Gut Microbiome Diversity and Abundance Correlate with Gray Matter Volume (GMV) in Older Adults with Depression
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI
2.3. Microbial Analysis
2.3.1. Intestinal Microbial Composition: Stool Collection, Processing, and Analysis of 16S rRNA Gene Sequencing Data
2.3.2. Within-Sample Diversity Analysis
2.3.3. Between-Sample Diversity Analysis
2.3.4. Differential Taxonomic Abundance Analysis
3. Results
3.1. Participant Characteristics
3.2. Gut Microbiota Diversity Association with GMV
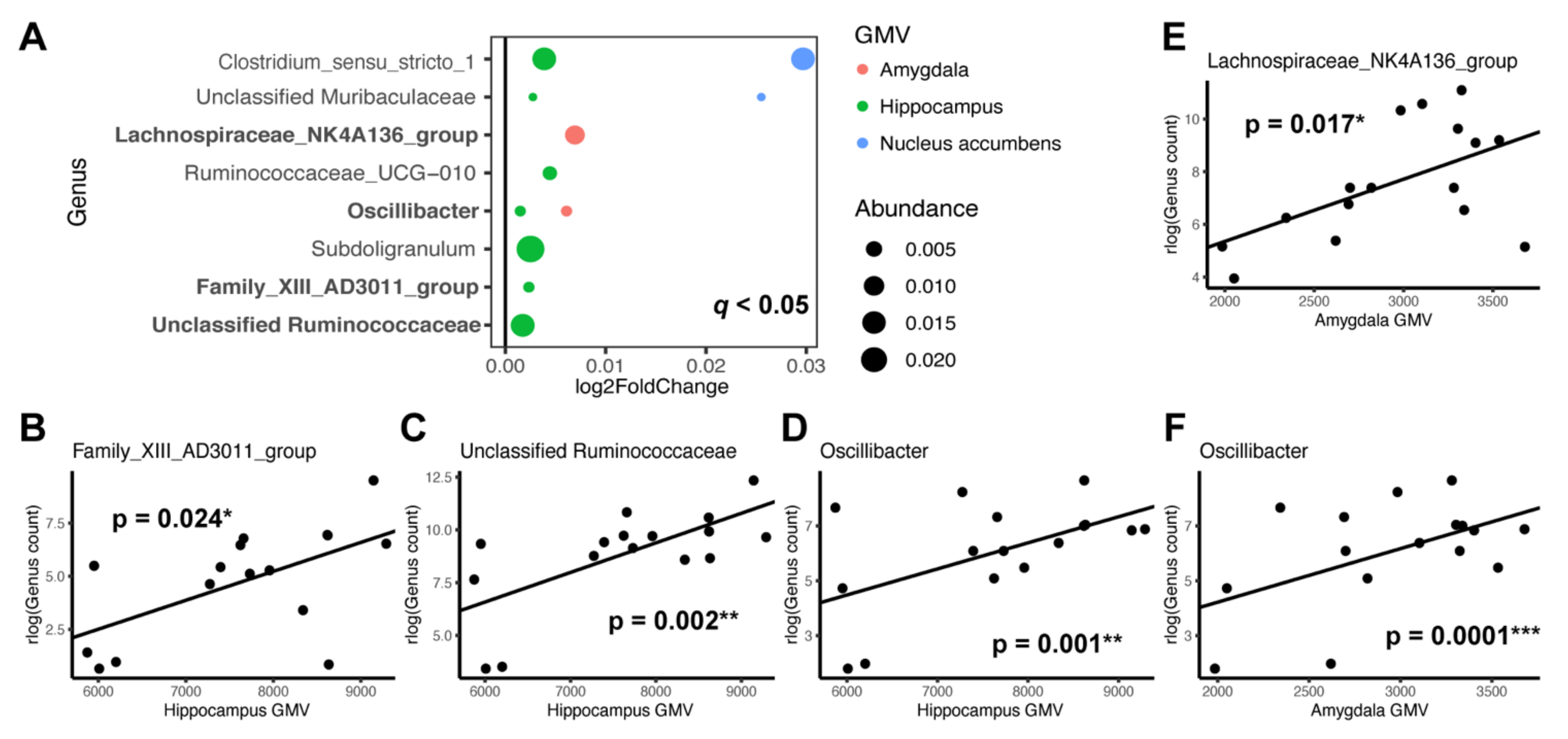
3.3. Individual Taxa Association with Gray Matter Volumes
4. Discussion
4.1. Gut Microbiome Diversity and Brain Structures
4.2. Microbial Taxa and Brain Structures
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar]
- Belmaker, R.H.; Agam, G. Major depressive disorder. N. Engl. J. Med. 2008, 358, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.; Steffens, D.C. What are the causes of late-life depression? Psychiatr. Clin. N. Am. 2013, 36, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.J.; Jayakody, D.M.P.; Bennett, R.J.; Eikelboom, R.H.; Gasson, N.; Friedland, P.L. Hearing Loss and Depression in Older Adults: A Systematic Review and Meta-analysis. Gerontologist 2020, 60, e137–e154. [Google Scholar] [CrossRef]
- Funes, C.M.; Lavretsky, H.; Ercoli, L.; St Cyr, N.; Siddarth, P. Apathy Mediates Cognitive Difficulties in Geriatric Depression. Am. J. Geriatr. Psychiatry 2018, 26, 100–106. [Google Scholar] [CrossRef]
- Mace, R.A.; Gansler, D.A.; Suvak, M.K.; Gabris, C.M.; Areán, P.A.; Raue, P.J.; Alexopoulos, G.S. Therapeutic relationship in the treatment of geriatric depression with executive dysfunction. J. Affect. Disord. 2017, 214, 130–137. [Google Scholar] [CrossRef][Green Version]
- Yi, H.A.; Möller, C.; Dieleman, N.; Bouwman, F.H.; Barkhof, F.; Scheltens, P.; van der Flier, W.M.; Vrenken, H. Relation between subcortical grey matter atrophy and conversion from mild cognitive impairment to Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2016, 87, 425–432. [Google Scholar] [CrossRef]
- Zhang, H.; Trollor, J.N.; Wen, W.; Zhu, W.; Crawford, J.D.; Kochan, N.A.; Slavin, M.J.; Brodaty, H.; Reppermund, S.; Kang, K.; et al. Grey matter atrophy of basal forebrain and hippocampus in mild cognitive impairment. J. Neurol. Neurosurg. Psychiatry 2011, 82, 487–493. [Google Scholar] [CrossRef]
- Amidfar, M.; Quevedo, J.; Réus, G.Z.; Kim, Y.-K. Grey matter volume abnormalities in the first depressive episode of medication-naïve adult individuals: A systematic review of voxel based morphometric studies. Int. J. Psychiatry Clin. Pract. 2020, 25, 407–420. [Google Scholar] [CrossRef]
- Abe, O.; Yamasue, H.; Kasai, K.; Yamada, H.; Aoki, S.; Inoue, H.; Takei, K.; Suga, M.; Matsuo, K.; Kato, T.; et al. Voxel-based analyses of gray/white matter volume and diffusion tensor data in major depression. Psychiatry Res. 2010, 181, 64–70. [Google Scholar] [CrossRef]
- Chang, C.C.; Yu, S.-C.; McQuoid, D.R.; Messer, D.F.; Taylor, W.D.; Singh, K.; Boyd, B.D.; Krishnan, K.R.; MacFall, J.R.; Steffens, D.C.; et al. Reduction of dorsolateral prefrontal cortex gray matter in late-life depression. Psychiatry Res. 2011, 193, 1–6. [Google Scholar] [CrossRef]
