Interrogating Patterns of Cancer Disparities by Expanding the Social Determinants of Health Framework to Include Biological Pathways of Social Experiences
Abstract
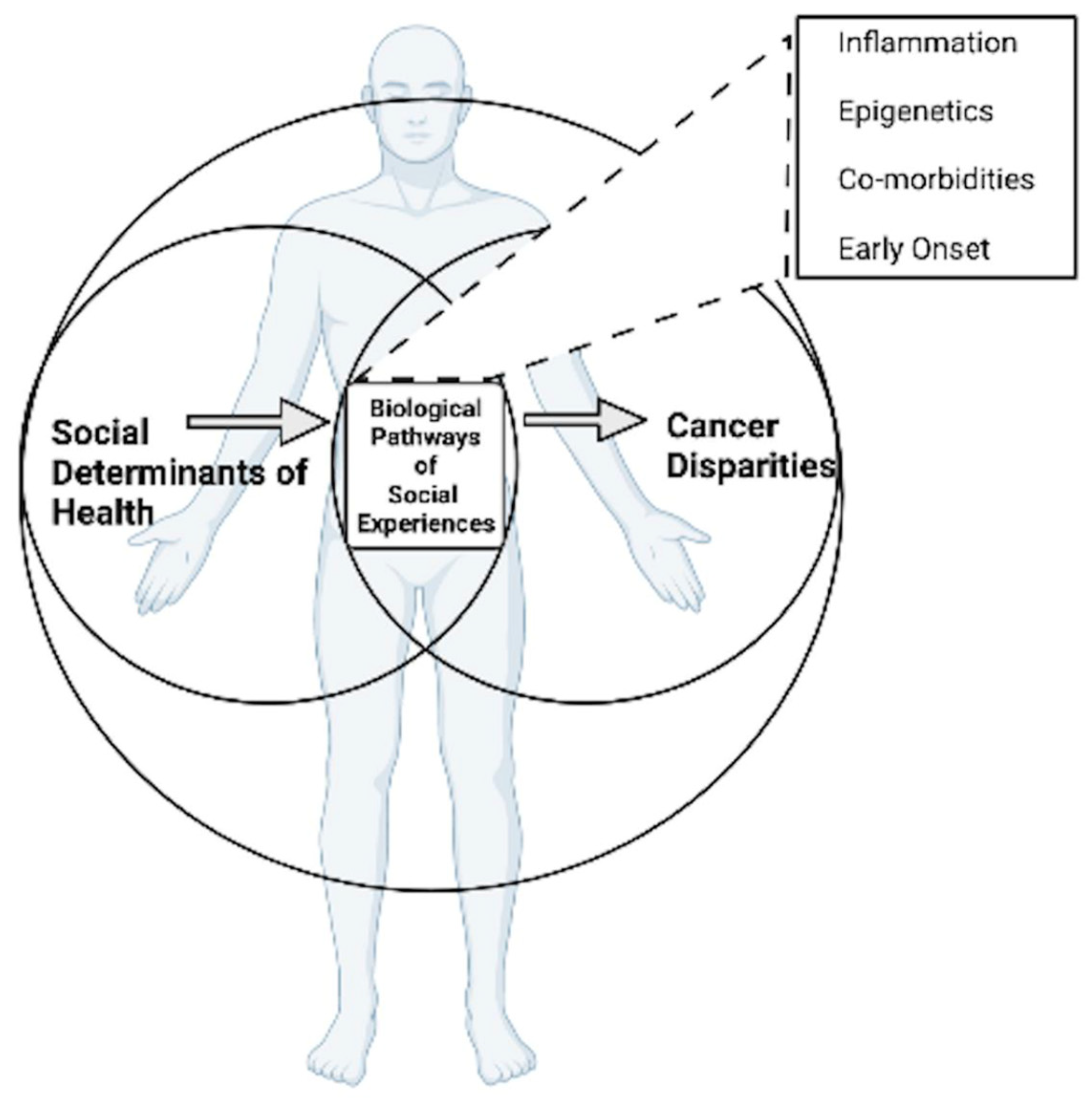
1. Introduction
2. Social Determinants of Health Framework
3. Biological Pathways of Social Experiences
4. Inflammation
5. Epigenetics
6. Complications from Co-Morbidities
7. Early-Onset Cancer Disparities
8. Conclusions
New Directions for the Field of Cancer Disparities
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Lifetime Probability of Developing or Dying from Cancer. Available online: https://www.cancer.org/cancer/cancer-basics/lifetime-probability-of-developing-or-dying-from-cancer.html (accessed on 15 November 2021).
