Epidemiology of Fungal Colonization in Children Treated at the Department of Oncology and Hematology: Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lanzkowsky, P.; Lipton, J.; Fish, J.D. Lanzkowsky’s Manual of Pediatric Hematology and Oncology, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Loeffen, E.A.H.; Knops, R.R.G.; Boerhof, J.; Feijen, E.A.M.L.; Merks, J.H.M.; Reedijk, A.M.J.; Lieverest, J.A.; Pieters, R.; Boezen, H.M.; Kremer, L.C.M.; et al. Treatment-related mortality in children with cancer: Prevalence and risk factors. Eur. J. Cancer 2019, 121, 113–122. [Google Scholar] [CrossRef]
- Alexander, S.; Pole, J.D.; Gibson, P.; Lee, M.; Hesser, T.; Chi, S.N.; Dvorak, C.C.; Fisher, B.; Hasle, H.; Kanerva, J.; et al. Classification of treatment-related mortality in children with cancer: A systematic assessment. Lancet Oncol. 2015, 16, e604–e610. [Google Scholar] [CrossRef]
- Badiee, P.; Kordbacheh, P.; Alborzi, A.; Zeini, F.; Mirhendy, H.; Mahmoody, M. Fungal infections in solid organ recipients. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2005, 3, 385–389. [Google Scholar]
- Carrol, K.C.; Pfaller, M.A.; Landry, M.L. Manual of Clinical Microbiology, 11th ed.; ASM Press: Washington, DC, USA, 2015. [Google Scholar]
- Baddley, J.W. Clinical risk factors for invasive aspergillosis. Med. Mycol. 2011, 49, S7–S12. [Google Scholar] [CrossRef]
- Badiee, P.; Alborzi, A.; Farhoudi, F. A case of Candida mediastinitis after dental extraction. J. Infect. Dev. Ctries. 2011, 5, 75–78. [Google Scholar] [CrossRef][Green Version]
- Pfaller, M.A.; Diekema, D.J. Rare and emerging opportunistic fungal pathogens: Concern for resistance beyond Candida albicans and Aspergillus fumigatus. J. Clin. Microbiol. 2004, 42, 4419–4431. [Google Scholar] [CrossRef]
- Badiee, P.; Hashemizadeh, Z. Opportunistic invasive fungal infections: Diagnosis & clinical management. Indian J. Med. Res. 2014, 139, 195–204. [Google Scholar]
- Angarone, M. Fungal infections in cancer patients. Cancer Treat Res. 2014, 161, 129–155. [Google Scholar]