- Du, M.; Liu, J.; Chen, Z.; Huang, X.; Li, J.; Kuang, W.; Yang, Y.; Zhang, W.; Zhou, D.; Bi, F.; et al. Brain grey matter volume alterations in late-life depression. J. Psychiatry Neurosci. 2014, 39, 397–406. [Google Scholar] [CrossRef]
- Du, M.Y.; Wu, Q.-Z.; Yue, Q.; Li, J.; Liao, Y.; Kuang, W.-H.; Huang, X.-Q.; Chan, R.C.; Mechelli, A.; Gong, Q.-Y. Voxelwise meta-analysis of gray matter reduction in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 36, 11–16. [Google Scholar] [CrossRef]
- Geerlings, M.I.; Gerritsen, L. Late-Life Depression, Hippocampal Volumes, and Hypothalamic-Pituitary-Adrenal Axis Regulation: A Systematic Review and Meta-analysis. Biol. Psychiatry 2017, 82, 339–350. [Google Scholar] [CrossRef]
- Lai, C.H. Gray matter volume in major depressive disorder: A meta-analysis of voxel-based morphometry studies. Psychiatry Res. 2013, 211, 37–46. [Google Scholar] [CrossRef]
- Sexton, C.E.; Mackay, C.E.; Ebmeier, K.P. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am. J. Geriatr. Psychiatry 2013, 21, 184–195. [Google Scholar] [CrossRef]
- Son, J.H.; Han, D.H.; Min, K.J.; Kee, B.S. Correlation between gray matter volume in the temporal lobe and depressive symptoms in patients with Alzheimer’s disease. Neurosci. Lett. 2013, 548, 15–20. [Google Scholar] [CrossRef]
- Xie, C.; Li, W.; Chen, G.; Ward, B.D.; Franczak, M.B.; Jones, J.L.; Antuono, P.G.; Li, S.-J.; Goveas, J.S. The co-existence of geriatric depression and amnestic mild cognitive impairment detrimentally affect gray matter volumes: Voxel-based morphometry study. Behav. Brain Res. 2012, 235, 244–250. [Google Scholar] [CrossRef]
- Zhou, H.; Li, R.; Ma, Z.; Rossi, S.; Zhu, X.; Li, J. Smaller gray matter volume of hippocampus/parahippocampus in elderly people with subthreshold depression: A cross-sectional study. BMC Psychiatry 2016, 16, 219. [Google Scholar] [CrossRef]
- Grieve, S.M.; Korgaonkar, M.S.; Koslow, S.H.; Gordon, E.; Williams, L.M. Widespread reductions in gray matter volume in depression. Neuroimage Clin. 2013, 3, 332–339. [Google Scholar] [CrossRef]
- Bouckaert, F.; De Winter, F.-L.; Emsell, L.; Dols, A.; Rhebergen, D.; Wampers, M.; Sunaert, S.; Stek, M.; Sienaert, P.; Vandenbulcke, M. Grey matter volume increase following electroconvulsive therapy in patients with late life depression: A longitudinal MRI study. J. Psychiatry Neurosci. 2016, 41, 105–114. [Google Scholar] [CrossRef]
- Droppa, K.; Karim, H.T.; Tudorascu, D.L.; Karp, J.F.; Reynolds, C.F., 3rd; Aizenstein, H.J.; Butters, M.A. Association between change in brain gray matter volume, cognition, and depression severity: Pre- and post- antidepressant pharmacotherapy for late-life depression. J. Psychiatr Res. 2017, 95, 129–134. [Google Scholar] [CrossRef]
- Marano, C.M.; Workman, C.I.; Lyman, C.H.; Munro, C.A.; Kraut, M.A.; Smith, G.S. Structural imaging in late-life depression: Association with mood and cognitive responses to antidepressant treatment. Am. J. Geriatr. Psychiatry 2015, 23, 4–12. [Google Scholar] [CrossRef]