- National Center for Health Statistics. Percentage of Any Type of Cancer for Adults Aged 18 and Over, United States, 2019–2020. National Health Interview Survey. Available online: https://wwwn.cdc.gov/NHISDataQueryTool/SHS_adult/index.html (accessed on 19 February 2022).
- Augustus, G.J.; Ellis, N.A. Colorectal Cancer Disparity in African Americans: Risk Factors and Carcinogenic Mechanisms. Am. J. Pathol. 2018, 188, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.K.; Jemal, A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950–2014: Over six decades of changing patterns and widening inequalities. J. Environ. Public Health 2017, 2017, 2819372. [Google Scholar] [CrossRef] [PubMed]
- Batai, K.; La Rosa, A.H.; Zeng, J.; Chipollini, J.J.; Gachupin, F.C.; Lee, B.R. Racial/ethnic disparities in renal cell carcinoma: Increased risk of early-onset and variation in histologic subtypes. Cancer Med. 2019, 8, 6780–6788. [Google Scholar] [CrossRef] [PubMed]
- Valencia, C.; Asmar, S.; Hsu, C.-H.; Gachupin, F.; Wong, A.; Chipollini, J.; Lee, B.; Batai, K. Renal Cell Carcinoma Health Disparities in Stage and Mortality among American Indians/Alaska Natives and Hispanic Americans: Comparison of National Cancer Database and Arizona Cancer Registry Data. Cancers 2021, 13, 990. [Google Scholar] [CrossRef] [PubMed]
- Haddad, D.N.; Sandler, K.L.; Henderson, L.M.; Rivera, M.P.; Aldrich, M.C. Disparities in Lung Cancer Screening: A Review. Ann. Am. Thorac. Soc. 2020, 17, 399–405. [Google Scholar] [CrossRef]
- Jiang, S.; Narayan, V.; Warlick, C. Racial disparities and considerations for active surveillance of prostate cancer. Transl. Androl. Urol. 2018, 7, 214–220. [Google Scholar] [CrossRef]
- Coughlin, S.S. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res. Treat. 2019, 177, 537–548. [Google Scholar] [CrossRef]
- Alcaraz, K.I.; Wiedt, T.L.; Daniels, E.C.; Yabroff, K.R.; Guerra, C.E.; Wender, R.C. Understanding and addressing social determinants to advance cancer health equity in the United States: A blueprint for practice, research, and policy. CA Cancer J. Clin. 2019, 70, 31–46. [Google Scholar] [CrossRef]
- Landrine, H.; Corral, I.; Lee, J.G.L.; Efird, J.T.; Hall, M.B.; Bess, J.J. Residential Segregation and Racial Cancer Disparities: A Systematic Review. J. Racial Ethn. Health Dispar. 2016, 4, 1195–1205. [Google Scholar] [CrossRef]
- Braveman, P.; Gottlieb, L. The social determinants of health: It’s time to consider the causes of the causes. Public Health Rep. 2014, 129, 19–31. [Google Scholar] [CrossRef]
- Krieger, N. Epidemiology and the web of causation: Has anyone seen the spider? Soc. Sci. Med. 1994, 39, 887–903. [Google Scholar] [CrossRef]
- Holden, C.E.; Wheelwright, S.; Harle, A.; Wagland, R. The role of health literacy in cancer care: A mixed studies systematic review. PLoS ONE 2021, 16, e0259815. [Google Scholar] [CrossRef] [PubMed]
- Kim-Mozeleski, J.E.; Pandey, R.; Tsoh, J.Y. Psychological distress and cigarette smoking among U.S. households by income: Considering the role of food insecurity. Prev. Med. Rep. 2019, 16, 100983. [Google Scholar] [CrossRef] [PubMed]
- Bailey, Z.D.; Krieger, N.; Agénor, M.; Graves, J.; Linos, N.; Bassett, M.T. Structural Racism and Health Inequities in the United States of America: Evidence and Interventions. In The Social Medicine Reader, 3rd ed.; Duke University Press: Durham, NC, USA, 2019; Volume II, pp. 209–234. [Google Scholar]
- Krieger, N.; Wright, E.; Chen, J.T.; Waterman, P.D.; Huntley, E.R.; Arcaya, M. Cancer Stage at Diagnosis, Historical Redlining, and Current Neighborhood Characteristics: Breast, Cervical, Lung, and Colorectal Cancers, Massachusetts, 2001–2015. Am. J. Epidemiol. 2020, 189, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Conching, A.K.S.; Thayer, Z. Biological pathways for historical trauma to affect health: A conceptual model focusing on epigenetic modifications. Soc. Sci. Med. 2019, 230, 74–82. [Google Scholar] [CrossRef]
