Overview of Renal Replacement Therapy Use in a General Intensive Care Unit
Abstract
1. Introduction
2. Materials and Methods
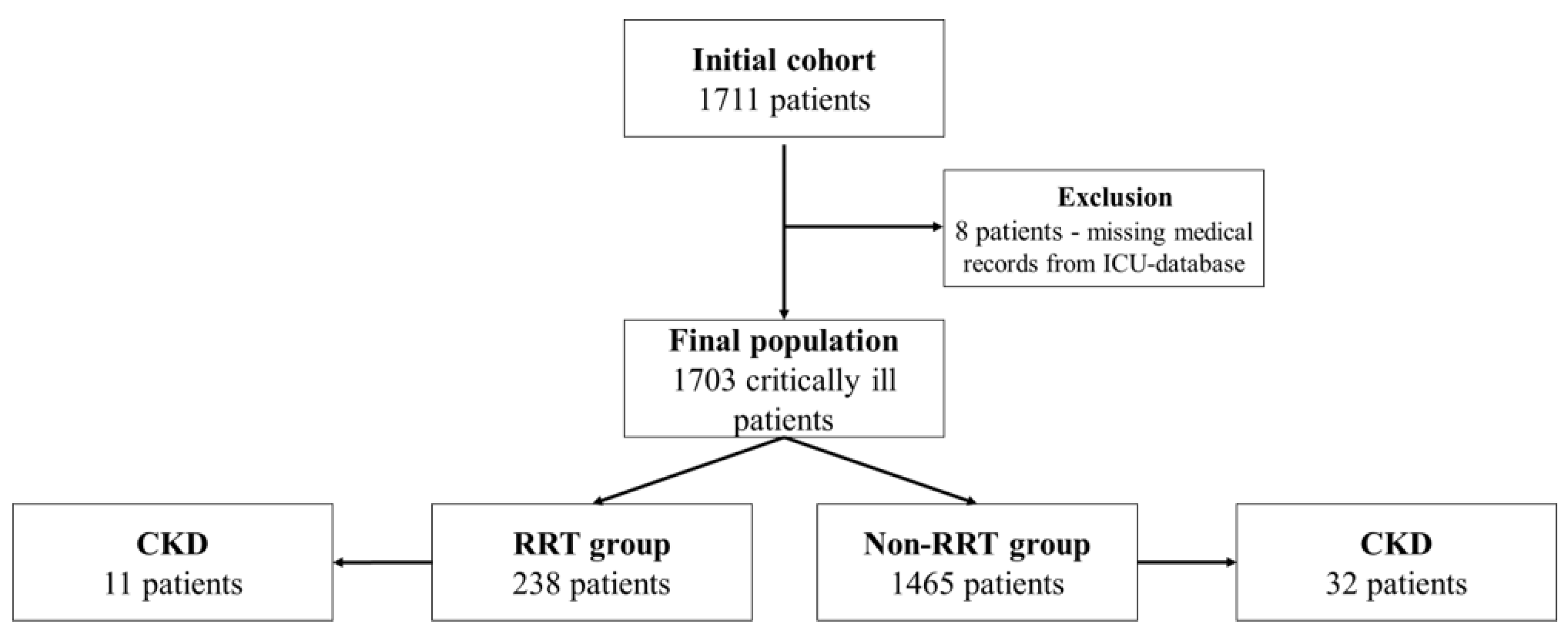
2.1. Patient Selection and Data Collection
2.2. Statistical Analysis
3. Results
3.1. Patient Demographics, Characteristics and the Use of RRT
3.2. Mortality
3.3. Length of ICU Stay
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, P.K.; Hsu, R.K.; Liu, K.D. Management of Acute Kidney Injury: Core Curriculum 2018. Am. J. Kidney Dis. 2018, 72, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Dennen, P.; Douglas, I.S.; Anderson, R. Acute kidney injury in the intensive care unit: An update and primer for the intensivist. Crit. Care Med. 2010, 38, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Zanella, M.; Brendolan, A.; Milan, M.; Canato, G.; Zamperetti, N.; Bellomo, R. Management of severe acute renal failure in critically ill patients: An international survey in 345 centres. Nephrol. Dial. Transplant. 2001, 16, 230–237. [Google Scholar] [CrossRef][Green Version]
- Ahmed, A.R.; Obilana, A.; Lappin, D. Renal Replacement Therapy in the Critical Care Setting. Crit. Care Res. Pract. 2019, 2019, 6948710. [Google Scholar] [CrossRef]
- Herrera-Gutiérrez, M.E.; Seller-Pérez, G.; Sánchez-Izquierdo-Riera, J.A.; Maynar-Moliner, J. COFRADE investigators group. Prevalence of acute kidney injury in intensive care units: The “COrte de prevalencia de disFunción RenAl y DEpuración en críticos” point-prevalence multicenter study. J. Crit. Care 2013, 28, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Wald, R.; McArthur, E.; Adhikari, N.K.; Bagshaw, S.M.; Burns, K.E.; Garg, A.X.; Harel, Z.; Kitchlu, A.; Mazer, C.D.; Nash, D.M.; et al. Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: A population-based cohort study. Am. J. Kidney Dis. 2015, 65, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.K.; McCulloch, C.E.; Dudley, R.A.; Lo, L.J.; Hsu, C.Y. Temporal changes in incidence of dialysis-requiring AKI. J. Am. Soc. Nephrol. 2013, 24, 37–42. [Google Scholar] [CrossRef]
- Siddiqui, N.F.; Coca, S.G.; Devereaux, P.J.; Jain, A.K.; Li, L.; Luo, J.; Parikh, C.R.; Paterson, M.; Philbrook, H.T.; Wald, R.; et al. Secular trends in acute dialysis after elective major surgery—1995 to 2009. CMAJ 2012, 184, 1237–1245. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Tang, Y.; Liu, F.; Yu, S.; Zhang, L.; Zeng, X.; Zhao, Y.; Fu, P. Effect of acute kidney injury on mortality and hospital stay in patient with severe acute pancreatitis. Nephrology 2015, 20, 485–491. [Google Scholar] [CrossRef]