- Ribeiz, S.R.; Duran, F.; Oliveira, M.C.; Bezerra, D.; Castro, C.C.; Steffens, D.C.; Busatto Filho, G.; Bottino, C.M.C. Structural brain changes as biomarkers and outcome predictors in patients with late-life depression: A cross-sectional and prospective study. PLoS ONE 2013, 8, e80049. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Z.; Bai, F.; You, J.; Yu, H.; Shi, Y.; Liu, W. Genetic variation in apolipoprotein E alters regional gray matter volumes in remitted late-onset depression. J. Affect. Disord. 2010, 121, 273–277. [Google Scholar] [CrossRef]
- Lavretsky, H.; Lesser, I.M.; Wohl, M.; Miller, B.L.; Mehringer, C.M.; Vinters, H.V. Apolipoprotein-E and white-matter hyperintensities in late-life depression. Am. J. Geriatr. Psychiatry 2000, 8, 257–261. [Google Scholar] [CrossRef]
- Lee, S.M.; Dong, T.S.; Krause-Sorio, B.; Siddarth, P.; Milillo, M.M.; Lagishetty, V.; Datta, T.; Aguilar-Faustino, Y.; Jacobs, J.P.; Lavretsky, H. The intestinal microbiota as a predictor for antidepressant treatment outcome in geriatric depression: A prospective pilot study. Int. Psychogeriatr. 2021, 1–13. [Google Scholar] [CrossRef]
- Alkasir, R.; Li, J.; Li, X.; Jin, M.; Zhu, B. Human gut microbiota: The links with dementia development. Protein Cell 2017, 8, 90–102. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and mental health: A review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Calvani, R.; Picca, A.; Lo Monaco, M.R.; Landi, F.; Bernabei, R.; Marzetti, E. Of Microbes and Minds: A Narrative Review on the Second Brain Aging. Front. Med. 2018, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Capuco, A.; Urits, I.; Hasoon, J.; Chun, R.; Gerald, B.; Wang, J.K.; Kassem, H.; Ngo, A.L.; Abd-Elsayed, A.; Simopoulos, T.; et al. Current Perspectives on Gut Microbiome Dysbiosis and Depression. Adv. Ther. 2020, 37, 1328–1346. [Google Scholar] [CrossRef]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef]
- Evrensel, A.; Unsalver, B.O.; Ceylan, M.E. Neuroinflammation, Gut-Brain Axis and Depression. Psychiatry Investig. 2020, 17, 2–8. [Google Scholar] [CrossRef]
- Jeffery, I.B.; Lynch, D.B.; O’Toole, P.W. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016, 10, 170–182. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef]
- Li, Y.; Hao, Y.; Fan, F.; Zhang, B. The Role of Microbiome in Insomnia, Circadian Disturbance and Depression. Front. Psychiatry 2018, 9, 669. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Hu, X.; Wang, T.; Jin, F. Recognizing Depression from the Microbiota(-)Gut(-)Brain Axis. Int. J. Mol. Sci. 2018, 19, 1592. [Google Scholar] [CrossRef]
- Lima-Ojeda, J.M.; Rupprecht, R.; Baghai, T.C. “I Am I and My Bacterial Circumstances”: Linking Gut Microbiome, Neurodevelopment, and Depression. Front. Psychiatry 2017, 8, 153. [Google Scholar] [CrossRef]
- Zhu, S.; Jiang, Y.; Xu, K.; Cui, M.; Ye, W.; Zhao, G.; Jin, L.; Chen, X. The progress of gut microbiome research related to brain disorders. J. Neuroinflamm. 2020, 17, 25. [Google Scholar] [CrossRef]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.-C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic Review of Gut Microbiota and Major Depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.-J.; Fan, S.-H.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.D.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef]