- Jacobs, W.; Amuta, A.O.; Jeon, K.C. Health information seeking in the digital age: An analysis of health information seeking behavior among US adults. Cogent Soc. Sci. 2017, 3, 1302785. [Google Scholar] [CrossRef]
- Galobardes, B.; Smith, G.D.; Lynch, J.W. Systematic Review of the Influence of Childhood Socioeconomic Circumstances on Risk for Cardiovascular Disease in Adulthood. Ann. Epidemiol. 2006, 16, 91–104. [Google Scholar] [CrossRef]
- Williams, D.R.; Mohammed, S.A.; Leavell, J.; Collins, C. Race, socioeconomic status and health: Complexities, ongoing challenges and research opportunities. Ann. N. Y. Acad. Sci. 2010, 1186, 69–101. [Google Scholar] [CrossRef]
- Gravlee, C.C. How race becomes biology: Embodiment of social inequality. Am. J. Phys. Anthr. 2009, 139, 47–57. [Google Scholar] [CrossRef]
- Marsland, A.L.; Walsh, C.; Lockwood, K.; John-Henderson, N.A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav. Immun. 2017, 64, 208–219. [Google Scholar] [CrossRef]
- De Baca, T.C.; Prather, A.A.; Lin, J.; Sternfeld, B.; Adler, N.; Epel, E.S.; Puterman, E. Chronic psychosocial and financial burden accelerates 5-year telomere shortening: Findings from the Coronary Artery Risk Development in Young Adults Study. Mol. Psychiatry 2020, 25, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Song, R.; Ye, Y.; Chow, W.-H.; Shen, J. Allostatic score and its associations with demographics, healthy behaviors, tumor characteristics, and mitochondrial DNA among breast cancer patients. Breast Cancer Res. Treat. 2021, 187, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Geronimus, A.T.; Hicken, M.; Keene, D.; Bound, J. “Weathering” and Age Patterns of Allostatic Load Scores Among Blacks and Whites in the United States. Am. J. Public Health 2006, 96, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Lacal, I.; Ventura, R. Epigenetic inheritance: Concepts, mechanisms and perspectives. Front. Mol. Neurosci. 2018, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, D.; Sohrabi, B.; Mosallaei, M.; Nariman-Saleh-Fam, Z.; Bastami, M.; Mansoori, Y.; Daraei, A.; Vahed, S.Z.; Navid, S.; Saadatian, Z.; et al. Epi-miRNAs: Regulators of the Histone Modification Machinery in Human Cancer. J. Oncol. 2022, 2022, 4889807. [Google Scholar] [CrossRef]
- Argentieri, M.A.; Nagarajan, S.; Seddighzadeh, B.; Baccarelli, A.A.; Shields, A.E. Epigenetic Pathways in Human Disease: The Impact of DNA Methylation on Stress-Related Pathogenesis and Current Challenges in Biomarker Development. eBioMedicine 2017, 18, 327–350. [Google Scholar] [CrossRef]
- Simons, R.L.; Lei, M.-K.; Klopach, E.; Berg, M.; Zhang, Y.; Beach, S.S.R. (Re)Setting Epigenetic Clocks: An Important Avenue Whereby Social Conditions Become Biologically Embedded across the Life Course. J. Health Soc. Behav. 2021, 62, 436–453. [Google Scholar] [CrossRef]
- Linnenbringer, E.; Gehlert, S.; Geronimus, A.T. Black-White Disparities in Breast Cancer Subtype: The Intersection of Socially Patterned Stress and Genetic Expression. AIMS Public Health 2017, 4, 526–556. [Google Scholar] [CrossRef]
- Guo, Y.; Nie, Q.; MacLean, A.L.; Li, Y.; Lei, J. Multiscale Modeling of Inflammation-Induced Tumorigenesis Reveals Competing Oncogenic and Oncoprotective Roles for Inflammation. Cancer Res. 2017, 77, 6429–6441. [Google Scholar] [CrossRef]
- Maiuri, A.; O’Hagan, H. Interplay between Inflammation and Epigenetic Changes in Cancer. Prog. Mol. Biol. Transl. Sci. 2016, 144, 69–117. [Google Scholar]
