The Salvador Primary Care Longitudinal Study of Child Development (CohortDICa) Following the Zika Epidemic: Study Protocol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Plan and the Territorial Location of Participants
2.2.1. Sample Size Calculation
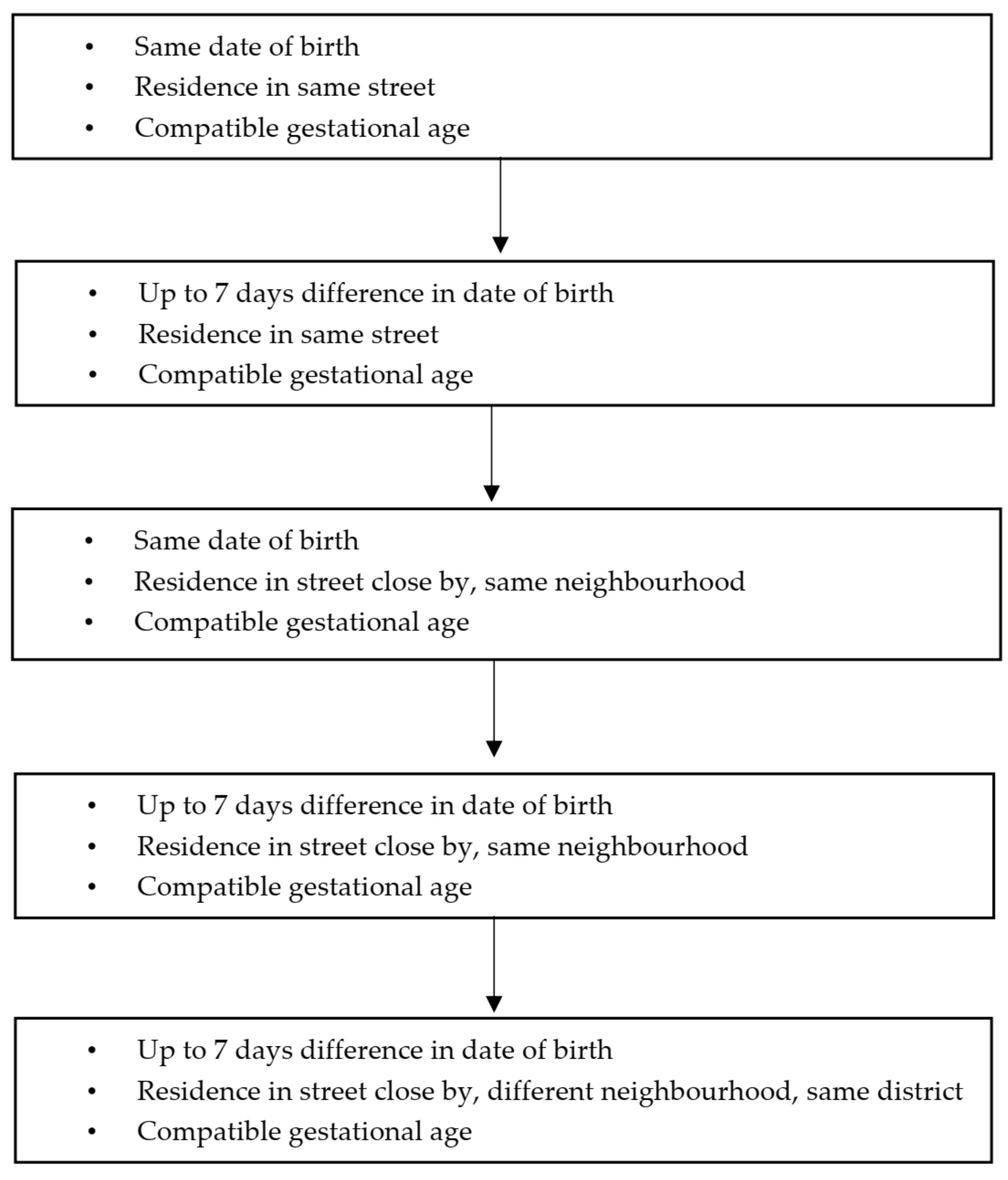
2.2.2. Definition of the Groups
2.3. Instruments and Procedures
2.4. Definition of Variables
Covariables
2.5. Baseline Study Procedures
2.6. Data Collection and Management
2.7. Data Collection Waves
2.8. Early Intervention
3. Discussion
3.1. Health Services and Recruiting the Cohort
3.2. Multidimensional Assessment of Development
3.3. Study Limitations and Notification Criteria
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brasil Ministério da Saúde. Secretaria de Vigilância em Saúde Departamento de Vigilância das Doenças Transmissíveis. Coordenação-Geral do Programa de Controle da Dengue. In Protocol for Surveillance and Response to the Occurrence of Microcephaly and/or Central Nervous System (CNS) Alterations, 2nd ed.; Ministério da Saúde: Brasilia, Brazil, 2016; p. 55. [Google Scholar]
- Albuquerque, M.S.V.; Lyra, T.M.; Melo, A.P.L.; Valongueiro, S.A.; Araújo, T.V.B.; Pimentel, C.; Moreira, M.C.N.; Mendes, C.H.F.; Nascimento, M.; Kuper, H.; et al. Access to healthcare for children with congenital Zika syndrome in Brazil: Perspectives of mothers and health professionals. Health Policy Plan. 2019, 34, 499–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunoni, D.; Blascovi-Assis, S.M.; Osório, A.A.C.; Seabra, A.G.; Amato, C.A.D.L.H.; Teixeira, M.C.T.V.; da Rocha, M.M.; Carreiro, L.R.R. Microcephaly and other Zika virus related events: The impact on children, families and health teams. Cien. Saude Colet. 2016, 21, 3297–3302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, A.A.M.; Ganz, J.S.S.; Sousa, P.S.; Doriqui, M.J.R.; Ribeiro, M.R.C.; Branco, M.R.F.C.; Queiroz, R.C.S.; Pacheco, M.J.T.; da Costa, F.R.V.; Silva, F.S.; et al. Early growth and neurologic outcomes of infants with probable congenital Zika virus syndrome. Emerg. Infect. Dis. 2016, 22, 1953–1956. [Google Scholar] [CrossRef]
- Shao, Q.; Herrlinger, S.; Yang, S.-L.; Lai, F.; Moore, J.M.; Brindley, M.A.; Chen, J.-F. Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development 2016, 143, 4127–4136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, A.; Brites, C.; Mochida, G.; Ventura, P.; Fernandes, A.; Lage, M.L.; Taguchi, T.; Brandi, I.; Silva, A.; Franceschi, G.; et al. Clinical and neurodevelopmental features in children with cerebral palsy and probable congenital Zika. Brain Dev. 2019, 41, 587–594. [Google Scholar] [CrossRef] [PubMed]
- de França, T.L.B.; Medeiros, W.R.; de Souza, N.L.; Longo, E.; Pereira, S.A.; de Oliveira França, T.B.; Sousa, K.G. Growth and development of children with microcephaly associated with congenital zika virus syndrome in Brazil. Int. J. Environ. Res. Public Health 2018, 15, 1990. [Google Scholar] [CrossRef] [Green Version]
- Alves, L.V.; Paredes, C.E.; Silva, G.C.; Mello, J.G.; Alves, J.G. Neurodevelopment of 24 children born in Brazil with congenital Zika syndrome in 2015: A case series study. BMJ Open 2018, 6, e021304. [Google Scholar] [CrossRef] [Green Version]
