 2019 (V) | LLIS 2 (subscales) and LVD r = 0.30–0.36, p < 0.05; LLIS 2 (total) and LVD r = 0.39, p < 0.01 (−) | Weak correlation with gold measurement standard (LVD) r < 0.40 (−) | N/A | N/A | N/A | N/A | N/A | N/A |
| Hypothesis testing (for construct validity) | Orhan 2019 (V) | Convergent validity → LLIS 2 and LYMQOL (subscales) r = 0.52–0.82, p < 0.01; LLIS 2 and EORTC QLQ-C30 (functional and symptom) r = 0.67 to −0.85, p < 0.01; LLIS 2 and Quick-DASH r = 0.68–0.84, p < 0.01; Divergent validity → there was a significant difference in total score and all subscale scores between LE and non-LE groups, p < 0.05 (4+) | Result in line with 7 hypotheses (+) | Devoogdt 2011 (V) | Convergent validity → Lymph-ICF-UL and SF-36 (bodily pain, mental health, physical functioning, social functioning) r = −0.33 to −0.70; Divergent validity → Lymph-ICF-UL and SF-36 (role-emotional, mental health, physical functioning, role-physical) r = 0.03 to −0.42; Known-groups validity → the scores on 26 of 29 questions were significantly higher for LE patients compared to non-LE patients, p < 0.05 (40+, 5-) | Result in line with 75 hypotheses, but not with 15 hypotheses (+) | Ridner 2015 (V) | Convergent validity → LSIDS-A and FACT-G rs = −0.20 to −0.53; LSIDS-A and FACT-B+4 rs = −0.41 to −0.50; LSIDS-A and ULL-27 rs = −0.29 to −0.52; LSIDS-A and FASQ rs = 0.25–0.47; LSIDS-A and CES-D rs = 0.29–0.65; LSIDS-A and FACT rs = −0.46 to −0.50; LSIDS-A and POMS-SF rs = 0.07–0.36; Divergent validity → LSIDS-A and MCSDS rs = 0.01 to −0.25 (8+, 6-) | Result in line with 9 hypotheses, but not with 6 hypotheses (−) |
| Sharour 2020 (V) | Convergent validity → LLIS 2 (total) and EORTC QLQ-C30 (functional and symptoms) r = 0.81 to −0.84; LLIS 2 (subscales) and EORTC QLQ-C30 (functional) r = −0.79 to −0.87; LLIS 2 (subscales) and EORTC QLQ-C30 (symptoms) r = 0.73–0.81 (3+) | De Vrieze 2019 (V) | Convergent validity → Lymph-ICF-UL and SF-36 (bodily pain, mental health, physical functioning, social functioning) r = −0.224 to −0.661; Divergent validity → Lymph-ICF-UL and SF-36 (role-emotional, mental health, physical functioning, role-physical) rs = −0.191 to −0.607 (11+, 3-) | Deveci 2021 (V) | Known groups validity → there was a significantly higher mean score in patients with active LE compared to patients with latent LE (1+) | ||||
| De Vrieze 2021 (V) | Convergent validity → Lymph-ICF-UL and SF-36 (bodily pain, mental health, physical functioning, social functioning) rs = −0.156 to −0.704; Divergent validity → Lymph-ICF-UL and SF-36 (role-emotional, mental health, physical functioning, role-physical) rs = −0.144 to −0.499 (9+, 5-) | ||||||||
| Zhao 2022 (V) | Convergent validity → Lymph-ICF-UL and SF-36 (bodily pain, mental health, physical functioning, social functioning) r = −0.371 to −0.563; Lymph-ICF-UL and EORTC-QLQ-C30 r = 0.230 to −0.457; Divergent validity → Lymph-ICF-UL and SF-36 (role-emotional, mental health, physical functioning, role-physical) r = −0.102 to −0.376; Discriminant validity → patients with LE showed more impairments than patients without LE (p < 0.001) (15+, 2-) | ||||||||