- Piotrowski, I.; Kulcenty, K.; Suchorska, W. Interplay between inflammation and cancer. Rep. Pract. Oncol. Radiother. 2020, 25, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Lutgendorf, S.K.; Andersen, B.L. Biobehavioral approaches to cancer progression and survival: Mechanisms and interventions. Am. Psychol. 2015, 70, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.L.; Golden-Kreutz, D.M.; Emery, C.F.; Thiel, D.L. Biobehavioral Intervention for Cancer Stress: Conceptualization, Components, and Intervention Strategies. Cogn. Behav. Pract. 2009, 16, 253–265. [Google Scholar] [CrossRef]
- Eckerling, A.; Ricon-Becker, I.; Sorski, L.; Sandbank, E.; Ben-Eliyahu, S. Stress and cancer: Mechanisms, significance and future directions. Nat. Cancer 2021, 21, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Richman, A.D. Concurrent Social Disadvantages and Chronic Inflammation: The Intersection of Race and Ethnicity, Gender, and Socioeconomic Status. J. Racial. Ethn. Health Dispar. 2017, 5, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Stepanikova, I.; Bateman, L.B.; Oates, G.R. Systemic inflammation in midlife: Race, socioeconomic status, and perceived discrimination. Am. J. Prev. Med. 2017, 52, S63–S76. [Google Scholar] [CrossRef]
- Mac Giollabhui, N.; Alloy, L.B.; Swistun, D.; Coe, C.L.; Ellman, L.M.; Moriarity, D.P.; Stumper, A.C.; Abramson, L.Y. Concurrent and Longitudinal Associations of Sex and Race with Inflammatory Biomarkers during Adolescence. J. Youth Adolesc. 2021, 50, 711–723. [Google Scholar] [CrossRef]
- Goosby, B.J.; Malone, S.; Richardson, E.A.; Cheadle, J.E.; Williams, D.T. Perceived discrimination and markers of cardiovascular risk among low-income A frican A merican youth. Am. J. Hum. Biol. 2015, 27, 546–552. [Google Scholar] [CrossRef]
- Schmeer, K.K.; Tarrence, J. Racial-ethnic disparities in inflammation: Evidence of weathering in childhood? J. Health Soc. Behav. 2018, 59, 411–428. [Google Scholar] [CrossRef]
- Shattuck, E.C. Networks, cultures, and institutions: Toward a social immunology. Brain Behav. Immun.-Health 2021, 18, 100367. [Google Scholar] [CrossRef]
- Adam, E.; Villaume, S.C.; Hittner, E. Reducing stress disparities: Pathways to equity through the study of stress biology. In Confronting Inequality: How Policies and Practices Shape Children’s Opportunities; American Psychological Association: Worcester, MA, USA, 2020; pp. 11–47. [Google Scholar]
- Schmeer, K.K.; Yoon, A. Socioeconomic status inequalities in low-grade inflammation during childhood. Arch. Dis. Child. 2016, 101, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.S.; Aiello, A.E.; Mensah, F.K.; Gasser, C.E.; Rueb, K.; Cordell, B.; Juonala, M.; Wake, M.; Burgner, D.P. Socioeconomic status in childhood and C reactive protein in adulthood: A systematic review and meta-analysis. J. Epidemiol. Community Health 2017, 71, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Muscatell, K.A.; Brosso, S.N.; Humphreys, K.L. Socioeconomic status and inflammation: A meta-analysis. Mol. Psychiatry 2018, 25, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Pageot, Y.K.; Stanton, A.L.; Ganz, P.A.; Irwin, M.R.; Cole, S.W.; Crespi, C.M.; Breen, E.C.; Kuhlman, K.R.; Bower, J.E. Socioeconomic Status and Inflammation in Women with Early-stage Breast Cancer: Mediation by Body Mass Index. Brain Behav. Immun. 2021, 99, 307–316. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Acheampong, T.; Jiang, L.; Ziogas, A.; Odegaard, A.O. Multi-Systemic Biological Risk and Cancer Mortality: The NHANES III Study. Sci. Rep. 2020, 10, 5047. [Google Scholar] [CrossRef]