- Parahym, A.M.R.D.C.; de Melo, L.R.B.; De Morais, V.L.L.; Neves, R.P. Candidiasis in pediatric patients with cancer interned in a university hospital. Braz. J. Microbiol. 2009, 40, 321–324. [Google Scholar] [CrossRef][Green Version]
- Steinbach, W.J. Epidemiology of invasive fungal infections in neonates and children. Clin. Microbiol. Infect. 2010, 16, 1321–1327. [Google Scholar] [CrossRef]
- Badiee, P.; Zareifar, S.; Haddadi, P.; Jafarian, H. Incidence of Fungal Infections in Pediatric Patients with Hematologic Neoplasms. Arch. Pediatr. Infect Dis. 2017, 5, e41317. [Google Scholar] [CrossRef]
- Georgiadou, S.P.; Pongas, G.; Fitzgerald, N.E.; Lewis, R.E.; Rytting, M.; Marom, E.M.; Kontoyiannis, D.P. Invasive mold infections in pediatric cancer patients reflect heterogeneity in etiology, presentation, and outcome: A 10-year, single-institution, retrospective study. J. Pediatr. Infect. Dis. Soc. 2012, 1, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Mesini, A.; Bandettini, R.; Caviglia, I.; Fioredda, F.; Amoroso, L.; Faraci, M.; Mattioli, G.; Piaggio, G.; Risso, F.M.; Moscatelli, A.; et al. Candida infections in paediatrics: Results from a prospective single-centre study in a tertiary care children’s hospital. Mycoses 2017, 60, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Wattier, R.L.; Dvorak, C.C.; Hoffman, J.A.; Brozovich, A.A.; Bin-Hussain, I.; Groll, A.H.; Castagnola, E.; Knapp, K.M.; Zaoutis, T.E.; Gustafsson, B.; et al. A Prospective, International Cohort Study of Invasive Mold Infections in Children. J. Pediatric Infect. Dis. Soc. 2015, 4, 313–322. [Google Scholar] [CrossRef]
- Moosa, M.Y.; Sobel, J.D. Non-albicans Candida infections in patients with hematologic malignancies. Semin. Respir. Infect. 2002, 17, 91–98. [Google Scholar] [CrossRef]
- Rosen, G.P.; Nielsen, K.; Glenn, S.; Abelson, J.; Deville, J.; Moore, T.B. Invasive fungal infections in pediatric oncology patients: 11-year experience at a single institution. J. Pediatric Hematol. Oncol. 2005, 27, 135–140. [Google Scholar] [CrossRef]
- Pagano, L.; Caira, M.; Candoni, A.; Offidani, M.; Fianchi, L.; Martino, B.; Pastore, D.; Picardi, M.; Bonini, A.; Chierchini, A.; et al. The epidemiology of fungal infections in patients with hematologic malignancies: The SEIFEM-2004 study. Haematologica 2006, 91, 1068–1075. [Google Scholar]