| Responsiveness | N/A | N/A | N/A | De Vrieze 2020 (V) | Internal responsiveness → there were: a significant changes in mean total score between pre- and postintensive treatment (p < 0.05); no significant difference in mean total scores between pre- and posttreatment in stable group (p > 0.05); moderate responsiveness for total score (SRM = 0.65); External responsiveness → there were: a significant difference in mean change score between responders and non-responders after intensive treatment (p < 0.001); weak correlation between Δ-Lymph-ICF-UL and the GPE scores; MCID (total scores) = 9% (5+, 1-) | Results in line with 5 hypotheses, but not with 1 hypothesis (+) | N/A | N/A | N/A |
| (c) | |||||||||
| COSMIN Measurement Properties | ULL27 [56,57] | ULL-QoL [58] | LYMPH-Q Upper Extremity [59] | ||||||
| Studies (Meth Qual Rating) | Results (Rating) | Summary of Results (Overall Rating) | Studies (Meth Qual Rating) | Results (Rating) | Summary of Results (Overall Rating) | Studies (Meth Qual Rating) | Results (Rating) | Summary of Results (Overall Rating) | |
| V/A/D/I * | +/−/? ** | +/−/±/? ** | V/A/D/I * | +/−/? ** | +/−/±/? ** | V/A/D/I * | +/−/? ** | +/−/±/? ** | |
| Content validity | Viehoff 2008 (D) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | Content validity: (+) | Williams 2018 (D) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | Content validity: (+) | Klassen 2021 (D) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | Content validity: (+) |
| Vatansever 2020 (D) | Relevance: (+) Comprehensiveness: (+) Comprehensibility: (+) | ||||||||
| Structural validity | Vatansever 2020 (I) | CFA → RMSEA = 0.074; CFI = 0.97; IFI = 0.97; GFI = 0.96 (+) | Model fit was acceptable (+) | Williams 2018 (A) | EFA → factor 1 = 0.348–0.852; factor 2 = 0.375–0.870 (+) | 2 factors with acceptable factor loadings (+) | Klassen 2021 (A) | Rasch: item fit was within ±2.5 for 27 of the 68 items (−) | Not all model fit was reported (−) |
| Internal consistency | Viehoff 2008 (V) | Cronbach’s α = 0.78–0.92 (?) | Cronbach’s α = 0.75–0.93 (+) | Williams 2018 (V) | Cronbach’s α = 0.87 (+) | (+) | Klassen 2021 (V) | Cronbach’s α (scales) = 0.89–0.97 (?) | (?) |
| Vatansever 2020 (V) | Cronbach’s α (dimensions) = 0.75–0.90; Cronbach’s α (total) = 0.93 (+) | ||||||||
| Cross-cultural validity/measurement invariance | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Reliability | Vatansever 2020 (I) | Test-retest r = 0.40, p > 0.05 (?) | r = 0.40, p > 0.05 (?) | Williams 2018 (A) | Test-retest ICC (total) = 0.93 (+) | (+) | Klassen 2021 (D) | Test-retest ICC (scales) = 0.92–0.96 (+) | (+) |
| Measurement error | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Criterion validity | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Hypothesis testing (for construct validity) | Viehoff 2008 (V) | Convergent validity → ULL-27 and RAND-36 rs = 0.45–0.69; Discriminant validity → there was a significant difference in total scores and all domain scores between LE and non-LE groups, p < 0.001 (10+,1-) | Result in line with 11 hypotheses, but not with 14 hypotheses (−) | Williams 2018 (V) | Convergent validity → ULL-QoL and EQ-5D-3L r = −0.44 to −0.59; ULL-QoL (physical well-being) and SF-36 (PCS) r = −0.57; Divergent validity → ULL-QoL and % excess limb volume r = 0.12–0.18; ULL-QoL and SF-36 r = −0.31 to −0.43; ULL-QoL (emotional well-being) and EQ-5D-3L (utility scores) r = −0.50 (7+,1-) | Result in line with 7 hypotheses, but not with 1 hypothesis (+) | Klassen 2021 (V) | The correlation between symptoms, function, appearance, psychological, arm sleeve with each other was higher than with information (r = >0.50); All six scales were associated with increased severity of arm swelling, reporting of arm problem caused by cancer treatments, and wearing of a compression sleeve to reduce or prevent swelling in the past 12 months (3+, 1-) | Result in line with 3 hypotheses, but not with 1 hypothesis (+) |
