Associations between Long-Term Air Pollution Exposure and Risk of Osteoporosis-Related Fracture in a Nationwide Cohort Study in South Korea
Abstract
:1. Introduction
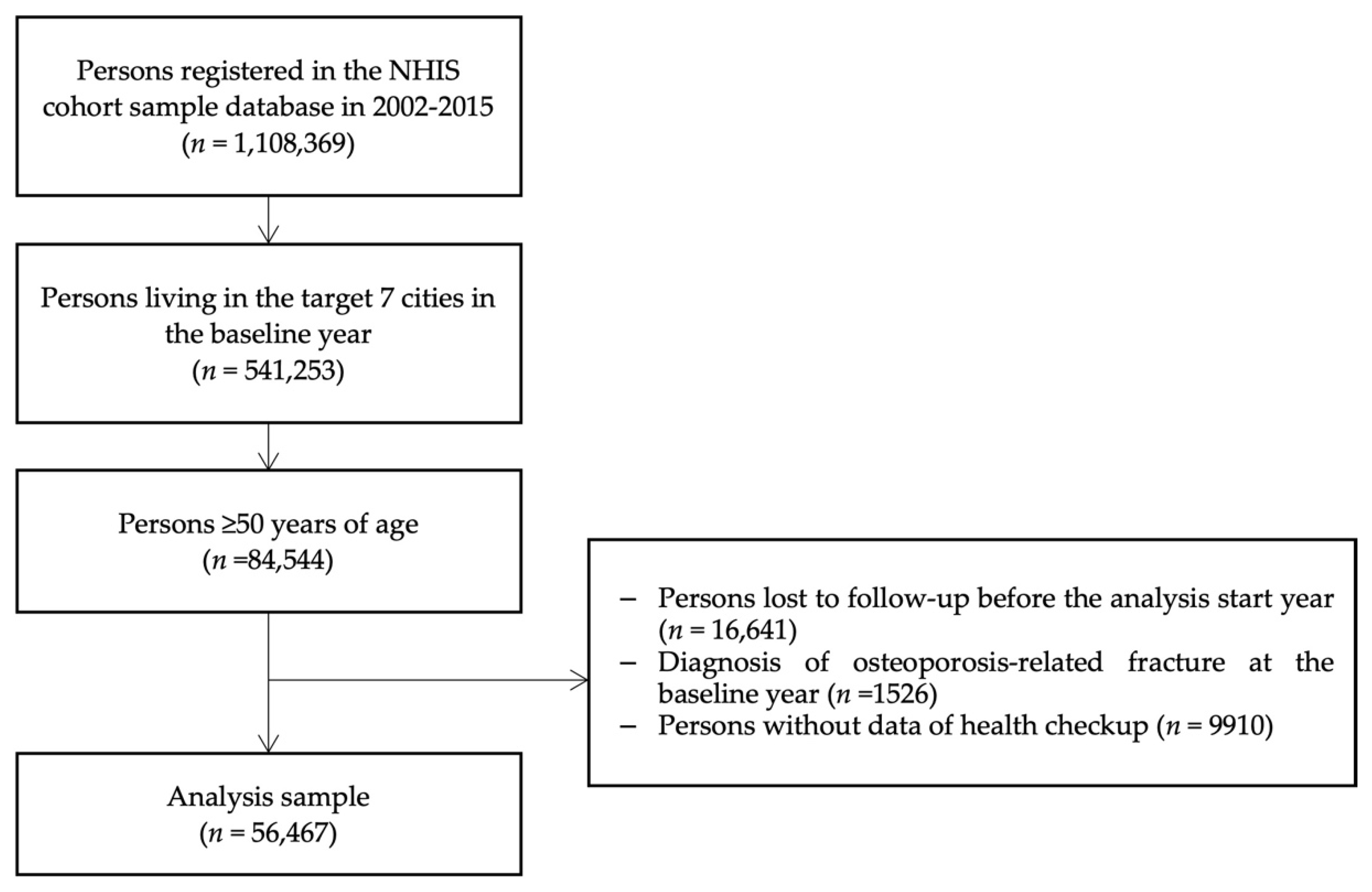
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Air Pollution Assessment
2.3. Covariates
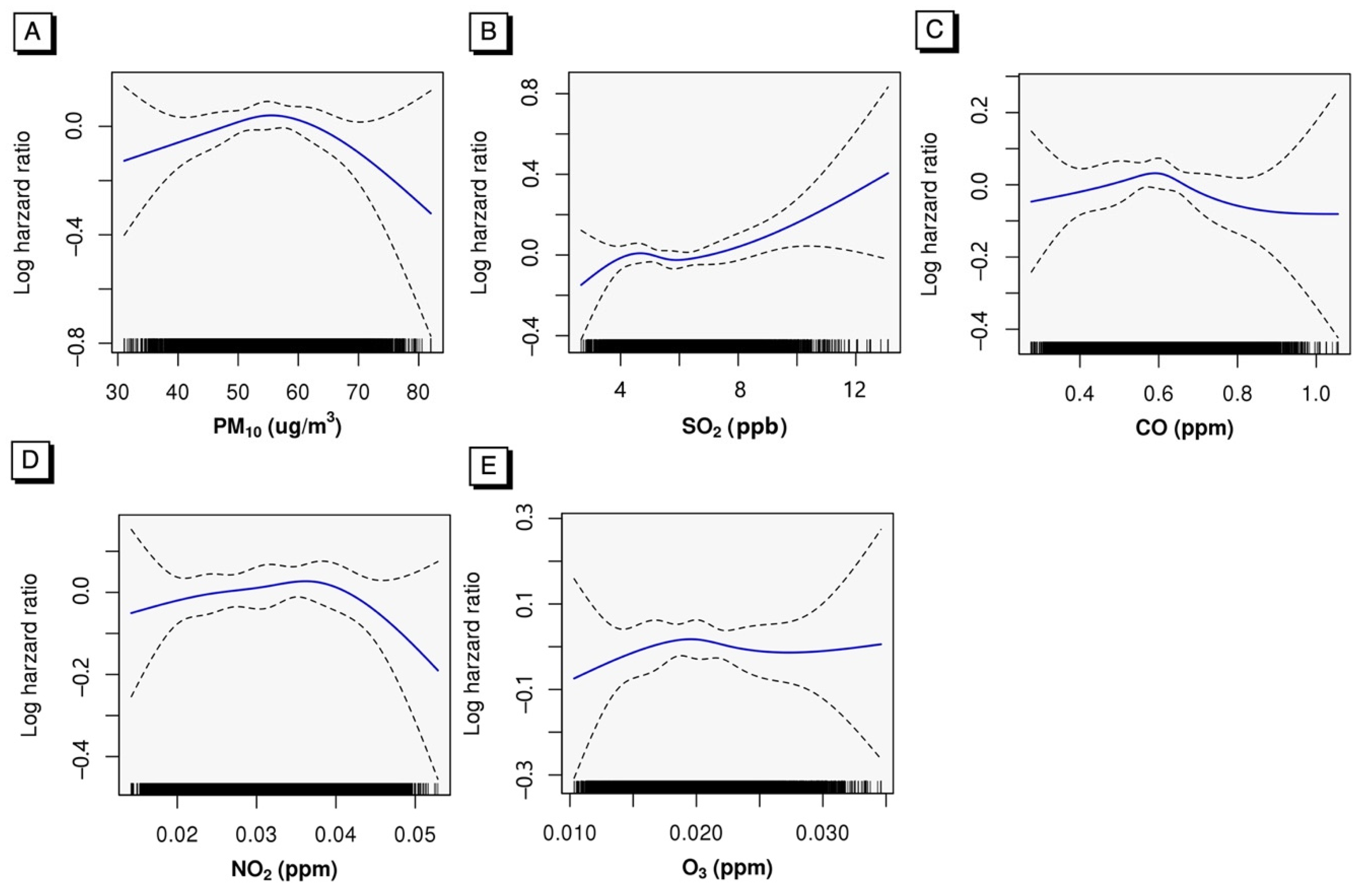
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiu, Y.C.; Lin, Y.T.; Hsia, Y.F.; Jung, C.R.; Lo, Y.C.; Chen, T.M.; Chan, J.C.; Wang, Y.C.; Kuo, C.C.; Hwang, B.F. Long-Term Exposure to Fine Particulate Matter and Osteoporotic Fracture: A Case–Control Study in Taiwan. Environ. Res. 2021, 196, 110888. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Leung, J.; Yu, B.; Woo, J.; Kwok, T.; Ka-Lun Lau, K. Association of Green Space with Bone Mineral Density Change and Incident Fracture in Elderly Hong Kong Chinese: Mr. OS and Ms. OS Study. Environ. Res. 2021, 201, 111547. [Google Scholar] [CrossRef]
- Nguyen, V.H. Environmental Air Pollution and the Risk of Osteoporosis and Bone Fractures. J. Prev. Med. Public Health 2018, 51, 217–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, C.-L.; Ang, S.B.; Chadha, M.; Chow, E.S.-L.; Chung, Y.-S.; Hew, F.L.; Jaisamrarn, U.; Ng, H.; Takeuchi, Y.; Wu, C.-H.; et al. An Updated Hip Fracture Projection in Asia: The Asian Federation of Osteoporosis Societies Study. Osteoporos. Sarcopenia 2018, 4, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Peden, D.B. The Unexpected Health Effects of Air Pollution. North Carol. Med. J. 2018, 79, 309–311. [Google Scholar] [CrossRef]
- Schraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; De Matteis, S.; Jung, S.H.; Mortimer, K.; Perez-Padilla, R.; Rice, M.B.; Riojas-Rodriguez, H.; Sood, A.; et al. Air Pollution and Noncommunicable Diseases: A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: Air Pollution and Organ Systems. Chest 2019, 155, 417–426. [Google Scholar] [CrossRef]