- Castagnola, E.; Cesaro, S.; Giacchino, M.; Livadiotti, S.; Tucci, F.; Zanazzo, G.; Caselli, D.; Caviglia, I.; Parodi, S.; Rondelli, R.; et al. Fungal infections in children with cancer: A prospective, multicenter surveillance study. Pediatric Infect. Dis. J. 2006, 25, 634–639. [Google Scholar] [CrossRef]
- Yeh, T.C.; Liu, H.C.; Wang, L.Y.; Chen, S.H.; Liang, D.C. Invasive fungal infection in children undergoing chemotherapy for cancer. Ann. Trop. Paediatr. 2007, 27, 141–147. [Google Scholar] [CrossRef]
- Marr, K.A. Fungal infections in oncology patients: Update on epidemiology, prevention and treatment. Curr. Opin. Oncol. 2010, 22, 138–142. [Google Scholar]
- Pana, Z.D.; Roilides, E.; Warris, A.; Groll, A.H.; Zaoutis, T. Epidemiology of Invasive Fungal Disease in Children. J. Pediatric Infect. Dis. Soc. 2017, 6, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Mantadakis, E.; Pana, Z.D.; Zaoutis, T. Candidemia in children: Epidemiology, prevention and management. Mycoses 2018, 61, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Gomez, S.M.; Caniza, M.; Fynn, A.; Vescina, C.; Ruiz, C.-D.; Iglesias, D.; Sosa, F.; Sung, L. Fungal infections in hematopoietic stem cell transplantation in children at a pediatric children’s hospital in Argentina. Transpl. Infect Dis. 2018, 20, e12913. [Google Scholar] [CrossRef]
- Silvester, E.J.; Watanabe, M.M.Y.; Pittet, L.F.; Boast, A.; Bryant, P.A.; Haeusler, G.M.; Daley, A.J.; Curtis, N.; Gwee, A. Candidemia in Children: A 16-year Longitudinal Epidemiologic Study. Pediatric Infect Dis. J. 2021, 40, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Ozsevik, S.N.; Sensoy, G.; Karli, A.; Albayrak, C.; Dagdemir, A.; Belet, N.; Elli, M.; Fisgin, T.; Ozyurek, E.; Duru, F.; et al. Invasive fungal infections in children with hematologic and malignant diseases. J. Pediatric Hematol. Oncol. 2015, 37, e69–e72. [Google Scholar] [CrossRef] [PubMed]
- Meidani, M.; Baniasadi, M.; Khorvash, F. Prevalence of Fungemia in Pediatric Patients with Febrile Neutropenia. Adv. Biomed. Res. 2018, 7, 88. [Google Scholar]