| Vatansever 2020 (V) | Convergent validity → ULL-27 and EORTC QLQ-C30 (QL2, PF2, RF2, EF, SF, FA, NV, PA, DY, SL, AP) r = −0.221 to −0.546, p < 0.001; ULL-27 and EORTC QLQ-BR23 (BRBI, BRFU, BRST) r = −0.248 (p < 0.005) to 0.348 (p < 0.001) (1+, 13-) | ||||||||
| Responsiveness | N/A | N/A | N/A | Williams 2018 (D) | LE transition to better → Mean change (SD of changes scores) = −5.4 (19.0) to −8.9 (17.7); MSRM = 0.30–0.64; LE transition to worse → Mean change (SD of changes scores) = 8.4 (13.8)–15.0 (27.7); MSRM = 0.61–0.83 (2+) | Result in line with 2 hypotheses (+) | N/A | N/A | N/A |
| PROM * (ref) | Quality of Evidence Rating (GRADE **) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content Validity | Structural Validity | Internal Consistency | Cross-Cultural Validity | Reliability | Measurement Error | Criterion Validity | Hypothesis Testing | Responsiveness | |||
| Relevance | Comprehensiveness | Comprehensibility | |||||||||
| LYMQOL-Arm A [41,42] | Moderate | Moderate | Low | Moderate | High | N/A | Low | N/A | N/A | High | N/A |
| LYMQOL-Arm B [43] | Low | Low | Low | Very Low | High | N/A | Moderate | N/A | N/A | High | N/A |
| LLIS 1 [44,45] | Moderate | Moderate | Moderate | Moderate | Moderate | N/A | Moderate | N/A | N/A | Moderate | N/A |
| LLIS 2 [46,47] | Low | Low | Low | Low | Moderate | N/A | Very Low | N/A | Moderate | High | N/A |
| Lymph-ICF-UL [48,49,50,51,52,53] | High | High | High | Moderate | High | N/A | High | Low | N/A | High | Moderate |
| LSIDS-A [54,55] | Low | Moderate | Low | Moderate | High | N/A | Very Low | N/A | N/A | High | N/A |
| ULL-27 [56,57] | Low | Low | Low | Very Low | High | N/A | Very Low | N/A | N/A | High | N/A |
| ULL-QoL [58] | High | High | Moderate | Moderate | High | N/A | Very Low | N/A | N/A | High | Very Low |
| LYMPH-Q Upper Extremity [59] | Moderate | Moderate | Moderate | Moderate | High | N/A | Very Low | N/A | N/A | High | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meilani, E.; Zanudin, A.; Mohd Nordin, N.A. Psychometric Properties of Quality of Life Questionnaires for Patients with Breast Cancer-Related Lymphedema: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 2519. https://doi.org/10.3390/ijerph19052519
Meilani E, Zanudin A, Mohd Nordin NA. Psychometric Properties of Quality of Life Questionnaires for Patients with Breast Cancer-Related Lymphedema: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(5):2519. https://doi.org/10.3390/ijerph19052519
Chicago/Turabian StyleMeilani, Estu, Asfarina Zanudin, and Nor Azlin Mohd Nordin. 2022. "Psychometric Properties of Quality of Life Questionnaires for Patients with Breast Cancer-Related Lymphedema: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 5: 2519. https://doi.org/10.3390/ijerph19052519
APA StyleMeilani, E., Zanudin, A., & Mohd Nordin, N. A. (2022). Psychometric Properties of Quality of Life Questionnaires for Patients with Breast Cancer-Related Lymphedema: A Systematic Review. International Journal of Environmental Research and Public Health, 19(5), 2519. https://doi.org/10.3390/ijerph19052519