- Prada, D.; López, G.; Solleiro-Villavicencio, H.; Garcia-Cuellar, C.; Baccarelli, A.A. Molecular and Cellular Mechanisms Linking Air Pollution and Bone Damage. Environ. Res. 2020, 185, 109465. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Wang, C.-F.; Chiu, H.; Lai, B.-C.; Tu, H.-P.; Wu, P.-Y.; Huang, J.-C.; Chen, S.-C. Air Pollutants Interaction and Gender Difference on Bone Mineral Density T-Score in Taiwanese Adults. Int. J. Environ. Res. Public Health 2020, 17, 9165. [Google Scholar] [CrossRef]
- Kheirouri, S. Effects of Sulfur Dioxide, Ozone, and Ambient Air Pollution on Bone Metabolism Related Biochemical Parameters in a Rat Model. Sulfur Dioxide 2020, 35, e2020023. [Google Scholar] [CrossRef]
- Meng, Z. Oxidative Damage of Sulfur Dioxide on Various Organs of Mice: Sulfur Dioxide Is a Systemic Oxidative Damage Agent. Inhal. Toxicol. 2003, 15, 181–195. [Google Scholar] [CrossRef]
- Manicourt, D.-H.; Devogelaer, J.-P. Urban Tropospheric Ozone Increases the Prevalence of Vitamin D Deficiency among Belgian Postmenopausal Women with Outdoor Activities during Summer. J. Clin. Endocrinol. Metab. 2008, 93, 3893–3899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cevei, M.; Stoicănescu, D. Air Pollution and Genetic Influences on Bone Mineral Density and Osteoporosis. Analele Universității din Oradea, Fascicula Biologie 2010, 17, 84–89. [Google Scholar]
- Chang, K.H.; Chang, M.Y.; Muo, C.H.; Wu, T.N.; Hwang, B.F.; Chen, C.Y.; Lin, T.H.; Kao, C.H. Exposure to Air Pollution Increases the Risk of Osteoporosis: A Nationwide Longitudinal Study. Medicine 2015, 94, e733. [Google Scholar] [CrossRef] [PubMed]