- Young, L.Y.; Hull, C.M.; Heitman, J. Disruption of ergosterol biosynthesis confers resistance to amphotericin B in Candida lusitaniae. Antimicrob. Agents Chemother. 2003, 47, 2717–2724. [Google Scholar] [CrossRef]
- Atkinson, B.J.; Russell, L.E.; Kontoyiannis, D.P. Candida lusitaniae fungemia in cancer patients: Risk factors for amphotericin B failure and outcome. Med. Mycol. 2008, 46, 541–546. [Google Scholar] [CrossRef]
- Bennett, J.E.; Izumikawa, K.; Marr, K.A. Mechanism of increased fluconazole resistance in Candida glabrata during prophylaxis. Antimicrob. Agents Chemother. 2004, 48, 1773–1777. [Google Scholar] [CrossRef]
- Amanati, A.; Badiee, P.; Jafarian, H.; Ghasemi, F.; Nematolahi, S.; Haghpanah, S.; Hamzavi, S.S. Impact of antifungal stewardship interventions on the susceptibility of colonized Candida species in pediatric patients with malignancy. Sci. Rep. 2021, 11, 14099. [Google Scholar] [CrossRef]
- Thygesen, J.B.; Glerup, H.; Tarp, B. Saccharomyces boulardii fungemia caused by treatment with a probioticum. BMJ Case Rep. 2012, 2012, bcr0620114412. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, A.J.; Calvo, M.A.; Guzmán, A.M.; Depix, M.S.; Garcia, P.M.D.; Perez, C.M.D.; Arrese, M.M.D.; Labarca, J.A.M.D. Saccharomyces cerevisiae fungemia after Saccharomyces boulardii treatment in immunocompromised patients. J. Clin. Gastroenterol. 2003, 36, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.; Parashar, A.; Acharyya, S. Saccharomyces cerevisiae Sepsis Following Probiotic Therapy in an Infant. Indian Pediatrics 2019, 56, 971–972. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.A.; Slavinski, S.A.; Morgan, J.; Lott, T.; Arthington-Skaggs, B.A.; Brandt, M.E.; Webb, R.M.; Currier, M.; Flowers, R.H.; Fridkin, S.K.; et al. Epidemiologic and molecular characterization of an outbreak of Candida parapsilosis bloodstream infections in a community hospital. J. Clin. Microbiol. 2004, 42, 4468–4472. [Google Scholar] [CrossRef]
- Rojas de Morales, T.; Zambrano, O.; Rivera, L.; Navas, R.; Chaparro, N.; Bernardonni, C.; Rivera, F.; Fonseca, N.; Tirado, D.M. Oral-disease prevention in children with cancer: Testing preventive protocol effectiveness. Med. Oral 2001, 6, 326–334. [Google Scholar]