- Leal, M.C.; Muniz, L.F.; Ferreira, T.S.A.; Santos, C.M.; Almeida, L.C.; Van Der Linden, V.; Ramos, R.C.F.; Rodrigues, L.C.; Neto, S.S.C. Hearing Loss in Infants with Microcephaly and Evidence of Congenital Zika Virus Infection—Brazil, November 2015–May 2016. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 917–919. [Google Scholar] [CrossRef] [Green Version]
- Fox, S.E.; Levitt, P.; Nelson, C.A., III. How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010, 81, 28–40. [Google Scholar] [CrossRef]
- Cioni, G.; Inguaggiato, E.; Sgandurra, G. Early intervention in neurodevelopmental disorders: Underlying neural mechanisms. Dev. Med. Child Neurol. 2016, 58 (Suppl. 4), 61–66. [Google Scholar] [CrossRef] [Green Version]
- Shonkoff, J.P.; Boyce, W.T.; McEwen, B.S. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA 2009, 301, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- França, G.V.A.; Schuler-Faccini, L.; Oliveira, W.K.; Henriques, C.M.P.; Carmo, E.H.; Pedi, V.D.; Nunes, M.L.; Castro, M.C.; Serruya, S.; Silveira, M.F.; et al. Congenital Zika virus syndrome in Brazil: A case series of the first 1501 livebirths with complete investigation. Lancet 2016, 388, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Sanders Pereira Pinto, P.; de Almeida, T.M.; Monteiro, L.; Souza, M.M.D.S.; dos Santos, G.A.; Cardoso, C.W.; dos Santos, L.M.; Ribeiro, G.S.; dos Santos, D.N. Brain abnormalities on neuroimaging in Children with Congenital Zika Syndrome in Salvador, Brazil, and its possible implications on neuropsychological development. Int. J. Dev. Neurosci. 2020, 80, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.A.; Santos, D.N.; Bastos, A.C.; Pedromônico, M.R.M.; de Almeida-Filho, N.; Barreto, M.L. Family environment and child’s cognitive development: An epidemiological approach. Rev. Saude Publica 2005, 39, 606–611. [Google Scholar] [CrossRef] [Green Version]
- Michalec, D. Bayley Scales of Infant Development: Third Edition. Encycl. Child Behav. Dev. 2011, 215. [Google Scholar] [CrossRef]
- Bradley, R.H.; Corwyn, R.F.; McAdoo, H.P.; Coll, C.G. The home environments of children in the United States part I: Variations by age, ethnicity, and poverty status. Child Dev. 2001, 72, 1844–1867. [Google Scholar] [CrossRef]
- Wagnild, G. A review of the Resilience Scale. J. Nurs. Meas. 2009, 17, 105–113. [Google Scholar] [CrossRef]
- Barreto do Carmo, M.B.; dos Santos, L.M.; Feitosa, C.A.; Fiaccone, R.L.; da Silva, N.B.; dos Santos, D.N.; Barreto, M.L.; Amorim, L.D. Screening for common mental disorders using the SRQ-20 in Brazil: What are the alternative strategies for analysis? Rev. Bras. Psiquiatr. 2018, 40, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Santos, I.S.; Tavares, B.F.; Munhoz, T.N.; de Almeida, L.S.P.; da Silva, N.T.B.; Tams, B.D.; Patella, A.M.; Matijasevich, A. Sensitivity and specificity of the Patient Health Questionnaire-9 (PHQ-9) among adults from the general population. Cad. Saude Publica 2013, 29, 1533–1543. [Google Scholar] [CrossRef]