- Prada, D.; Zhong, J.; Colicino, E.; Zanobetti, A.; Schwartz, J.; Dagincourt, N.; Fang, S.C.; Kloog, I.; Zmuda, J.M.; Holick, M.; et al. Association of Air Particulate Pollution with Bone Loss over Time and Bone Fracture Risk: Analysis of Data from Two Independent Studies. Lancet Planet. Health 2017, 1, e337–e347. [Google Scholar] [CrossRef]
- Qiao, D.; Pan, J.; Chen, G.; Xiang, H.; Tu, R.; Zhang, X.; Dong, X.; Wang, Y.; Luo, Z.; Tian, H.; et al. Long-Term Exposure to Air Pollution Might Increase Prevalence of Osteoporosis in Chinese Rural Population. Environ. Res. 2020, 183, 109264. [Google Scholar] [CrossRef]
- Alver, K.; Meyer, H.E.; Falch, J.A.; Søgaard, A.J. Outdoor Air Pollution, Bone Density and Self-Reported Forearm Fracture: The Oslo Health Study. Osteoporos. Int. 2010, 21, 1751–1760. [Google Scholar] [CrossRef]
- Mazzucchelli, R.; Crespi Villarias, N.; Perez Fernandez, E.; Durban Reguera, M.L.; Garcia-Vadillo, A.; Quiros, F.J.; Guzon, O.; Rodriguez Caravaca, G.; Gil de Miguel, A. Short-Term Association between Outdoor Air Pollution and Osteoporotic Hip Fracture. Osteoporos. Int. 2018, 29, 2231–2241. [Google Scholar] [CrossRef]
- Sung, J.H.; Kim, K.; Cho, Y.; Choi, S.; Chang, J.; Kim, S.M.; Kim, S.R.; Lee, G.; Son, J.S.; Park, S.M. Association of Air Pollution with Osteoporotic Fracture Risk among Women over 50 Years of Age. J. Bone Min. Metab. 2020, 38, 839–847. [Google Scholar] [CrossRef]
- Kim, H.Y.; Jang, E.J.; Park, B.; Kim, T.-Y.; Shin, S.-A.; Ha, Y.-C.; Jang, S. Development of a Korean Fracture Risk Score (KFRS) for Predicting Osteoporotic Fracture Risk: Analysis of Data from the Korean National Health Insurance Service. PLoS ONE 2016, 11, e0158918. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-K.; Yoon, B.-H.; Koo, K.-H. Epidemiology of Osteoporosis and Osteoporotic Fractures in South Korea. Endocrinol. Metab. 2013, 28, 90. [Google Scholar] [CrossRef]