- Mor, M.; Gilad, G.; Kornreich, L.; Fisher, S.; Yaniv, I.; Levy, I. Invasive fungal infections in pediatric oncology. Pediatric Blood Cancer 2011, 56, 1092–1097. [Google Scholar] [CrossRef]
- Behzadi, P.; Behzadi, E.; Ranjbar, R. Urinary tract infections and Candida albicans. Cent. Eur. J. Urol. 2015, 68, 96–101. [Google Scholar] [CrossRef]
- Binelli, C.A.; Moretti, M.L.; Assis, R.S.; Sauaia, N.; Menezes, P.R.; Ribeiro, E.; Geiger, D.C.P.; Mikami, Y.; Miyaji, M.; Oliveira, M.S.; et al. Investigation of the possible association between nosocomial candiduria and candidaemia. Clin. Microbiol. Infec. 2006, 12, 538–543. [Google Scholar] [CrossRef][Green Version]
- Lass-Flörl, C. Current Challenges in the Diagnosis of Fungal Infections. In Human Fungal Pathogen Identification. Methods in Molecular Biology; Lion, T., Ed.; Humana Press: New York, NY, USA, 2017; Volume 1508. [Google Scholar]
- Quindos, G.; Moragues, M.D.; Ponton, J. Is there a role for antibody testing in the diagnosis of invasive candidiasis? Rev. Iberoam. Micol. 2004, 21, 10–14. [Google Scholar]
- Arvantis, M.; Anagnostou, T.; Fuchs, B.B.; Caliendo, A.M.; Mylonakis, E. Molecular an Nonmolecular Diagnostic Methods ofor Invasive Fungal Infections. Clin. Microbiol. Rev. 2020, 27, 490–526. [Google Scholar] [CrossRef]
- Cesaro, S.; Tridello, G.; Castagnola, E.; Calore, E.; Carraro, F.; Mariotti, I.; Colombini, A.; Perrucio, K.; Decembrino, N.; Maximova, N.; et al. Retrospective study on the incidence and outcome of proven and probable invasive fungal infections in high-risk pediatric onco-hematological patients. Eur. J. Haematol. 2017, 99, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Hodgman, E.I.; Compton, J.; Qureshi, F.G.; Murphy, J.T. Diagnosis of Invasive Fungal Infection Among Pediatric Oncology Patients. J. Pediatric Hematol. Oncol. 2019, 41, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.; Nouér, S.A.; Morales, H.; Simões, B.; Solza, C.; Queiroz‐Telles, F.; Nucci, M. Epidemiology of invasive fungal disease in haematologic patients. Mycoses 2021, 64, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, P.J.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367. [Google Scholar] [CrossRef] [PubMed]