- Palevsky, P.M. Kidney Replacement Therapy (Dialysis) in Acute Kidney Injury in Adults: Indications, Timing, and Dialysis Dose. UpToDate. 2021. Available online: https://www.uptodate.com/contents/kidney-replacement-therapy-dialysis-in-acute-kidney-injury-in-adults-indications-timing-and-dialysis-dose.com (accessed on 30 November 2021).
- Valdenebro, M.; Martín-Rodríguez, L.; Tarragón, B.; Sánchez-Briales, P.; Portolés, J. Renal replacement therapy in critically ill patients with acute kidney injury: 2020 nephrologist’s perspective. Nefrologia 2021, 41, 102–114. [Google Scholar] [CrossRef]
- Luft, J.; Boes, A.A.; Lazzari, D.D.; Nascimento, E.R.P.; Busana, J.A.; Canever, B.P. Chronic kidney injury at an intensive care service: Clinical characteristics and outcomes. Cogitare Enferm. 2016, 21, 1–9. [Google Scholar] [CrossRef]
- Hammond, D.A.; Smith, M.N.; Painter, J.T.; Meena, N.K.; Lusardi, K. Comparative Incidence of Acute Kidney Injury in Critically Ill Patients Receiving Vancomycin with Concomitant Piperacillin-Tazobactam or Cefepime: A Retrospective Cohort Study. Pharmacotherapy 2016, 36, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Peres, L.A.; Wandeur, V.; Matsuo, T. Predictors of acute kidney injury and mortality in an Intensive Care Unit. J. Bras. Nefrol. 2015, 37, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Luna, L.D.; Soares Dde, S.; Junior, G.B.; Cavalcante, M.G.; Malveira, L.R.; Meneses, G.C.; Pereira, E.D.; Daher, E.F. Clinical characteristics, outcomes and risk factors for death among critically ill patients with HIV-related acute kidney injury. Rev. Inst. Med. Trop. 2016, 58, 52. [Google Scholar] [CrossRef][Green Version]
- Medina-Liabres, K.R.P.; Jeong, J.C.; Oh, H.J.; An, J.N.; Lee, J.P.; Kim, D.K.; Ryu, D.R.; Kim, S. Mortality predictors in critically ill patients with acute kidney injury requiring continuous renal replacement therapy. Kidney Res. Clin. Pract. 2021, 40, 401–410. [Google Scholar] [CrossRef]
- Piccinni, P.; Cruz, D.N.; Gramaticopolo, S.; Garzotto, F.; Dal Santo, M.; Aneloni, G.; Rocco, M.; Alessandri, E.; Giunta, F.; Michetti, V.; et al. NEFROINT Investigators. Prospective multicenter study on epidemiology of acute kidney injury in the ICU: A critical care nephrology Italian collaborative effort (NEFROINT). Minerva Anestesiol. 2011, 77, 1072–1083. [Google Scholar]
- Fujii, T.; Uchino, S.; Doi, K.; Sato, T.; Kawamura, T. JAKID study group. Diagnosis, management, and prognosis of patients with acute kidney injury in Japanese intensive care units: The JAKID study. J. Crit. Care 2018, 47, 185–191. [Google Scholar] [CrossRef]