- Nguyen, T.V. Air Pollution: A Largely Neglected Risk Factor for Osteoporosis. Lancet Planet. Health 2017, 1, e311–e312. [Google Scholar] [CrossRef]
- Yu, A.; Yang, Y.J.; Jeong, S.R.; Kim, J.; Kim, Y.J.; Kwon, O.; Oh, S.-Y.; Kim, J.H. Calcium Intakes in Korean and American Populations (in Korean). J. Korean Diet. Assoc. 2013, 19, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Kim, O.; Kim, S.-Y.; Kwon, H.-Y.; Kim, H. Data Issues and Suggestions in the National Health Insurance Service-National Sample Cohort for Assessing the Long-term Health Effects of Air Pollution Focusing on Mortality. J. Health Inform. Stat. 2017, 42, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Jo, Y.-H.; Lee, B.-G.; Kim, H.-S.; Kim, J.-H.; Lee, C.-H.; Kim, S.-J.; Choi, W.-S.; Lee, J.-H.; Lee, K.-H. Incidence and Seasonal Variation of Distal Radius Fractures in Korea: A Population-Based Study. J. Korean Med. Sci. 2018, 33, e48. [Google Scholar] [CrossRef]
- Yoon, H.-K.; Park, C.; Jang, S.; Jang, S.; Lee, Y.-K.; Ha, Y.-C. Incidence and Mortality Following Hip Fracture in Korea. J. Korean Med. Sci. 2011, 26, 1087–1092. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.-S.; Nho, J.-H.; Ha, Y.-C.; Jang, S.; Kim, H.-Y.; Yoo, J.-I.; Park, S.-M.; Lee, Y.-K. Incidence of Osteoporotic Refractures Following Proximal Humerus Fractures in Adults Aged 50 Years and Older in Korea. J. Bone Metab. 2019, 26, 105–111. [Google Scholar] [CrossRef]
- Park, S.-M.; Ahn, S.H.; Kim, H.-Y.; Jang, S.; Ha, Y.-C.; Lee, Y.-K.; Chung, H.-Y. Incidence and Mortality of Subsequent Vertebral Fractures: Analysis of Claims Data of the Korea National Health Insurance Service from 2007 to 2016. Spine J. 2020, 20, 225–233. [Google Scholar] [CrossRef]
- The National Ambient air Monitoring Information System (NAMIS). Available online: https://airkorea.or.kr/web (accessed on 9 September 2021).