- Chan, J.E.; Mann, A.K.; Kapp, D.S.; Rehkopf, D.H. Income, inflammation and cancer mortality: A study of U.S. National Health and Nutrition Examination Survey mortality follow-up cohorts. BMC Public Health 2020, 20, 1805. [Google Scholar] [CrossRef]
- Thayer, Z.M.; Non, A.L. Anthropology Meets Epigenetics: Current and Future Directions. Am. Anthr. 2015, 117, 722–735. [Google Scholar] [CrossRef]
- Smith, J.A.; Zhao, W.; Wang, X.; Mukherjee, B.; Kardia, S.L.R.; Seeman, T.E.; Liu, Y.; Needham, B.L.; Shively, C.A.; Roux, A.V.D. Life Course Socioeconomic Status and DNA Methylation in Genes Related to Stress Reactivity and Inflammation: The Multi-Ethnic Study of Atherosclerosis. Epigenetics 2015, 10, 958–969. [Google Scholar]
- McDade, T.W.; Ryan, C.P.; Jones, M.; Hoke, M.K.; Borja, J.; Miller, G.E.; Kuzawa, C.W.; Kobor, M. Genome-wide analysis of DNA methylation in relation to socioeconomic status during development and early adulthood. Am. J. Phys. Anthr. 2019, 169, 3–11. [Google Scholar] [CrossRef]
- Reuben, A.; Sugden, K.; Arseneault, L.; Corcoran, D.L.; Danese, A.; Fisher, H.L.; Moffitt, T.E.; Newbury, J.B.; Odgers, C.; Prinz, J.; et al. Association of Neighborhood Disadvantage in Childhood With DNA Methylation in Young Adulthood. JAMA Netw. Open 2020, 3, e206095. [Google Scholar] [CrossRef] [PubMed]
- Borghol, N.; Suderman, M.; McArdle, W.; Racine, A.; Hallett, M.; Pembrey, M.; Hertzman, C.; Power, C.; Szyf, M. Associations with early-life socio-economic position in adult DNA methylation. Int. J. Epidemiol. 2011, 41, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.P.; Bhattacharya, A.; Martin, E.M.; Addo, K.; Psioda, M.; Smeester, L.; Joseph, R.M.; Hooper, S.R.; Frazier, J.A.; Kuban, K.C.; et al. Epigenome-wide DNA methylation in placentas from preterm infants: Association with maternal socioeconomic status. Epigenetics 2019, 14, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Rehkopf, D.H.; Needham, B.L. The impact of race and ethnicity in the social epigenomic regulation of disease. Nutr. Epigenomics 2019, 14, 51–65. [Google Scholar]
- Fiorito, G.; Polidoro, S.; Dugue, P.-A.; Kivimaki, M.; Ponzi, E.; Matullo, G.; Guarrera, S.; Assumma, M.B.; Georgiadis, P.; Kyrtopoulos, S.A.; et al. Social adversity and epigenetic aging: A multi-cohort study on socioeconomic differences in peripheral blood DNA methylation. Sci. Rep. 2017, 7, 16266. [Google Scholar] [CrossRef]
- Lau, C.E.; Robinson, O. DNA methylation age as a biomarker for cancer. Int. J. Cancer 2021, 148, 2652–2663. [Google Scholar] [CrossRef]
- Lerner, L.; Winn, R.; Hulbert, A. Lung cancer early detection and health disparities: The intersection of epigenetics and ethnicity. J. Thorac. Dis. 2018, 10, 2498–2507. [Google Scholar] [CrossRef]
- Vick, A.D.; Burris, H.H. Epigenetics and Health Disparities. Curr. Epidemiol. Rep. 2017, 4, 31–37. [Google Scholar] [CrossRef]
- Wu, Y.; Sarkissyan, M.; Vadgama, J.V. Epigenetics in Breast and Prostate Cancer. Cancer Epigenetics 2014, 1238, 425–466. [Google Scholar]
- Mills, M.C.; Tropf, F.C. Sociology, Genetics, and the Coming of Age of Sociogenomics. Annu. Rev. Sociol. 2020, 46, 553–581. [Google Scholar] [CrossRef]
- Steele, C.B.; Thomas, C.C.; Henley, S.J.; Massetti, G.M.; Galuska, D.A.; Agurs-Collins, T.; Puckett, M.; Richardson, L.C. Vital signs: Trends in incidence of cancers associated with overweight and obesity—United States, 2005–2014. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1052. [Google Scholar] [CrossRef] [PubMed]
- Zdemir, B.C.; Dotto, G.-P. Racial differences in cancer susceptibility and survival: More than the color of the skin? Trends Cancer 2017, 3, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Cooksey-Stowers, K.; Schwartz, M.B.; Brownell, K.D. Food Swamps Predict Obesity Rates Better Than Food Deserts in the United States. Int. J. Environ. Res. Public Health 2017, 14, 1366. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, C.; Ziliak, J.P. Food Insecurity and Health Outcomes. Health Aff. 2015, 34, 1830–1839. [Google Scholar] [CrossRef]
