Preliminary Effectiveness of mHealth App-Based Pelvic Floor Muscle Training among Pregnant Women to Improve Their Exercise Adherence: A Pilot Randomised Control Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design Overview
2.2. Participants
2.3. Intervention
- Educational video: Participants watched a PFMT educational video demonstrated by a certified physiotherapist for six minutes. The video has been approved for education in the rehabilitation department tertiary hospital.
- Training timer: Participants performed the exercise according to the tailored timer performance ability (beginner: 2 s contraction, intermediate: 6 s contraction, and expert: 10 s contraction) and 6 s rest between each repetition, every day for three times daily. There were slow-velocity, close-to-maximum contractions of exercise. The first (beginner) performed the quick muscle contractions for two seconds and rested for six seconds while breathing normally. The quick contractions were required to perform 3 times daily, for 10 repetitions each cycle. After gaining confidence and skills, they proceeded to the longer durations, where the same muscles they contracted with longer durations of 6 to 10 seconds, for 10 repetitions, 3 times daily.
- Symptoms calendar charting: Participants recorded their UI symptoms for their self-monitoring.
- Progress chart: Participants could self-monitor their progress of UI symptoms and PFMT adherence.
- Frequently asked questions: Participants could read further the details of anatomy and PFMT techniques.
- Notification reminder: Participants received a daily notification to remind their pelvic exercise.
2.4. Control Group
2.5. Outcome Measures (Preliminary Effectiveness)
2.6. Sample Size
2.7. Randomisation and Blinding
2.8. Statistical Methods
3. Results
3.1. Participant Characteristics
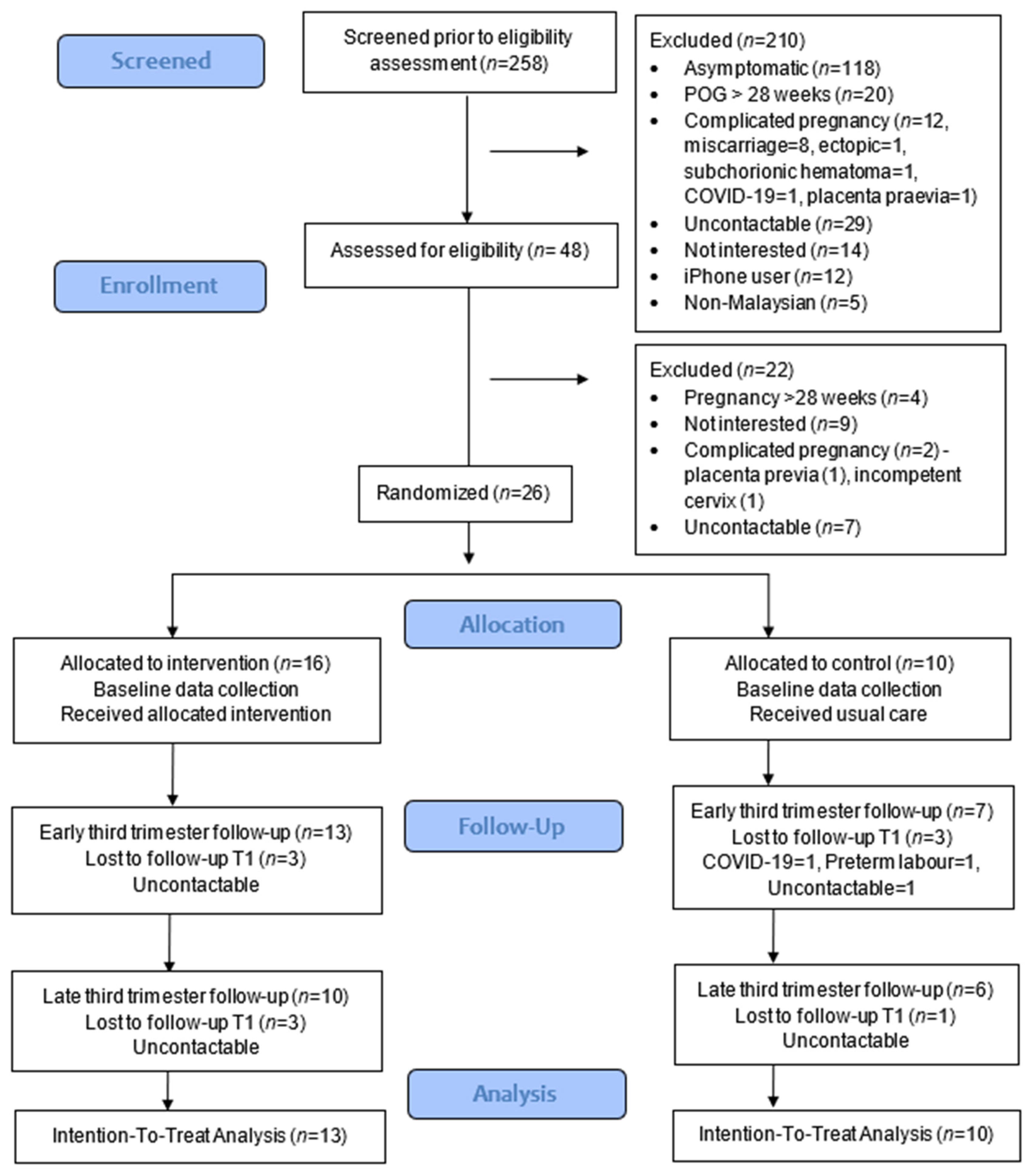
3.2. Feasibility of the Study
3.3. Primary Outcome
3.4. Secondary Outcomes
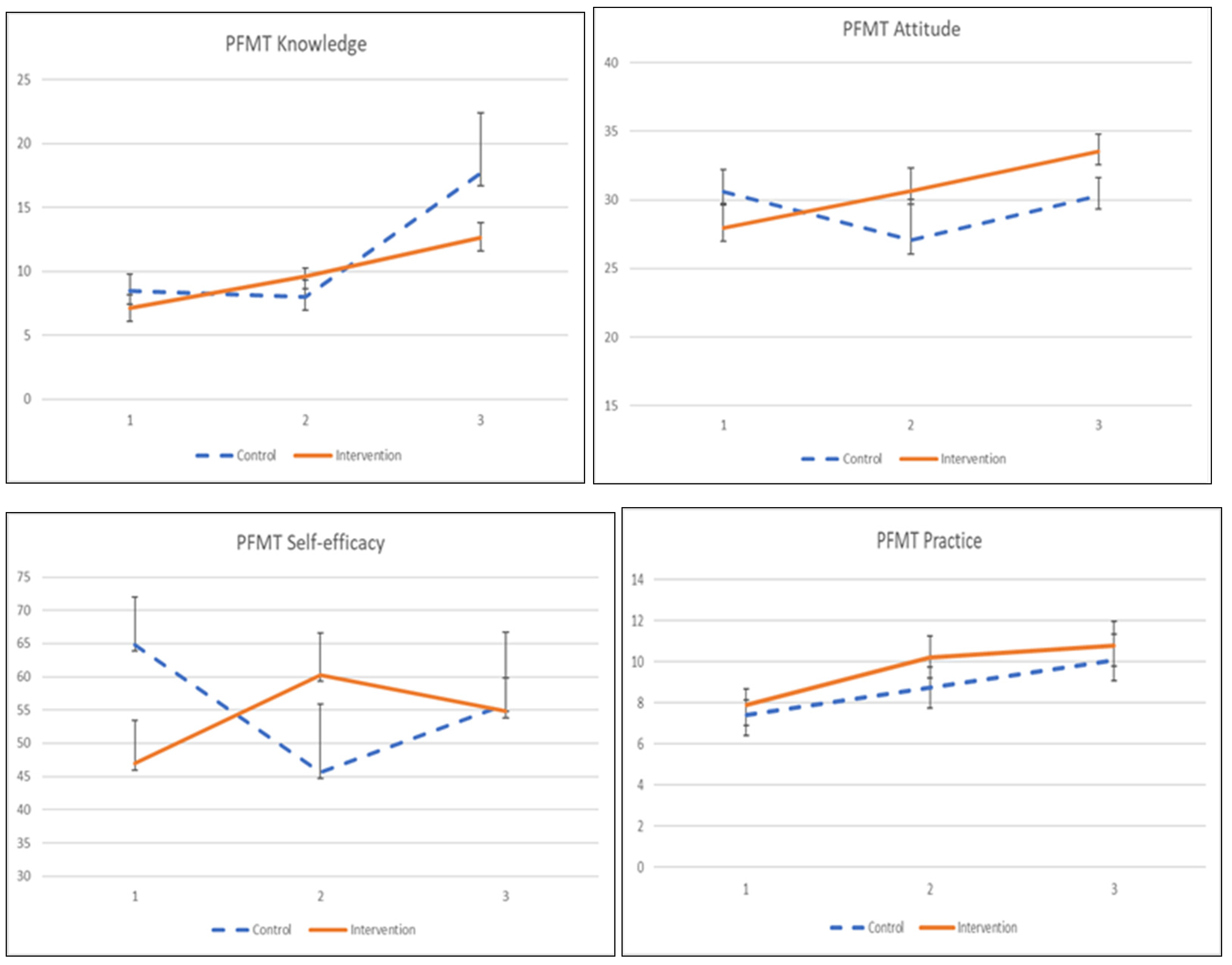
3.4.1. PFMT Knowledge, Attitude, Practice, and Self-Efficacy
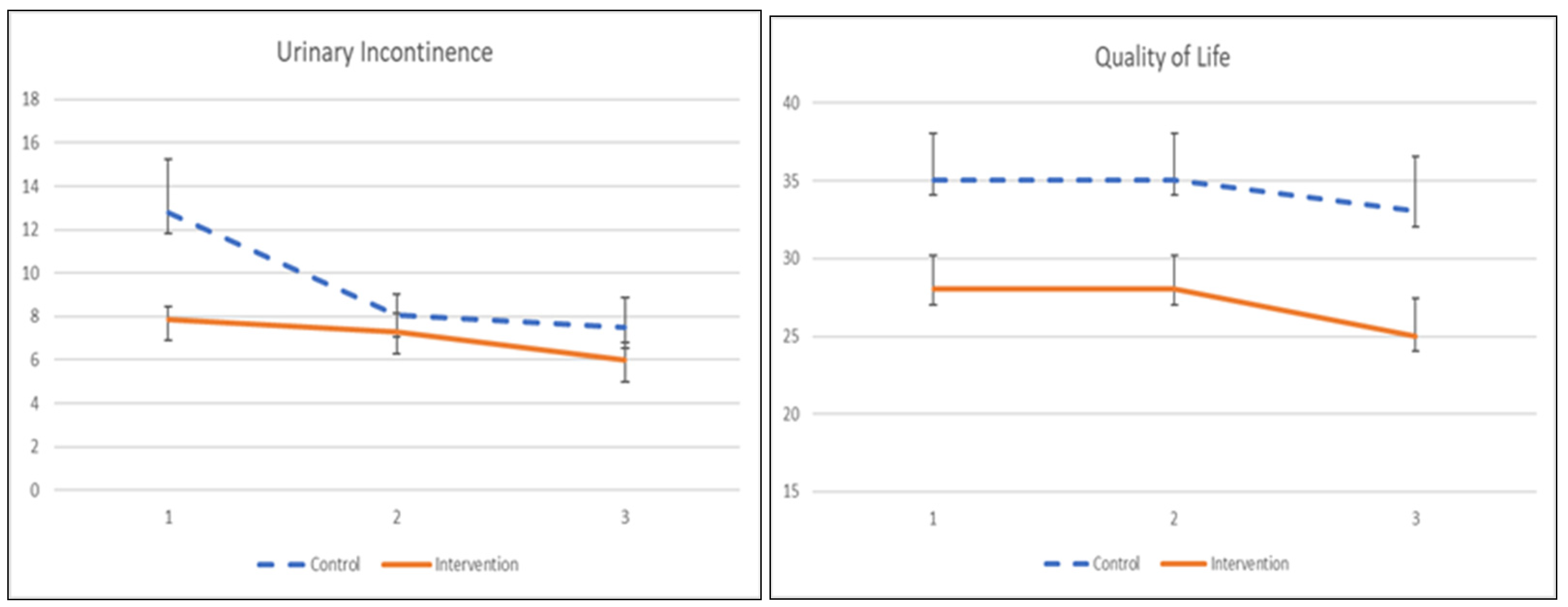
3.4.2. Urinary Incontinence and Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jacomo, R.H.; Nascimento, T.R.; Da Siva, M.L.; Salata, M.C.; Alves, A.T.; Da Cruz, P.R.C.; De Sousa, J.B. Exercise regimens other than pelvic floor muscle training cannot increase pelvic muscle strength-a systematic review. J. Bodyw. Mov. Ther. 2020, 24, 568–574. [Google Scholar] [CrossRef]
- Woodley, S.J.; Lawrenson, P.; Boyle, R.; Cody, J.D.; Mørkved, S.; Kernohan, A.; Hay-Smith, E.J.C. Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst. Rev. 2020, 2020, 1–216. [Google Scholar]