- Friis-Holmberg, T.; Rubin, K.H.; Brixen, K.; Tolstrup, J.S.; Bech, M. Fracture Risk Prediction Using Phalangeal Bone Mineral Density or FRAX®?—A Danish Cohort Study on Men and Women. J. Clin. Densitom. 2014, 17, 7–15. [Google Scholar] [CrossRef]
- González-Macías, J.; Marin, F.; Vila, J.; Díez-Pérez, A. Probability of Fractures Predicted by FRAX® and Observed Incidence in the Spanish ECOSAP Study Cohort. Bone 2012, 50, 373–377. [Google Scholar] [CrossRef]
- Glasheen, W.P.; Cordier, T.; Gumpina, R.; Haugh, G.; Davis, J.; Renda, A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am. Health Drug Benefits 2019, 12, 188–197. [Google Scholar]
- Eriksen, E.F. Cellular Mechanisms of Bone Remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rulli, E.; Ghilotti, F.; Biagioli, E.; Porcu, L.; Marabese, M.; D’Incalci, M.; Bellocco, R.; Torri, V. Assessment of Proportional Hazard Assumption in Aggregate Data: A Systematic Review on Statistical Methodology in Clinical Trials Using Time-to-Event Endpoint. Br. J. Cancer 2018, 119, 1456–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Fu, S.; Jiang, J.; Tang, X. Association between Outdoor Particulate Air Pollution and the Risk of Osteoporosis: A Systematic Review and Meta-Analysis. Osteoporos. Int. 2021, 32, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; An, J.; He, Y.; Zheng, J. An Overview of Emissions of SO2 and NOx and the Long-Range Transport of Oxidized Sulfur and Nitrogen Pollutants in East Asia. J. Environ. Sci. 2016, 44, 13–25. [Google Scholar] [CrossRef]
- Pang, K.-L.; Ekeuku, S.O.; Chin, K.-Y. Particulate Air Pollution and Osteoporosis: A Systematic Review. Risk Manag. Healthc. Policy 2021, 14, 2715. [Google Scholar] [CrossRef]
- Kim, H.; Byun, G.; Choi, Y.; Kim, S.; Kim, S.-Y.; Lee, J.-T. Effects of Long-Term Exposure to Air Pollution on All-Cause Mortality and Cause-Specific Mortality in Seven Major Cities of South Korea: Korean National Health and Nutritional Examination Surveys with Mortality Follow-Up. Environ. Res. 2021, 192, 110290. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Lee, J.-T. Effect of Air Pollutant Emission Reduction Policies on Hospital Visits for Asthma in Seoul, Korea; Quasi-Experimental Study. Environ. Int. 2019, 132, 104954. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Lee, J.-T. Spatial Variation in Lag Structure in the Short-Term Effects of Air Pollution on Mortality in Seven Major South Korean Cities, 2006–2013. Environ. Int. 2019, 125, 595–605. [Google Scholar] [CrossRef]
- Evangelopoulos, D.; Katsouyanni, K.; Keogh, R.H.; Samoli, E.; Schwartz, J.; Barratt, B.; Zhang, H.; Walton, H. PM2.5 and NO2 Exposure Errors Using Proxy Measures, Including Derived Personal Exposure from Outdoor Sources: A Systematic Review and Meta-Analysis. Environ. Int. 2020, 137, 105500. [Google Scholar] [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Oh, T.K.; Song, I.-A. Exposure to Air Pollution and Risk of Hip Fracture: A Population-Based Cohort Study With a 6-Year Follow-Up in South Korea. J. Occup. Environ. Med. 2020, 62, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Mirchandani, S.; Aharonoff, G.; Hiebert, R.; Capla, E.; Zuckerman, J.D.; Koval, K.J. The Effects of Weather and Seasonality of Hip Fracture Incidence in Older Adults. Orthopedics 2005, 28, 149–155. [Google Scholar] [PubMed]