- Hernandez, D.C.; Reesor, L.M.; Murillo, R. Food insecurity and adult overweight/obesity: Gender and race/ethnic disparities. Appetite 2017, 117, 373–378. [Google Scholar] [CrossRef]
- DU, M.; Zhang, X. Urban greening: A new paradox of economic or social sustainability? Land Use Policy 2020, 92, 104487. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Bandrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef]
- Pinheiro, P.S.; Callahan, K.E.; Siegel, R.L.; Jin, H.; Morris, C.R.; Trapido, E.J.; Gomez, S.L. Cancer Mortality in Hispanic Ethnic Groups. Cancer Epidemiol. Biomark. Prev. 2017, 26, 376–382. [Google Scholar] [CrossRef]
- Dietze, E.C.; Chavez, T.A.; Seewaldt, V.L. Obesity and Triple-Negative Breast Cancer: Disparities, Controversies, and Biology. Am. J. Pathol. 2018, 188, 280–290. [Google Scholar] [CrossRef]
- Fane, M.; Weeraratna, A.T. How the ageing microenvironment influences tumour progression. Nat. Cancer 2019, 20, 89–106. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Maurer, H.; Reed, K.; Selagamsetty, R. Diabetes and cancer: Two diseases with obesity as a common risk factor. Diabetes Obes. Metab. 2013, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Lega, I.C.; Lipscombe, L.L. Review: Diabetes, Obesity, and Cancer-Pathophysiology and Clinical Implications. Endocr. Rev. 2020, 41, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.J.; George, A.S.; Subrahmanyan, A.N.; Pappachan, J.M. Epidemiological link between obesity, type 2 diabetes mellitus and cancer. World J. Methodol. 2021, 11, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Gebreab, S.Y.; Hickson, D.A.; Sims, M.; Wyatt, S.B.; Davis, S.K.; Correa, A.; Diez-Roux, A.V. Neighborhood social and physical environments and type 2 diabetes mellitus in African Americans: The Jackson Heart Study. Health Place 2016, 43, 128–137. [Google Scholar] [CrossRef]
- Aguayo-Mazzucato, C.; Diaque, P.; Hernandez, S.; Rosas, S.; Kostic, A.; Caballero, A.E. Understanding the growing epidemic of type 2 diabetes in the Hispanic population living in the United States. Diabetes/Metab. Res. Rev. 2018, 35, e3097. [Google Scholar] [CrossRef]
- DeBruyn, L.; Fullerton, L.; Satterfield, D.; Frank, M. Peer reviewed: Integrating culture and history to promote health and help prevent Type 2 diabetes in American Indian/Alaska Native communities: Traditional foods have become a way to talk about health. Prev. Chronic Dis. 2020, 17, E12. [Google Scholar] [CrossRef]
- Peyrot, M.; Egede, L.E.; Funnell, M.M.; Hsu, W.C.; Ruggiero, L.; Siminerio, L.M.; Stuckey, H.L. US ethnic group differences in self-management in the 2nd diabetes attitudes, wishes and needs (DAWN2) study. J. Diabetes Complicat. 2018, 32, 586–592. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Zheng, X.; Peng, J.; Chen, Y.; Yu, K.; Yang, Y.; Wang, X.; Yang, X.; Qian, J.; et al. Deaths from COPD in patients with cancer: A population-based study. Aging 2021, 13, 12641–12659. [Google Scholar] [CrossRef]