- Harris, D.G.; McCrone, M.P.; Koo, G.; Weltz, A.S.; Chiu, W.C.; Scalea, T.M.; Diaz, J.J.; Lissauer, M.E. Epidemiology and outcomes of acute kidney injury in critically ill surgical patients. J. Crit. Care 2015, 30, 102–106. [Google Scholar] [CrossRef]
- Oweis, A.O.; Alshelleh, S.A.; Momany, S.M.; Samrah, S.M.; Khassawneh, B.Y.; Al Ali, M.A.K. Incidence, Risk Factors, and Outcome of Acute Kidney Injury in the Intensive Care Unit: A Single-Center Study from Jordan. Crit. Care Res. Pract. 2020, 2020, 8753764. [Google Scholar] [CrossRef]
- Truche, A.S.; Ragey, S.P.; Souweine, B.; Bailly, S.; Zafrani, L.; Bouadma, L.; Clec’h, C.; Garrouste-Orgeas, M.; Lacave, G.; Schwebel, C.; et al. ICU survival and need of renal replacement therapy with respect to AKI duration in critically ill patients. Ann. Intensive Care 2018, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Lohse, R.; Damholt, M.B.; Wiis, J.; Perner, A.; Lange, T.; Ibsen, M. Long term end-stage renal disease and death following acute renal replacement therapy in the ICU. Acta Anaesthesiol. Scand. 2016, 60, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Czempik, P.; Cieśla, D.; Knapik, P.; Krzych, Ł. Mortality of patients with acute kidney injury requiring renal replacement therapy. Adv. Clin. Exp. Med. 2018, 27, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Gaião, S.M.; Gomes, A.A.; Paiva, J.A. Prognostics factors for mortality and renal recovery in critically ill patients with acute kidney injury and renal replacement therapy. Rev. Bras. Ter. Intensiva 2016, 28, 70–77. [Google Scholar] [CrossRef]
- Al-Dorzi, H.M.; Alhumaid, N.A.; Alwelyee, N.H.; Albakheet, N.M.; Nazer, R.I.; Aldakhil, S.K.; AlSaif, S.A.; Masud, N. Anemia, Blood Transfusion, and Filter Life Span in Critically Ill Patients Requiring Continuous Renal Replacement Therapy for Acute Kidney Injury: A Case-Control Study. Crit Care Res Pract. 2019, 2019, 3737083. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Jiang, L.; Du, B.; Wen, Y.; Wang, M.; Xi, X. Beijing Acute Kidney Injury Trial (BAKIT) workgroup. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit. Care 2014, 18, R144. [Google Scholar] [CrossRef]
- Podoll, A.S.; Kozar, R.; Holcomb, J.B.; Finkel, K.W. Incidence and outcome of early acute kidney injury in critically-ill trauma patients. PLoS ONE 2013, 8, e77376. [Google Scholar] [CrossRef]
- Iwagami, M.; Yasunaga, H.; Noiri, E.; Horiguchi, H.; Fushimi, K.; Matsubara, T.; Yahagi, N.; Nangaku, M.; Doi, K. Current state of continuous renal replacement therapy for acute kidney injury in Japanese intensive care units in 2011: Analysis of a national administrative database. Nephrol. Dial. Transplant. 2015, 30, 988–995. [Google Scholar] [CrossRef]