- Koren, L.; Barak, A.; Norman, D.; Sachs, O.; Peled, E. Effect of Seasonality, Weather and Holidays on the Incidence of Proximal Hip Fracture. Isr. Med. Assoc. J. 2014, 16, 299–302. [Google Scholar] [PubMed]
- Bischoff-Ferrari, H.A.; Orav, J.E.; Barrett, J.A.; Baron, J.A. Effect of Seasonality and Weather on Fracture Risk in Individuals 65 Years and Older. Osteoporos. Int. 2007, 18, 1225–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, J.-Y.; Jeong, H.-S. Determinants of Health Screening and Its Effects on Health Behaviors. Health Policy Manag. 2012, 22, 49–64. [Google Scholar] [CrossRef]
| Osteoporosis-Related Fracture | |||
|---|---|---|---|
| Total Participants (n = 56,498) | No Event (n = 51,100) | Event (n = 5398) | |
| Entry year | |||
| Sex (n, %) | |||
| Men | 26,243 (46.5) | 25,041 (49.0) | 1202 (22.3) * |
| Women | 30,224 (53.5) | 26,030 (51.0) | 4194 (77.7) |
| Age in the entry year (n, %) | |||
| 50–59 y | 20,673 (36.6) | 19,301 (37.8) | 1372 (25.4) * |
| 60–64 y | 15,183 (26.9) | 13,929 (27.3) | 1254 (23.2) |
| 65–69 y | 11,510 (20.4) | 10,235 (20.0) | 1275 (23.6) |
| 70–74 y | 5851 (10.4) | 5001 (9.8) | 850 (15.8) |
| 75–79 y | 2449 (4.3) | 1987 (3.9) | 462 (8.6) |
| 80–84 y | 720 (1.3) | 551 (1.1) | 169 (3.1) |
| 85+ y | 81 (0.1) | 67 (0.1) | 14 (0.3) |
| During follow-up | |||
| Charlson Comorbidity Index in the first year of follow-up (mean ± SD) | 0.36 ± 0.82 | 0.35 ± 0.82 | 0.44 ± 0.86 * |
| BMI (mean ± SD) | 24.2 ± 3.1 | 24.2 ± 3.0 | 24.1 ± 3.2 * |
| Smoking status (n, %) | |||
| Never-smoker | 39,255 (69.8) | 34,818 (68.4) | 4437 (82.8) * |
| Former smoker | 9593 (17.1) | 9145 (18.0) | 448 (8.4) |
| Current smoker | 7399 (13.2) | 6925 (13.6) | 474 (8.8) |
| Frequency of exercise per week (n, %) | |||
| 0 time | 9488 (16.9) | 8272 (16.3) | 1216 (22.7) * |
| 1–2 times | 4146 (7.4) | 3718 (7.3) | 428 (8.0) |
| 3–4 times | 2331 (4.1) | 2109 (4.1) | 222 (4.1) |
| 5–6 times | 736 (1.3) | 650 (1.3) | 86 (1.6) |
| 7 times or more | 39,551 (70.3) | 36,140 (71.0) | 3411 (63.6) |
| High alcohol intake | |||
| Yes | 2106 (4.2) | 1965 (4.4) | 141 (3.0) * |
| No | 47,560 (95.8) | 43,071 (95.6) | 4489 (97.0) |
| Income-based insurance fee in the entry year (n, %) | |||
| 0–19th percentiles | 4644 (8.4) | 4150 (8.3) | 494 (9.4) * |
| 20–39th percentiles | 7454 (13.5) | 6751 (13.5) | 703 (13.4) |
| 40–59th percentiles | 9175 (16.6) | 8292 (16.6) | 883 (16.8) |
| 60–79th percentiles | 9923 (18.0) | 8925 (17.9) | 998 (19.0) |
| 80–100th percentiles | 24,003 (43.5) | 21,826 (43.7) | 2177 (41.4) |
| Follow-up years (mean, SD) | 9.5 (3.0) | 9.8 (2.8) | 6.4 (3.1) * |
| Diagnosis of rheumatoid arthritis during follow-up (n, %) | |||
| Yes | 5913 (10.5) | 5251 (10.3) | 662 (12.3) * |
| No | 50,554 (89.5) | 45,820 (89.7) | 4734 (87.7) |
| Diagnosis of secondary causes of osteoporosis during follow-up (n, %) | |||
| Yes | 7733 (13.7) | 6997 (13.7) | 736 (13.6) |
| No | 48,732 (86.3) | 44,074 (86.3) | 4660 (86.4) |
| Exposure to oral glucocorticoids during follow-up (n, %) | |||
| Yes | 9197 (16.3) | 8432 (16.5) | 765 (14.2) * |