- Van de Schans, S.A.; Janssen-Heijnen, M.L.; Biesma, B.; Smeenk, F.W.; Van de Poll-Franse, L.V.; Seynaeve, C.; Coebergh, J.W. COPD in cancer patients: Higher prevalence in the elderly, a different treatment strategy in case of primary tumours above the diaphragm, and a worse overall survival in the elderly patient. Eur. J. Cancer 2007, 43, 2194–2202. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Chiang, C.-C.; Peng, M.-T.; Huang, Y.-T.; Huang, J.-L.; Chang, S.-H.; Yang, H.-T.; Chen, W.-C.; Kuo, J.-J.; Hwang, T.-L. Chronic Obstructive Pulmonary Disease Increases the Risk of Mortality among Patients with Colorectal Cancer: A Nationwide Population-Based Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 8742. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Lund, J.L.; Weaver, M.A.; Wood, W.A.; Olshan, A.F.; Nichols, H.B. Noncancer mortality among adolescents and young adults with cancer. Cancer 2019, 125, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Pleasants, A.R.; Riley, I.L.; Mannino, D. Defining and targeting health disparities in chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2475–2496. [Google Scholar] [CrossRef] [PubMed]
- Assari, S.; Chalian, H.; Bazargan, M. High Education Level Protects European Americans but Not African Americans Against Chronic Obstructive Pulmonary Disease: National Health Interview Survey. Int. J. Biomed. Eng. Clin. Sci. 2019, 5, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.A.; Beebe, L.A.; Thompson, D.M.; Wagener, T.L.; Terrell, D.R.; Campbell, J.E. A structural equation modeling approach to understanding pathways that connect socioeconomic status and smoking. PLoS ONE 2018, 13, e0192451. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ko, H.; Yoon, C.; Lee, N.-H.; Sung, J. Social Determinants of Smoking Behavior: The Healthy Twin Study, Korea. J. Prev. Med. Public Health 2012, 45, 29–36. [Google Scholar] [CrossRef][Green Version]
- Assari, S.; Mistry, R.; Bazargan, M. Race, Educational Attainment, and E-Cigarette Use. J. Med. Res. Innov. 2019, 4, e000185. [Google Scholar] [CrossRef]
- Cambron, C.; Kosterman, R.; Hawkins, J.D. Neighborhood Poverty Increases Risk for Cigarette Smoking From Age 30 to 39. Ann. Behav. Med. 2018, 53, 858–864. [Google Scholar] [CrossRef]
- Parada, H.; Vu, A.H.; Pinheiro, P.S.; Thompson, C.A. Comparing Age at Cancer Diagnosis between Hispanics and Non-Hispanic Whites in the United States. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1904–1912. [Google Scholar] [CrossRef]
- Annangi, S.; Nutalapati, S.; Foreman, M.G.; Pillai, R.; Flenaugh, E.L. Potential Racial Disparities Using Current Lung Cancer Screening Guidelines. J. Racial Ethn. Health Dispar. 2018, 6, 22–26. [Google Scholar] [CrossRef]
- Muller, C.; Ihionkhan, E.; Stoffel, E.; Kupfer, S. Disparities in Early-Onset Colorectal Cancer. Cells 2021, 10, 1018. [Google Scholar] [CrossRef] [PubMed]
- Sighoko, D.; Hunt, B.R.; Irizarry, B.; Watson, K.; Ansell, D.; Murphy, A.M. Disparity in breast cancer mortality by age and geography in 10 racially diverse US cities. Cancer Epidemiol. 2018, 53, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Pellom, S.T.; Arnold, T.; Williams, M.; Brown, V.L.; Samuels, A.D. Examining breast cancer disparities in African Americans with suggestions for policy. Cancer Causes Control 2020, 31, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, E.M.; Murphy, C.C. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology 2020, 158, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Juo, Y.-Y.; Gibbons, M.A.M.; Dutson, E.; Lin, A.Y.; Yanagawa, J.; Hines, O.J.; Eibl, G.; Chen, Y. Obesity Is Associated with Early Onset of Gastrointestinal Cancers in California. J. Obes. 2018, 2018, 7014073. [Google Scholar] [CrossRef]
- Sung, H.; Siegel, R.L.; Rosenberg, P.; Jemal, A. Emerging cancer trends among young adults in the USA: Analysis of a population-based cancer registry. Lancet Public Health 2019, 4, e137–e147. [Google Scholar] [CrossRef]
- Liu, P.-H.; Wu, K.; Ng, K.; Zauber, A.G.; Nguyen, L.; Song, M.; He, X.; Fuchs, C.S.; Ogino, S.; Willett, W.C.; et al. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol. 2019, 5, 37–44. [Google Scholar] [CrossRef]