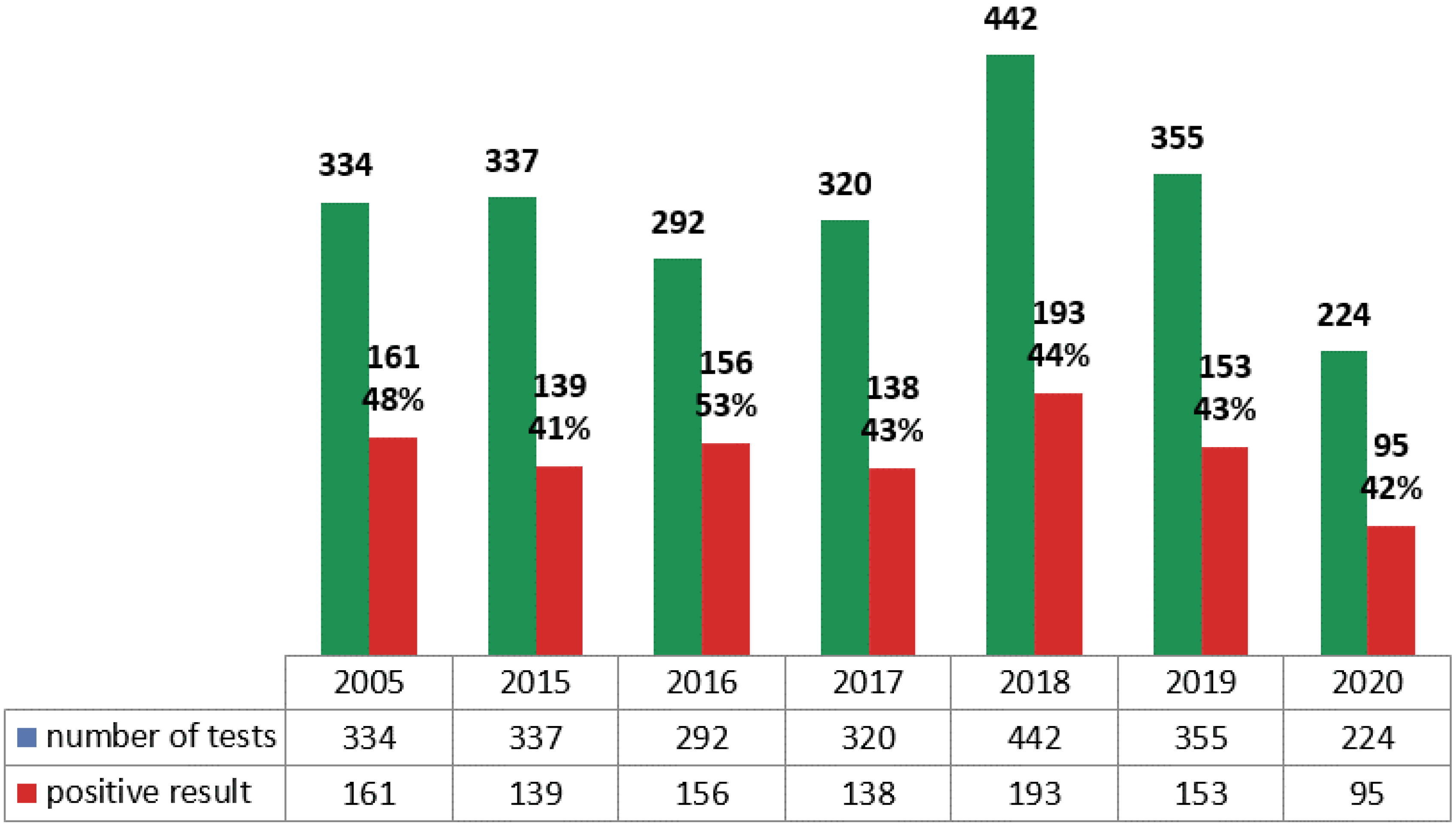
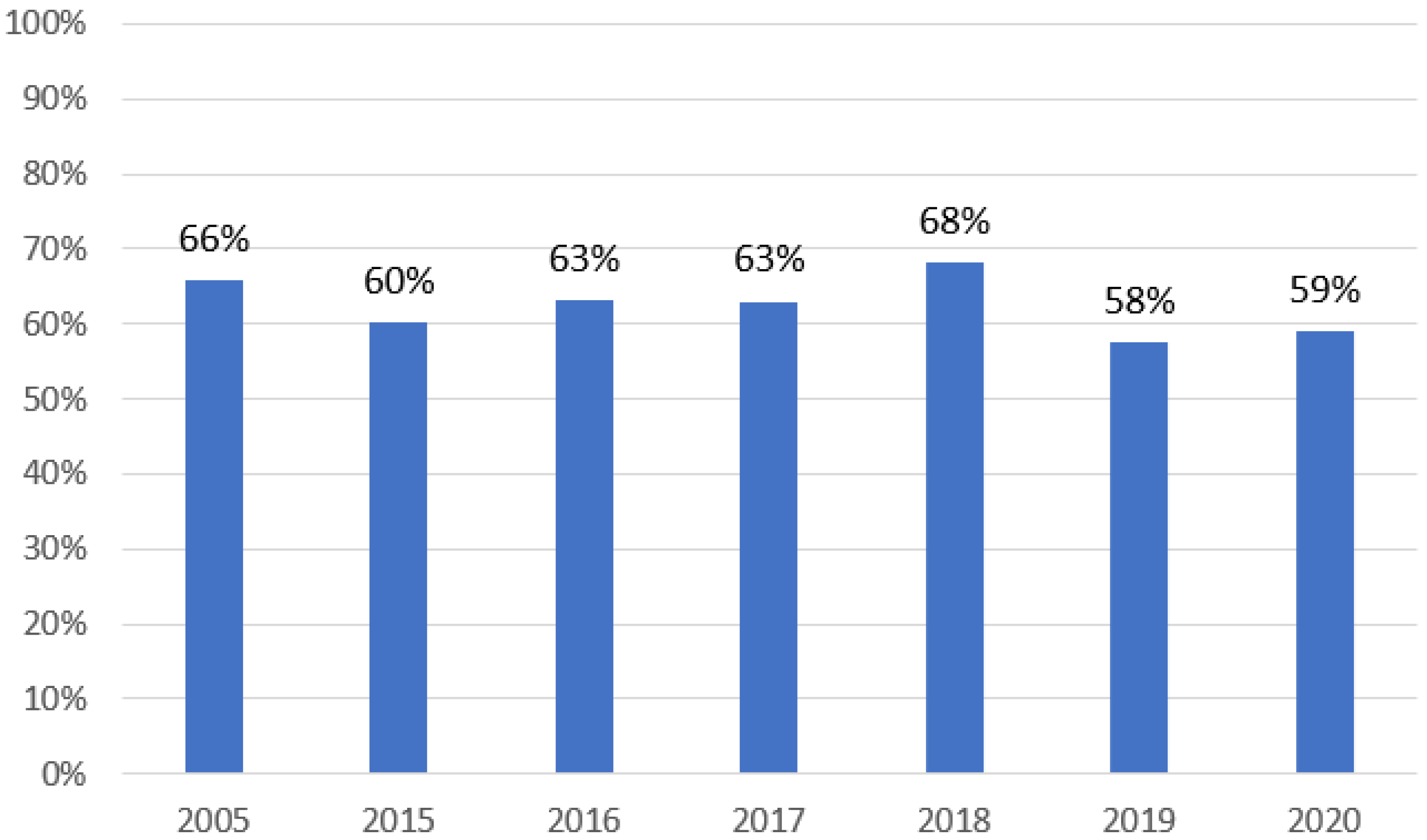
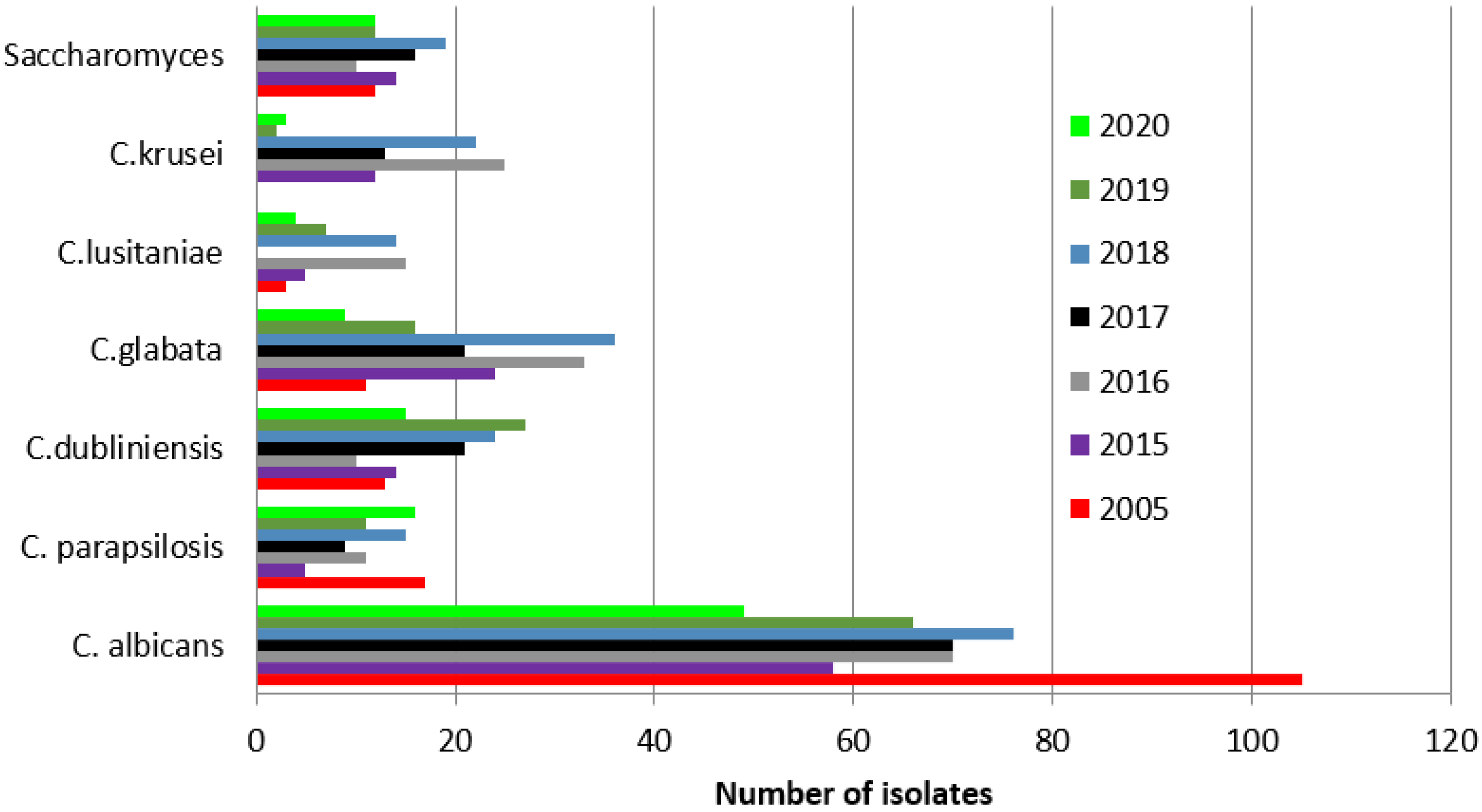
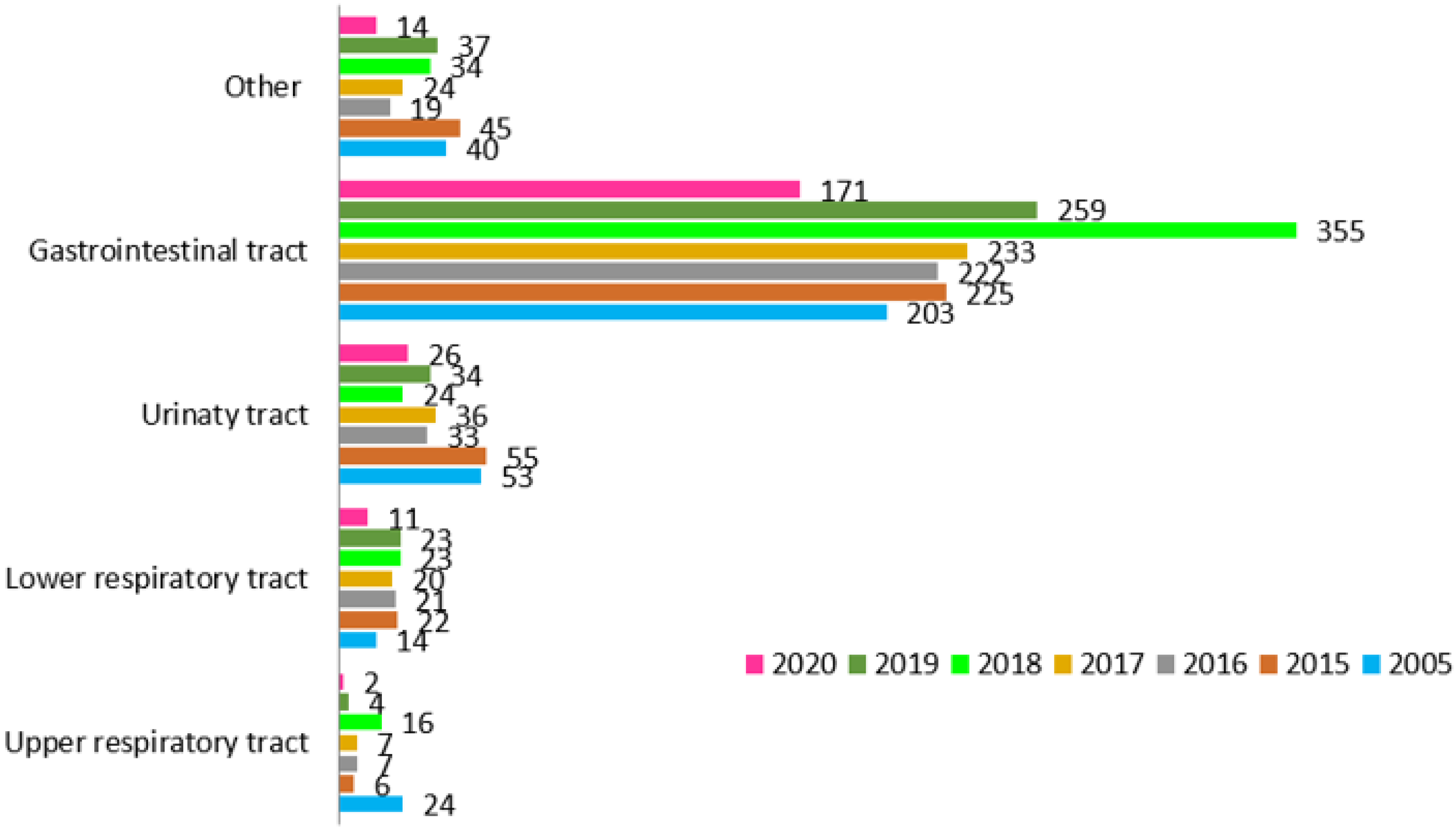
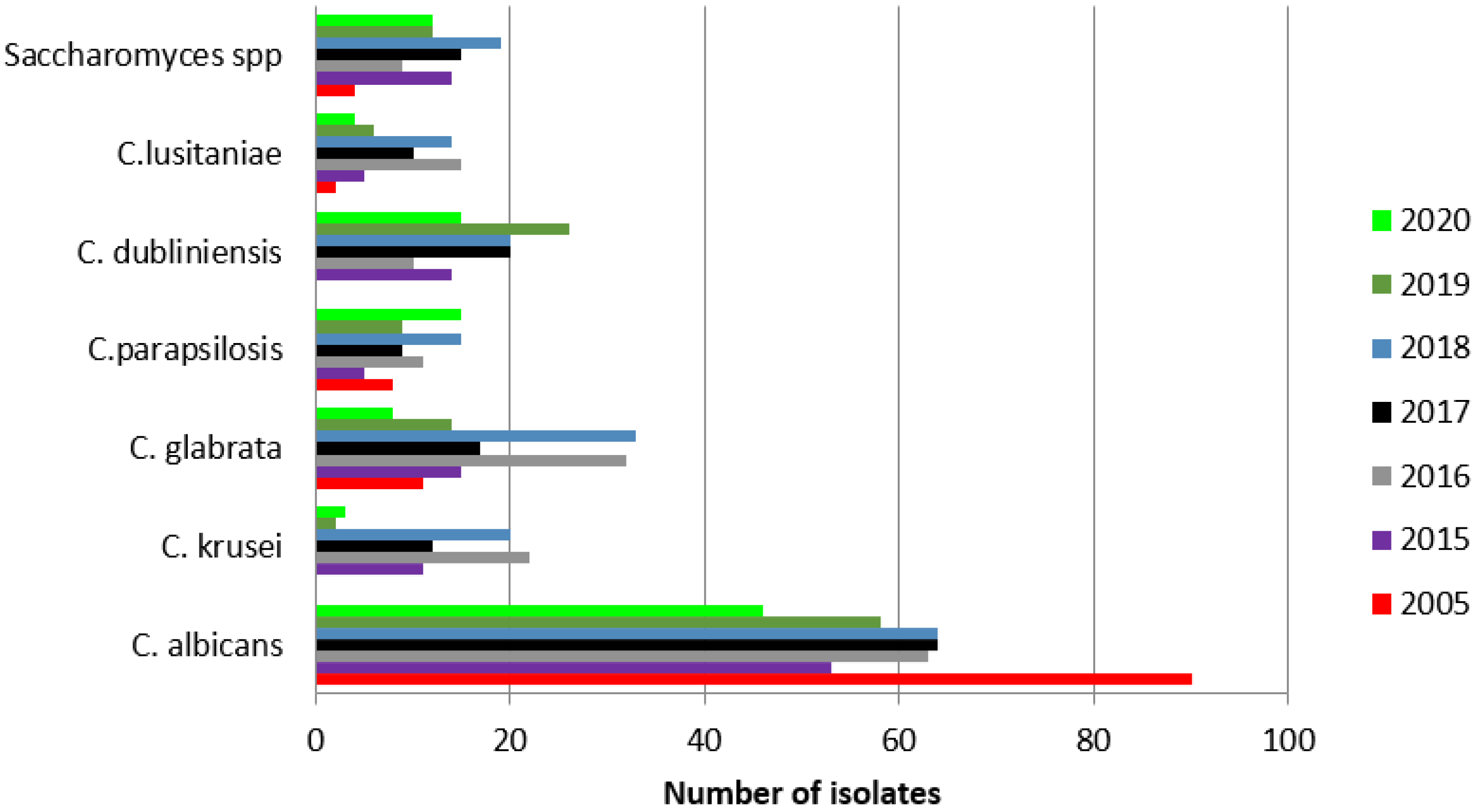
| 2005 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
|---|---|---|---|---|---|---|---|
| Number of patients (cultures) | 111 | 133 | 114 | 119 | 138 | 132 | 100 |
| Male (%) | 69 (62.16) | 60 (45.11) | 62 (54.36) | 68 (57.14) | 76 (55.1) | 52 (39.4) | 57 (57) |
| Number of mycological tests | 334 | 337 | 292 | 320 | 442 | 355 | 234 |
| Number of patients (serology) | 0 | 63 | 68 | 58 | 67 | 68 | 68 |
| Male (%) | 0 | 30 (47.62) | 36 (52.94) | 39 (67.24) | 40 (59.7) | 41 (60.29) | 38 (55.88) |
| Number of serological tests | 0 | 494 | 497 | 344 | 584 | 884 | 641 |
| Year (Amount of Tests) | 2005 (24) | 2015 (6) | 2016 (7) | 2017 (7) | 2018 (16) | 2019 (4) | 2020 (2) |
|---|---|---|---|---|---|---|---|
| Species | Amount of Isolates (Percentage of Tests) | ||||||
| Candida albicans | 6 (25%) | 1 (17%) | 3 (43%) | 0 | 2 (12%) | 3 (75%) | 0 |
| Candida glabrata | 1 (4%) | 0 | 0 | 0 | 1 (6%) | 0 | 0 |
| Candida parapsilosis | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Candida krusei | 0 | 0 | 1 (14%) | 0 | 0 | 0 | 0 |
| Candida dubliniensis | 0 | 0 | 1 (14%) | 1 (14%) | 0 | 0 | 0 |
| Year (Amount of Tests) | 2005 (14) | 2015 (22) | 2016 (21) | 2017 (20) | 2018 (23) | 2019 (24) | 2020 (11) |
|---|---|---|---|---|---|---|---|
| Species | Amount of Isolates (Percentage of Tests) | ||||||
| Candida albicans | 1 (7%) | 0 | 0 | 0 | 3 (13%) | 0 | 1 (9%) |
| Candida glabrata | 0 | 4 (18%) | 0 | 0 | 1 (4%) | 1 (4%) | 0 |
| Candida krusei | 0 | 0 | 0 | 1 (5%) | 0 | 0 | 0 |
| Candida kefyr | 3 (21%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Candida inconspicua | 3 (21%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Candida tropicalis | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 |
| Saccharomyces | 3 (21%) | 0 | 1 (5%) | 0 | 0 | 0 | 0 |