- Barroso, S.M.; de Andrade, V.S.; Midgett, A.H.; de Carvalho, R.G.N. Evidence of validity of Brazilian UCLA Loneliness Scale. J. Bras. Psiquiatr. 2016, 65, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Mari, J.J.; Williams, P. A validity study of a psychiatric screening questionnaire (SRQ-20) in primary care in the city of Sao Paulo. Br. J. Psychiatry 1986, 148, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.D. Measurement of growth in children with developmental disabilities. Dev. Med. Child Neurol. 1996, 38, 855–860. [Google Scholar] [CrossRef]
- Stevenson, R.D.; Conaway, M.; Chumlea, W.C.; Rosenbaum, P.; Fung, E.B.; Henderson, R.C.; Worley, G.; Liptak, G.; O’Donnell, M.; Samson-Fang, L.; et al. Measurement of growth in children with developmental disabilities. Pediatrics 2006, 118, 1010–1018. [Google Scholar] [CrossRef]
- Etges, C.L.; Barbosa, L.D.R.; Cardoso, M.C.d.A.F. Development of the Pediatric Dysphagia Risk Screening Instrument (PDRSI). CoDAS 2020, 32, e20190061. [Google Scholar] [CrossRef]
- Dumas, H.M.; Fragala-Pinkham, M.A.; Rosen, E.L.; Lombard, K.A.; Farrell, C. Pediatric Evaluation of Disability Inventory Computer Adaptive Test (PEDI-CAT) and Alberta Infant Motor Scale (AIMS): Validity and Responsiveness. Phys. Ther. 2015, 95, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, L.R.; Wyatt, C.C.; Brondani, M.M. Brondan A potential Gingival Inflammation Index for Frail Elders. Can. J. Dent. Hyg. 2010, 44, 118–123. [Google Scholar]
- Frencken, J.E.; de Amorim, R.G.; Faber, J.; Leal, S.C. The Caries Assessment Spectrum and Treatment (CAST) index: Rational and development. Int. Dent. J. 2011, 61, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Das Alecrim, M.D.; de Amorim, M.M.R.; de Araújo, T.V.B.; Brasil, P.; Brickley, E.B.; da Castilho, M.D.; Coelho, B.P.; da Cunha, A.J.L.A.; Duarte, G.; Estofolete, C.F.; et al. Zika Brazilian Cohorts (ZBC) Consortium: Protocol for an Individual Participant Data Meta-Analysis of Congenital Zika Syndrome after Maternal Exposure during Pregnancy. Viruses 2021, 16, 687. [Google Scholar] [CrossRef]
- Lopes, T.S.C.; Dos Santos, G.A.A.; Dos Santos, L.M.; Madaschi, V.; Jurdi, A.P.S.; Santos, D.N. Dos Reliability of the Bayley Scale in the Zika Virus Epidemic: A cohort study. Rev. Avaliação Psicológica, 2021; in press. [Google Scholar]
- Araújo, C.F.; Cabral, C.B.; Dantas, J.; de Oliveira, K.N.R.; Flores, M.C.M.; de Almeida, T.M.; da Silva, T.C.L.; dos Santos, L.M.; Santos, D.N. Coord Sensory and Motor Facilitation Protocol Manual for Using the Bayley Scale of Child Development in Children with Congenital Zika Virus Syndrome; 2 UFBA: Salvador, Brazil, 2020. [Google Scholar]

| Variables | Baseline April 2017–March 2018 (n = 274) | Follow-Up 1 May 2018–March 2019 (n = 238) | Follow-Up 2 March 2019–August 2019 (n = 222) | Instruments/Procedures |
|---|---|---|---|---|
| -Family’s social features, child’s perinatal history and results of tests on the Registry of Public Health Emergencies (42/5.000 Resultados de tradução Register of Public Health Emergencies: RESP) |  | Socio-demographic Questionnaire | ||
| -Quality of stimulation in the home and caregiver-child interaction |  | Home Observation for Measurement of the Environment (HOME) Inventory [17]. | ||