- Brivet, F.G.; Kleinknecht, D.J.; Loirat, P.; Landais, P.J. Acute renal failure in intensive care units--causes, outcome, and prognostic factors of hospital mortality; A prospective, multicenter study. French Study Group on Acute Renal Failure. Crit. Care Med. 1996, 24, 192–198. [Google Scholar] [CrossRef]
- Allegretti, A.S.; Steele, D.J.; David-Kasdan, J.A.; Bajwa, E.; Niles, J.L.; Bhan, I. Continuous renal replacement therapy outcomes in acute kidney injury and end-stage renal disease: A cohort study. Crit. Care 2013, 17, R109. [Google Scholar] [CrossRef]
- Kao, C.C.; Yang, J.Y.; Chen, L.; Chao, C.T.; Peng, Y.S.; Chiang, C.K.; Huang, J.W.; Hung, K.Y. Factors associated with poor outcomes of continuous renal replacement therapy. PLoS ONE 2017, 12, e0177759. [Google Scholar] [CrossRef] [PubMed]

| Age Interval | RRT Group (n = 238) | Non-RRT Group (n = 1465) |
|---|---|---|
| 17–20 | 4 (1.7%) | 17 (1.2%) |
| 21–30 | 9 (3.8%) | 63 (4.3%) |
| 31–40 | 7 (2.9%) | 100 (6.8%) |
| 41–50 | 25 (10.5%) | 185 (12.6%) |
| 51–60 | 40 (16.8%) | 211 (14.4%) |
| 61–70 | 54 (22.7%) | 305 (20.8%) |
| 71–80 | 61 (25.6%) | 323 (22.0%) |
| 81–90 | 35 (14.7%) | 233 (15.9%) |
| 91–100 | 3 (1.3%) | 28 (1.9%) |
| Data | RRT Group (n = 238) | Non-RRT Group (n = 1465) | p Value | |
|---|---|---|---|---|
| Age | 64.73 ± 16.65 SD | 63.40 ± 17.67 SD | p = 0.279 | |
| Gender | male | 149 (62.6%) | 881 (60.1%) | p = 0.470 |
| female | 89 (37.4%) | 584 (39.9%) | ||
| ICU admission from various medical wards | cardiology | 14 (5.8%) | 88 (6.0%) | p = 0.9451 |
| vascular surgery | 4 (1.6%) | 6 (0.4%) | p = 0.0548 | |
| general surgery | 98 (41.1%) | 360 (24.5%) | p < 0.001 | |
| internal medicine | 58 (24.3%) | 340 (23.2%) | p = 0.7568 | |
| neurosurgery | 13 (5.4%) | 365 (24.9%) | p < 0.001 | |
| neurology | 5 (2.1%) | 77 (5.2%) | p = 0.0514 | |
| orthopedic surgery | 21 (8.8%) | 125 (8.5%) | p = 0.9813 | |
| plastic surgery | 10 (4.2%) | 31 (2.1%) | p = 0.0868 | |
| gastroenterology | 14 (5.8%) | 73 (4.9%) | p = 0.6699 | |
| toxicology | 1 (0.4%) | 0 | - | |
| Primary organ dysfunction at ICU admission * | cardiac dysfunction | 27 (11.3%) | 153 (10.4%) | p = 0.7602 |
| respiratory dysfunction | 45 (18.9%) | 253 (17.2%) | p = 0.5991 | |
| renal dysfunction | 14 (5.8%) | 33 (2.2%) | p = 0.0031 | |
| gastrointestinal dysfunction | 53 (22.2%) | 226 (15.4%) | p = 0.0108 | |
| neurologic dysfunction | 14 (5.8%) | 416 (28.3%) | p < 0.001 | |
| multiple trauma | 24 (10.1%) | 158 (10.7%) | p = 0.8328 | |
| surgery | 36 (15.1%) | 140 (9.5%) | p = 0.0123 | |
| oncological patients | 25 (10.5%) | 86 (5.8%) | p = 0.0110 | |