- Micaily, I.; Hackbart, H.; Butryn, M.; Abu-Khalaf, M.M. Obesity in early onset breast cancer in African American patients. Breast J. 2021, 27, 603–607. [Google Scholar] [CrossRef]
- Murphy, C.C.; Cirillo, P.M.; Krigbaum, N.Y.; Singal, A.G.; Lee, M.; Zaki, T.; Burstein, E.; Cohn, B.A. Maternal obesity, pregnancy weight gain, and birth weight and risk of colorectal cancer. Gut 2021. [Google Scholar] [CrossRef]
- Diaz-Santana, M.V.; O’Brien, K.M.; D’Aloisio, A.A.; Regalado, G.; Sandler, D.P.; Weinberg, C.R. Perinatal and postnatal exposures and risk of young-onset breast cancer. Breast Cancer Res. 2020, 22, 88. [Google Scholar] [CrossRef]
- Mellemkjær, L.; Olsen, M.L.; Sørensen, H.T.; Thulstrup, A.M.; Olsen, J.; Olsen, J.H. Birth weight and risk of early-onset breast cancer (Denmark). Cancer Causes Control 2003, 14, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Hayashi, T.; Matsushita, M.; Uemura, M.; Nonomura, N. Obesity, inflammation, and prostate cancer. J. Clin. Med. 2019, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.L.; Gilman, S.E.; Cheng, T.L.; Drury, S.S.; Hill, C.V.; Geronimus, A.T. Life Course Approaches to the Causes of Health Disparities. Am. J. Public Health 2019, 109, S48–S55. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Noda, M.; Sawada, N.; Kato, M.; Hidaka, A.; Mizoue, T.; Shimazu, T.; Yamaji, T.; Iwasaki, M.; Sasazuki, S.; et al. High hemoglobin A1c levels within the non-diabetic range are associated with the risk of all cancers. Int. J. Cancer 2015, 138, 1741–1753. [Google Scholar] [CrossRef]
- Around Him, D.M.; Gachupin, F.C.; Freeman, W.L. Research with American Indian and Alaska Native Individuals, Tribes, and Communities. In Institutional Review Board Management and Function, 3rd ed.; Bankert, E.A., Gordon, B.G., Hurley, E.A., Shriver, S.P., Eds.; Jones and Bartlett Publishers: Sudbury, MA, USA, 2021. [Google Scholar]
- Gachupin, F.C.; Freeman, W.L. Ethics of Biospecimen Research. In Conducting Health Research with Native American Communities; Solomon, T., Randall, L., Eds.; American Public Health Association: Washington, DC, USA, 2014. [Google Scholar]
- Gachupin, F.C. Protections to Consider When Engaging American Indians/Alaska Natives in Human Subjects Research. In Health and Social Issues of Native American Women; Joe, J., Gachupin, F., Eds.; Praeger Publishers: Santa Barbara, CA, USA, 2012. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia, C.I.; Gachupin, F.C.; Molina, Y.; Batai, K. Interrogating Patterns of Cancer Disparities by Expanding the Social Determinants of Health Framework to Include Biological Pathways of Social Experiences. Int. J. Environ. Res. Public Health 2022, 19, 2455. https://doi.org/10.3390/ijerph19042455
Valencia CI, Gachupin FC, Molina Y, Batai K. Interrogating Patterns of Cancer Disparities by Expanding the Social Determinants of Health Framework to Include Biological Pathways of Social Experiences. International Journal of Environmental Research and Public Health. 2022; 19(4):2455. https://doi.org/10.3390/ijerph19042455
Chicago/Turabian StyleValencia, Celina I., Francine C. Gachupin, Yamilé Molina, and Ken Batai. 2022. "Interrogating Patterns of Cancer Disparities by Expanding the Social Determinants of Health Framework to Include Biological Pathways of Social Experiences" International Journal of Environmental Research and Public Health 19, no. 4: 2455. https://doi.org/10.3390/ijerph19042455
APA StyleValencia, C. I., Gachupin, F. C., Molina, Y., & Batai, K. (2022). Interrogating Patterns of Cancer Disparities by Expanding the Social Determinants of Health Framework to Include Biological Pathways of Social Experiences. International Journal of Environmental Research and Public Health, 19(4), 2455. https://doi.org/10.3390/ijerph19042455