| -Cognitive, motor and language performance |  | Bayley Scale of Infant and Toddler Development (BSID-III), applied individually [16] | ||
| -Maternal Mental Health: Common Mental Disorders and Depressive Symptoms |  |  | SRQ-20, [19,22] PHQ-9, SANTOS, I. S et al. (2013) [20]. | |
| -Positive Personal Psychosocial Adaptation: Personal competence Acceptance of Self and Life |  | Resilience Scale [18] | ||
| -Anthropometric nutritional status, body fat distribution and food consumption |  | Nutritional assessment indicators, according to the North American Growth in Cerebral Palsy Project [23,24]. | ||
| -Early signs and symptoms of pediatric dysphagia |  | Pediatric Dysphagia Risk Screening Instrument (PDRSI). [25]. | ||
| -Child functioning: Daily life; Mobility; Cognition/Sociability and Responsibility |  | Pediatric Evaluation of Disability Inventory—Computerized Adaptive Testing (PEDI-CAT) [26]. | ||
| -Health history recorded in previous tests (n = 205) |  | Scanning the child’s booklet and previous tests submitted by the family | ||
| -Hearing health (n = 28) Peripheral and central hearing, behavioral hearing response to non-calibrated percussion instruments |  | Survey of Evoked Otoacoustic Emissions (AccuScreen OAE device), Immittance testing (otoflex device), Observation of behavioral responses non-calibrated percussion instruments | ||
| -Oral health (n = 28) Presence of plaque, gingival bleeding and tooth decay |  | Carter & Barnes flossing index—CBI [27]. Assessment Spectrum and Treatment. (CAST) [28] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, D.N.; de Araújo, T.M.; dos Santos, L.M.; Kuper, H.; Aquino, R.; Da Silveira, I.H.; Miranda, S.S.; Pereira, M.; Werneck, G.L. The Salvador Primary Care Longitudinal Study of Child Development (CohortDICa) Following the Zika Epidemic: Study Protocol. Int. J. Environ. Res. Public Health 2022, 19, 2514. https://doi.org/10.3390/ijerph19052514
Santos DN, de Araújo TM, dos Santos LM, Kuper H, Aquino R, Da Silveira IH, Miranda SS, Pereira M, Werneck GL. The Salvador Primary Care Longitudinal Study of Child Development (CohortDICa) Following the Zika Epidemic: Study Protocol. International Journal of Environmental Research and Public Health. 2022; 19(5):2514. https://doi.org/10.3390/ijerph19052514
Chicago/Turabian StyleSantos, Darci Neves, Tânia Maria de Araújo, Leticia Marques dos Santos, Hannah Kuper, Rosana Aquino, Ismael Henrique Da Silveira, Samilly Silva Miranda, Marcos Pereira, and Guilherme Loureiro Werneck. 2022. "The Salvador Primary Care Longitudinal Study of Child Development (CohortDICa) Following the Zika Epidemic: Study Protocol" International Journal of Environmental Research and Public Health 19, no. 5: 2514. https://doi.org/10.3390/ijerph19052514
APA StyleSantos, D. N., de Araújo, T. M., dos Santos, L. M., Kuper, H., Aquino, R., Da Silveira, I. H., Miranda, S. S., Pereira, M., & Werneck, G. L. (2022). The Salvador Primary Care Longitudinal Study of Child Development (CohortDICa) Following the Zika Epidemic: Study Protocol. International Journal of Environmental Research and Public Health, 19(5), 2514. https://doi.org/10.3390/ijerph19052514