| Mechanical ventilation | days, median (IQR) | 4.5 (9.00) | 4 (7.00) | p = 0.315 |
| number of patients (n = 1034) | 215 (90.3%) | 819 (55.9%) | - | |
| Specific ICU scores # at admission | APACHE II score (points), median (IQR) | 21 (7.00) | 18 (12.00) | p < 0.001 |
| SOFA score (points), median (IQR) | 10 (6.00) | 8 (7.00) | p < 0.001 | |
| ISS score (points), median (IQR) | 43 (20) | 26 (16) | p = 0.001 | |
| ICU stay, days, median (IQR) | 8 (11.75) | 6 (11.00) | p < 0.001 | |
| Vasopressor treatment (yes/no) (during ICU stay) | 206 (86.5%) | 685 (46.7%) | p < 0.001 | |
| Transfusions (yes/no) (during ICU stay) | 151 (63.4%) | 397 (27.1%) | p < 0.001 | |
| RRT sessions, mean ± SD | 2.01 ± 0.58 | - | - | |
| Primary Organ Dysfunction at ICU Admission * | RRT Group (n = 238) | Non-RRT Group (n = 1465) | p Values for RRT Group |
|---|---|---|---|
| cardiac dysfunction | 21 (8.80%) | 91 (6.20%) | p = 0.1720 |
| respiratory dysfunction | 26 (10.90%) | 100 (6.80%) | p = 0.0356 |
| renal dysfunction | 10 (4.20%) | 18 (1.20%) | p = 0.0022 |
| gastrointestinal dysfunction | 32 (13.40%) | 82 (5.60%) | p < 0.001 |
| neurologic dysfunction | 10 (4.20%) | 130 (8.90%) | p = 0.0211 |
| multiple trauma | 16 (6.70%) | 24 (1.60%) | p < 0.001 |
| surgery | 24 (10.10%) | 32 (2.18%) | p < 0.001 |
| oncologic patients | 20 (8.40%) | 21 (1.40%) | p < 0.001 |
| Variables | b | SE | Wald | p | Exp(b) | 95% CI of Exp(b) |
|---|---|---|---|---|---|---|
| age | 0.01765 | 0.002751 | 41.1718 | <0.001 | 1.0178 | 1.0124 to 1.0233 |
| cardiac dysfunction | 0.4950 | 0.1161 | 18.1855 | <0.001 | 1.6406 | 1.3082 to 2.0573 |
| gastrointestinal dysfunction | 0.2958 | 0.1158 | 6.5274 | 0.0106 | 1.3442 | 1.0725 to 1.6846 |
| male gender | −0.1610 | 0.08102 | 3.9511 | 0.0468 | 0.8512 | 0.7268 to 0.9969 |
| neurologic dysfunction | −0.2364 | 0.1124 | 4.4237 | 0.0354 | 0.7894 | 0.6341 to 0.9829 |
| multiple trauma | −0.5668 | 0.1790 | 10.0262 | 0.0015 | 0.5674 | 0.4002 to 0.8043 |
| renal dysfunction | 0.4104 | 0.2029 | 4.0891 | 0.0432 | 1.5074 | 1.0147 to 2.2391 |
| RRT | 0.4002 | 0.09518 | 17.6771 | <0.001 | 1.4921 | 1.2393 to 1.7964 |
| RRT Group | Non-RRT Group | Overall | ||||
|---|---|---|---|---|---|---|
| Survival Time * | Survival Proportion # | Standard Error | Survival Proportion # | Standard Error | Survival Proportion # | Standard Error |
| 1 | 0.937 | 0.0158 | 0.954 | 0.00550 | 0.951 | 0.00522 |
| 2 | 0.848 | 0.0233 | 0.901 | 0.00787 | 0.894 | 0.00753 |
| 3 | 0.791 | 0.0265 | 0.857 | 0.00954 | 0.847 | 0.00905 |
| 4 | 0.738 | 0.0288 | 0.823 | 0.0107 | 0.810 | 0.0101 |
| 5 | 0.702 | 0.0302 | 0.796 | 0.0116 | 0.781 | 0.0109 |