- Krause-Sorio, B.; Kilpatrick, L.; Siddarth, P.; Ercoli, L.; Laird, K.T.; Aguilar-Faustino, Y.; Milillo, M.M.; Narr, K.L.; Lavretsky, H. Cortical thickness increases with levomilnacipran treatment in a pilot randomised double-blind placebo-controlled trial in late-life depression. Psychogeriatrics 2020, 20, 140–148. [Google Scholar] [CrossRef]
- First, M.B. Diagnostic and statistical manual of mental disorders, and clinical utility. J. Nerv. Ment. Dis. 2013, 201, 727–729. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Hamilton, M. Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 1967, 6, 278–296. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, G.K.; Reynolds, N.C., Jr.; Warren, R.M. Qualitative assessment of Parkinson’s disease: Study of reliability and data reduction with an abbreviated Columbia Scale. Clin. Neuropharmacol. 1985, 8, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Tong, M.; Jacobs, J.P.; McHardy, I.H.; Braun, J. Sampling of intestinal microbiota and targeted amplification of bacterial 16S rRNA genes for microbial ecologic analysis. Curr. Protoc. Immunol. 2014, 107, 7.41.1–7.41.11. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Martino, C.; Morton, J.T.; Marotz, C.A.; Thompson, L.R.; Tripathi, A.; Knight, R.; Zengler, K. A Novel Sparse Compositional Technique Reveals Microbial Perturbations. mSystems 2019, 4, e00016-19. [Google Scholar] [CrossRef]
- McArdle, B.H.; Anderson, M.J. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology 2001, 82, 290–297. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Storey, J.D.; Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef]
- Jackson, M.A.; Jeffery, I.B.; Beaumont, M.; Bell, J.T.; Clark, A.G.; Ley, R.E.; O’Toole, P.W.; Spector, T.D.; Steves, C.J. Signatures of early frailty in the gut microbiota. Genome Med. 2016, 8, 8. [Google Scholar] [CrossRef]
- Lotze, M.; Domin, M.; Gerlach, F.H.; Gaser, C.; Lueders, E.; Schmidt, C.O.; Neumann, N. Novel findings from 2838 Adult Brains on Sex Differences in Gray Matter Brain Volume. Sci. Rep. 2019, 9, 1671. [Google Scholar] [CrossRef]
- Raz, N.; Rodrigue, K.M.; Head, D.; Kennedy, K.M.; Acker, J.D. Differential aging of the medial temporal lobe: A study of a five-year change. Neurology 2004, 62, 433–438. [Google Scholar] [CrossRef]
- Salat, D.H.; Buckner, R.L.; Snyder, A.Z.; Greve, D.N.; Desikan, R.S.R.; Busa, E.; Morris, J.C.; Dale, A.M.; Fischl, B. Thinning of the cerebral cortex in aging. Cereb. Cortex 2004, 14, 721–730. [Google Scholar] [CrossRef]
- Van Petten, C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta-analysis. Neuropsychologia 2004, 42, 1394–1413. [Google Scholar] [CrossRef]
- Wise, T.; Radua, J.; Via, E.; Cardoner, N.; Abe, O.; Adams, T.M.; Amico, F.; Cheng, Y.; Cole, J.H.; de Azevedo Marques Périco, C.; et al. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: Evidence from voxel-based meta-analysis. Mol. Psychiatry 2017, 22, 1455–1463. [Google Scholar] [CrossRef]
- Barch, D.M.; Tillman, R.; Kelly, D.; Whalen, D.; Gilbert, K.; Luby, J.L. Hippocampal volume and depression among young children. Psychiatry Res. Neuroimaging 2019, 288, 21–28. [Google Scholar] [CrossRef]