| Year (Amount of Tests) | 2005 (53) | 2015 (55) | 2016 (53) | 2017 (36) | 2018 (24) | 2019 (34) | 2020 (26) |
|---|---|---|---|---|---|---|---|
| Species | Amount of Isolates (Percentage of Tests) | ||||||
| Candida albicans | 6 (11%) | 0 | 3 (6%) | 4 (11%) | 4 (17%) | 2 (6%) | 2 (8%) |
| Candida glabrata | 4 (7%) | 5 (9%) | 1 (2%) | 0 | 1 (4%) | 1 (3%) | 0 |
| Candida parapsilosis | 2 (4%) | 0 | 0 | 0 | 1 (4%) | 1 (3%) | 1 (4%) |
| Candida dubliniensis | 0 | 0 | 0 | 0 | 2 (8%) | 2 (6%) | 0 |
| Candida lusitaniae | 0 | 0 | 0 | 0 | 0 | 1 (3%) | 0 |
| Result/Year | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|---|
| Aspergillus antigen (number of samples) | 129 | 144 | 92 | 157 | 220 | 157 |
| Negative (index < 0.5) | 123 | 140 | 91 | 155 | 218 | 139 |
| Positive (index > 0.5) | 6 (4.65%) | 4 (2.78%) | 1 (1.09%) | 2 (1.27%) | 2 (0.90%) | 18 (11.47%) |
| Anti-Aspergillus antibodies (number of samples) | 110 | 105 | 74 | 128 | 212 | 147 |
| Negative (<5 AU/mL) | 107 | 97 | 73 | 128 | 212 | 145 |
| Intermediate (5–10 AU/mL) | 2 (1.87%) | 8 (8.25%) | 1 (1.37%) | 0 | 0 | 2 (1.38%) |
| Positive (>10 AU/mL) | 1 (0.94%) | 0 | 0 | 0 | 0 | 0 |
| Candida antigen (number of samples) | 135 | 141 | 97 | 162 | 229 | 167 |
| Negative (<62.5 pg/mL) | 125 | 137 | 95 | 157 | 214 | 159 |
| Intermediate (62.5–125 pg/mL) | 4 (3.20%) | 1 (0.73%) | 0 | 2 (1.32%) | 9 (4.20%) | 3 (1.89%) |
| Positive (>125 pg/mL) | 6 (4.80%) | 3 (2.19%) | 2 (2.11%) | 3 (1.91%) | 6 (2.80%) | 5 (3.15%) |
| Anti-Candida antibodies (number of samples) | 120 | 101 | 80 | 137 | 220 | 166 |
| Negative (<5 AU/mL) | 73 | 69 | 54 | 98 | 132 | 73 |
| Intermediate (5-10 AU/mL) | 27 (22.50%) | 19 (27.54%) | 17 (31.48%) | 20 (20.40%) | 73 (55.30%) | 52 (71.23%) |
| Positive (>10 AU/mL) | 20 (27.40%) | 13 (18.84%) | 9 (16.67%) | 19 (19.40%) | 15 (11.36%) | 41 (56.16%) |
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | p | |
|---|---|---|---|---|---|---|---|
| Aspergillus antigen | 0.675938 | ||||||
| Number of patients | 63 | 69 | 57 | 66 | 68 | 65 | |
| Number of patients with a positive result (percentage) | 2 (3.2%) | 4 (5.8%) | 1 (1.7%) | 2 (3.0%) | 2 (2.9%) | 2 (3.1%) | |
| Anti-Aspergillus antibodies | 0.698505 | ||||||