| 6 | 0.659 | 0.0315 | 0.756 | 0.0129 | 0.740 | 0.0120 |
| 7 | 0.619 | 0.0326 | 0.734 | 0.0135 | 0.714 | 0.0126 |
| 8 | 0.583 | 0.0335 | 0.712 | 0.0142 | 0.689 | 0.0132 |
| 9 | 0.561 | 0.0339 | 0.686 | 0.0150 | 0.664 | 0.0138 |
| 10 | 0.524 | 0.0345 | 0.660 | 0.0158 | 0.635 | 0.0145 |
| 12 | 0.478 | 0.0351 | 0.622 | 0.0168 | 0.595 | 0.0153 |
| 20 | 0.259 | 0.0348 | 0.497 | 0.0205 | 0.444 | 0.0183 |
| 25 | 0.192 | 0.0330 | 0.463 | 0.0215 | 0.399 | 0.0191 |
| 35 | 0.170 | 0.0326 | 0.354 | 0.0254 | 0.313 | 0.0212 |
| 40 | 0.155 | 0.0331 | 0.338 | 0.0259 | 0.296 | 0.0216 |
| 50 | 0.133 | 0.0350 | 0.262 | 0.0286 | 0.233 | 0.0236 |
| 60 | 0.0885 | 0.0346 | 0.232 | 0.0300 | 0.196 | 0.0249 |
| Primary Organ Dysfunction at ICU Admission * | RRT Group (n = 238) | Non-RRT Group (n = 1465) | p Values for RRT Group |
|---|---|---|---|
| cardiac dysfunction | 7 (14.00) | 5 (8.00) | p = 0.221 |
| respiratory dysfunction | 7 (12.00) | 6 (9.00) | p = 0.297 |
| renal dysfunction | 10 (10.25) | 4 (4.50) | p = 0.019 |
| gastrointestinal dysfunction | 6 (12.50) | 4 (6.00) | p = 0.006 |
| neurologic dysfunction | 7 (10.00) | 6 (10.00) | p = 0.259 |
| multiple trauma | 12.50 (12.75) | 8 (12.25) | p = 0.041 |
| surgery | 7 (11.75) | 3 (4.00) | p < 0.001 |
| oncologic patients | 6 (9.50) | 5 (6.00) | p = 0.537 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiglis, M.; Peride, I.; Florea, I.A.; Niculae, A.; Petcu, L.C.; Neagu, T.P.; Checherita, I.A.; Grintescu, I.M. Overview of Renal Replacement Therapy Use in a General Intensive Care Unit. Int. J. Environ. Res. Public Health 2022, 19, 2453. https://doi.org/10.3390/ijerph19042453
Tiglis M, Peride I, Florea IA, Niculae A, Petcu LC, Neagu TP, Checherita IA, Grintescu IM. Overview of Renal Replacement Therapy Use in a General Intensive Care Unit. International Journal of Environmental Research and Public Health. 2022; 19(4):2453. https://doi.org/10.3390/ijerph19042453
Chicago/Turabian StyleTiglis, Mirela, Ileana Peride, Iulia Alexandra Florea, Andrei Niculae, Lucian Cristian Petcu, Tiberiu Paul Neagu, Ionel Alexandru Checherita, and Ioana Marina Grintescu. 2022. "Overview of Renal Replacement Therapy Use in a General Intensive Care Unit" International Journal of Environmental Research and Public Health 19, no. 4: 2453. https://doi.org/10.3390/ijerph19042453
APA StyleTiglis, M., Peride, I., Florea, I. A., Niculae, A., Petcu, L. C., Neagu, T. P., Checherita, I. A., & Grintescu, I. M. (2022). Overview of Renal Replacement Therapy Use in a General Intensive Care Unit. International Journal of Environmental Research and Public Health, 19(4), 2453. https://doi.org/10.3390/ijerph19042453