| No | 47,270 (83.7) | 42,639 (83.5) | 4631 (85.8) |
| Air pollution exposure in the last 3 years before the index year (mean ± SD) | |||
| PM10 (μg/m3) | 48.7 ± 7.3 | 48.2 ± 7.0 | 53.6 ± 7.9 * |
| SO2 (ppb) | 5.67 ± 1.37 | 5.66 ± 1.36 | 5.80 ± 1.47 * |
| CO (ppm) | 0.553 ± 0.119 | 0.549 ± 0.118 | 0.592 ± 0.121 * |
| NO2 (ppb) | 30.3 ± 8.2 | 30.3 ± 8.2 | 30.9 ± 7.9 * |
| O3 (ppb) | 22.3 ± 4.5 | 22.5 ± 4.4 | 20.4 ± 4.1 * |
| Air pollution exposure in the last 3 years before the index year (n, %) | |||
| PM10, Q1 (31.1–44.3 μg/m3) | 14,882 (26.4) | 14,298 (28.0) | 584 (10.8) * |
| PM10, Q2 (44.4–47.3 μg/m3) | 14,076 (24.9) | 13,385 (26.2) | 691 (12.8) |
| PM10, Q3 (47.4–51.2 μg/m3) | 13,752 (24.4) | 12,755 (25.0) | 997 (18.5) |
| PM10, Q4 (51.3–82.0 μg/m3) | 13,757 (24.4) | 10,633 (20.8) | 3124 (57.9) |
| SO2, Q1 (2.7–4.8 ppb) | 14,130 (25.0) | 12,566 (24.6) | 1564 (29.0) * |
| SO2, Q2 (4.9–5.3 ppb) | 14,777 (26.2) | 13,868 (27.2) | 909 (16.8) |
| SO2, Q3 (5.4–6.3 ppb) | 13,870 (24.6) | 12,423 (24.3) | 1447 (26.8) |
| SO2, Q4 (6.4–13.1 ppb) | 13,690 (24.2) | 12,214 (23.9) | 1476 (27.4) * |
| CO, Q1 (0.276–0.470 ppm) | 14,295 (25.3) | 13,426 (26.3) | 869 (16.1) |
| CO, Q2 (0.271–0.568 ppm) | 14,318 (25.4) | 13,113 (25.7) | 1205 (22.3) |
| CO, Q3 (0.569–0.630 ppm) | 13,745 (24.3) | 12,318 (24.1) | 1427 (26.4) |
| CO, Q4 (0.631–1.055 ppm) | 14,109 (25.0) | 12,214 (23.9) | 1895 (35.1) |
| NO2, Q1 (14.2–23.5 ppb) | 14,642 (25.9) | 13,415 (26.3) | 1227 (22.7) * |
| NO2, Q2 (23.6–29.9 ppb) | 14,269 (25.3) | 12,944 (25.3) | 1325 (24.6) |
| NO2, Q3 (30.0–36.5 ppb) | 13,439 (23.8) | 11,992 (23.5) | 1447 (26.8) |
| NO2, Q4 (36.6–51.3 ppb) | 14,117 (25.0) | 12,720 (24.9) | 1397 (25.9) |
| O3, Q1 (10.6–19.1 ppb) | 14,559 (25.8) | 12,501 (24.5) | 2058 (38.1) * |
| O3, Q2 (19.2–22.1 ppb) | 13,703 (24.3) | 11,999 (23.5) | 1704 (31.6) |
| O3, Q3 (22.2–25.5 ppb) | 14,130 (25.0) | 13,038 (25.5) | 1092 (20.2) |
| O3, Q4 (25.6–34.6 ppb) | 14,075 (24.9) | 13,533 (26.5) | 542 (10.0) |
| Exposure (IQR) | HR (95% CI) | |||
|---|---|---|---|---|
| 1-yr Moving Average with No Lag | 2-yr Moving Average with No Lag | 3-yr Moving Average with No Lag | 2-yr Moving Average with a 1-yr Lag | |
| PM10 (13.7μg/m3) | 0.99 (0.92, 1.05) | 0.98 (0.92, 1.05) | 1.00 (0.93, 1.07) | 1.01 (0.944, 1.07) |
| SO2 (2 ppb) | 1.04 (1.00, 1.08) | 1.04 (1.00, 1.08) | 1.04 (1.00, 1.09) | 1.04 (1.00, 1.08) |
| CO (0.192 ppm) | 0.99 (0.94, 1.03) | 0.99 (0.94, 1.03) | 0.99 (0.94, 1.04) | 0.99 (0.95, 1.04) |
| NO2 (0.012 ppm) | 0.99 (0.95, 1.03) | 0.99 (0.95, 1.04) | 1.00 (0.96, 1.05) | 1.01 (0.97, 1.05) |
| O3 (0.007 ppm) | 1.01 (0.97, 1.06) | 1.01 (0.96, 1.06) | 1.00 (0.95, 1.06) | 1.00 (0.95, 1.05) |
| PM10 (IQR = 13.7 μg/m3) | SO2 (IQR = 2 ppb) | CO (IQR = 0.192 ppm) | NO2 (IQR = 0.012 ppm) | O3 (IQR = 0.007 ppm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-Int. | HR (95% CI) | p-Int. | HR (95% CI) | p-Int. | HR (95% CI) | p-Int. | HR (95% CI) | p-Int. | |
| Sex | ||||||||||
| Men | 0.99 (0.89, 1.11) | 0.884 | 1.00 (0.92, 1.09) | 0.300 | 0.93 (0.85, 1.02) | 0.142 | 1.00 (0.91, 1.09) | 0.929 | 1.03 (0.93, 1.13) | 0.614 |
| Women | 1.00 (0.93, 1.08) | 1.06 (1.01, 1.11) ** | 1.01 (0.95, 1.07) | 1.00 (0.95, 1.06) | 1.00 (0.94, 1.06) | |||||