| Number of patients | 57 | 57 | 49 | 62 | 65 | 64 | |
| Number of patients with a positive result (percentage) | 1 (1,7%) | 1 (1,7%) | 0 | 0 | 0 | 1 (1.6%) | |
| Candida antigen | 0.346278 | ||||||
| Number of patients | 63 | 69 | 57 | 67 | 68 | 68 | |
| Number of patients with a positive result (percentage) | 2 (3.2%) | 1 (1.4%) | 2 (3.5%) | 3 (4.5%) | 5 (7.3%) | 6 (8.8%) | |
| Anti-Candida antibodies | 0.531438 | ||||||
| Number of patients | 57 | 56 | 50 | 62 | 65 | 66 | |
| Number of patients with a positive result (percentage) | 9 (15.8%) | 11 (19.6%) | 7 (14.0%) | 10 (16.1%) | 8 (12.3%) | 16 (24.2%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klepacka, J.; Zakrzewska, Z.; Czogała, M.; Wojtaszek-Główka, M.; Krzysztofik, E.; Czogała, W.; Skoczeń, S. Epidemiology of Fungal Colonization in Children Treated at the Department of Oncology and Hematology: Single-Center Experience. Int. J. Environ. Res. Public Health 2022, 19, 2485. https://doi.org/10.3390/ijerph19042485
Klepacka J, Zakrzewska Z, Czogała M, Wojtaszek-Główka M, Krzysztofik E, Czogała W, Skoczeń S. Epidemiology of Fungal Colonization in Children Treated at the Department of Oncology and Hematology: Single-Center Experience. International Journal of Environmental Research and Public Health. 2022; 19(4):2485. https://doi.org/10.3390/ijerph19042485
Chicago/Turabian StyleKlepacka, Joanna, Zuzanna Zakrzewska, Małgorzata Czogała, Magdalena Wojtaszek-Główka, Emil Krzysztofik, Wojciech Czogała, and Szymon Skoczeń. 2022. "Epidemiology of Fungal Colonization in Children Treated at the Department of Oncology and Hematology: Single-Center Experience" International Journal of Environmental Research and Public Health 19, no. 4: 2485. https://doi.org/10.3390/ijerph19042485
APA StyleKlepacka, J., Zakrzewska, Z., Czogała, M., Wojtaszek-Główka, M., Krzysztofik, E., Czogała, W., & Skoczeń, S. (2022). Epidemiology of Fungal Colonization in Children Treated at the Department of Oncology and Hematology: Single-Center Experience. International Journal of Environmental Research and Public Health, 19(4), 2485. https://doi.org/10.3390/ijerph19042485