- Belleau, E.L.; Treadway, M.T.; Pizzagalli, D.A. The Impact of Stress and Major Depressive Disorder on Hippocampal and Medial Prefrontal Cortex Morphology. Biol. Psychiatry 2019, 85, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Carlezon, W.A., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 2006, 59, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Song, J.; Ke, P.; Kong, L.; Lei, B.; Zhou, J.; Huang, Y.; Li, H.; Li, G.; Chen, J.; et al. The gut microbiome is associated with brain structure and function in schizophrenia. Sci. Rep. 2021, 11, 9743. [Google Scholar] [CrossRef] [PubMed]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef]
- Iino, T.; Mori, K.; Tanaka, K.; Suzuki, K.-I.; Harayama, S. Oscillibacter valericigenes gen. nov., sp. nov., a valerate-producing anaerobic bacterium isolated from the alimentary canal of a Japanese corbicula clam. Int. J. Syst. Evol. Microbiol. 2007, 57 Pt 8, 1840–1845. [Google Scholar] [CrossRef]

| Variables 1 | Cohort (N = 16) |
|---|---|
| Age (years), mean +/− SD | 70.6 +/− 5.7 |
| Female, n (%) | 6 (37.5) |
| Education (years), mean +/− SD | 16.0 +/− 1.5 |
| Age of onset (years), mean +/− SD | 47.2 +/− 25.0 |
| BMI (kg/m2), mean +/− SD | 26.6 +/− 3.6 |
| MMSE, mean +/− SD | 28.9 +/− 3.6 |
| MADRS, mean +/− SD | 14.6 +/− 3.6 |
| HAMD, mean +/− SD | 18.6 +/− 2.4 |
| GMV Region | Chao1 | Faith’s PD | Shannon | |||
|---|---|---|---|---|---|---|
| β | p | β | p | β | p | |
| Hippocampus | 0.571 | 0.032 * | 0.617 | 0.028 * | 0.528 | 0.097 |
| Amygdala | 0.516 | 0.116 | 0.495 | 0.159 | 0.401 | 0.303 |
| Nucleus accumbens | 0.791 | 0.006 ** | 0.752 | 0.018 * | 0.809 | 0.020 * |
| Pericalcarine (control) | −0.148 | 0.554 | −0.256 | 0.328 | 0.173 | 0.549 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.M.; Milillo, M.M.; Krause-Sorio, B.; Siddarth, P.; Kilpatrick, L.; Narr, K.L.; Jacobs, J.P.; Lavretsky, H. Gut Microbiome Diversity and Abundance Correlate with Gray Matter Volume (GMV) in Older Adults with Depression. Int. J. Environ. Res. Public Health 2022, 19, 2405. https://doi.org/10.3390/ijerph19042405
Lee SM, Milillo MM, Krause-Sorio B, Siddarth P, Kilpatrick L, Narr KL, Jacobs JP, Lavretsky H. Gut Microbiome Diversity and Abundance Correlate with Gray Matter Volume (GMV) in Older Adults with Depression. International Journal of Environmental Research and Public Health. 2022; 19(4):2405. https://doi.org/10.3390/ijerph19042405
Chicago/Turabian StyleLee, Sungeun Melanie, Michaela M. Milillo, Beatrix Krause-Sorio, Prabha Siddarth, Lisa Kilpatrick, Katherine L. Narr, Jonathan P. Jacobs, and Helen Lavretsky. 2022. "Gut Microbiome Diversity and Abundance Correlate with Gray Matter Volume (GMV) in Older Adults with Depression" International Journal of Environmental Research and Public Health 19, no. 4: 2405. https://doi.org/10.3390/ijerph19042405
APA StyleLee, S. M., Milillo, M. M., Krause-Sorio, B., Siddarth, P., Kilpatrick, L., Narr, K. L., Jacobs, J. P., & Lavretsky, H. (2022). Gut Microbiome Diversity and Abundance Correlate with Gray Matter Volume (GMV) in Older Adults with Depression. International Journal of Environmental Research and Public Health, 19(4), 2405. https://doi.org/10.3390/ijerph19042405