| Age (years) | ||||||||||
| 50–69 y | 1.02 (0.94, 1.11) | 0.300 | 1.06 (1.00, 1.11) * | 0.441 | 1.01 (0.95, 1.07) | 0.395 | 1.02 (0.96, 1.08) | 0.437 | 0.99 (0.93, 1.06) | 0.650 |
| ≥70 y | 0.96 (0.88, 1.06) | 1.02 (0.96, 1.09) | 0.97 (0.90, 1.04) | 0.98 (0.91, 1.05) | 1.02 (0.94, 1.10) | |||||
| Sex and age (years) | ||||||||||
| Men, age 50–69 y | 1.02 (0.89, 1.17) | 0.753 | 0.96 (0.86, 1.07) | 0.130 | 0.94 (0.84, 1.05) | 0.372 | 0.95 (0.85, 1.07) | 0.260 | 1.02 (0.90, 1.15) | 0.940 |
| Men, age ≥70 y | 0.93 (0.77, 1.13) | 1.08 (0.94, 1.24) | 0.92 (0.79, 1.08) | 1.08 (0.93, 1.25) | 1.03 (0.88, 1.21) | |||||
| Women, age 50–69 y | 1.02 (0.93, 1.11) | 1.09 (1.03, 1.16) ** | 1.03 (0.96, 1.11) | 1.04 (0.97, 1.11) | 0.99 (0.91, 1.06) | |||||
| Women, age ≥70 y | 0.97 (0.88, 1.08) | 1.01 (0.94, 1.08) | 0.98 (0.90, 1.06) | 0.95 (0.88, 1.03) | 1.01 (0.93, 1.10) | |||||
| Frequency of exercise per week | ||||||||||
| Never | 0.93 (0.83, 1.04) | 0.064 | 1.04 (0.96, 1.12) | 0.980 | 0.94 (0.86, 1.03) | 0.446 | 0.98 (0.89, 1.07) | 0.803 | 1.02 (0.93, 1.13) | 0.900 |
| 1–4 times | 0.92 (0.79, 1.06) | 1.04 (0.92, 1.18) | 1.01 (0.89, 1.15) | 1.02 (0.90, 1.15) | 0.99 (0.87, 1.13) | |||||
| 5 times or more | 1.05 (0.97, 1.13) | 1.05 (0.99, 1.10) * | 1.01 (0.95, 1.07) | 1.01 (0.95, 1.07) | 1.00 (0.94, 1.07) | |||||
| Income-based insurance fee | ||||||||||
| 0–39th percentile | 1.02 (0.91, 1.14) | 0.177 | 1.03 (0.95, 1.12) | 0.429 | 1.03 (0.94, 1.14) | 0.395 | 1.02 (0.93, 1.13) | 0.617 | 1.01 (0.91, 1.12) | 0.611 |
| 40–79th percentile | 0.93 (0.84, 1.03) | 1.01 (0.94, 1.09) | 1.01 (0.93, 1.09) | 0.97 (0.89, 1.05) | 1.04 (0.95, 1.14) | |||||
| 80–100th percentile | 1.04 (0.95, 1.13) | 1.07 (1.01, 1.14) ** | 0.96 (0.89, 1.03) | 1.01 (0.94, 1.08) | 0.98 (0.91, 1.06) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, S.; Kim, H.; Kim, S.; Choe, S.-A.; Byun, G.; Lee, J.-T.; Bell, M.L. Associations between Long-Term Air Pollution Exposure and Risk of Osteoporosis-Related Fracture in a Nationwide Cohort Study in South Korea. Int. J. Environ. Res. Public Health 2022, 19, 2404. https://doi.org/10.3390/ijerph19042404
Heo S, Kim H, Kim S, Choe S-A, Byun G, Lee J-T, Bell ML. Associations between Long-Term Air Pollution Exposure and Risk of Osteoporosis-Related Fracture in a Nationwide Cohort Study in South Korea. International Journal of Environmental Research and Public Health. 2022; 19(4):2404. https://doi.org/10.3390/ijerph19042404
Chicago/Turabian StyleHeo, Seulkee, Honghyok Kim, Sera Kim, Seung-Ah Choe, Garam Byun, Jong-Tae Lee, and Michelle L. Bell. 2022. "Associations between Long-Term Air Pollution Exposure and Risk of Osteoporosis-Related Fracture in a Nationwide Cohort Study in South Korea" International Journal of Environmental Research and Public Health 19, no. 4: 2404. https://doi.org/10.3390/ijerph19042404
APA StyleHeo, S., Kim, H., Kim, S., Choe, S.-A., Byun, G., Lee, J.-T., & Bell, M. L. (2022). Associations between Long-Term Air Pollution Exposure and Risk of Osteoporosis-Related Fracture in a Nationwide Cohort Study in South Korea. International Journal of Environmental Research and Public Health, 19(4), 2404. https://doi.org/10.3390/ijerph19042404