- Du, Y.; Xu, L.; Ding, L.; Wang, Y.; Wang, Z. The effect of antenatal pelvic floor muscle training on labor and delivery outcomes: A systematic review with meta-analysis. Int. Urogynecol. J. 2015, 26, 1415–1427. [Google Scholar] [CrossRef]
- Ren, S.; Gao, Y.; Yang, Z.; Li, J.; Xuan, R.; Liu, J.; Chen, X.; Thirupathi, A. The Effect of Pelvic Floor Muscle Training on Pelvic Floor Dysfunction in Pregnant and Postpartum Women. Phys. Act. Health 2020, 4, 130–141. [Google Scholar] [CrossRef]
- Hadizadeh-Talasaz, Z.; Sadeghi, R.; Khadivzadeh, T. Effect of pelvic floor muscle training on postpartum sexual function and quality of life: A systematic review and meta-analysis of clinical trials. Taiwan J. Obstet. Gynecol. 2019, 58, 737–747. [Google Scholar] [CrossRef]
- Abrams, P.; Andersson, K.; Apostolidis, A.; Birder, L.; Bliss, D.; Brubaker, L. 6th International Consultation on Incontinence. Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse and faecal incontinence. Neurourol. Urodyn. 2018, 37, 2271–2272. [Google Scholar] [CrossRef] [Green Version]
- Jaffar, A.; Mohd-Sidik, S.; Nien, F.C.; Fu, G.Q.; Talib, N.H. Urinary incontinence and its association with pelvic floor muscle exercise among pregnant women attending a primary care clinic in Selangor, Malaysia. PLoS ONE 2020, 15, e0236140. [Google Scholar] [CrossRef]
- Moossdorff-Steinhauser, H.F.A.; Berghmans, B.C.M.; Spaanderman, M.E.A.; Bols, E.M.J. Prevalence, incidence and bothersomeness of urinary incontinence in pregnancy: A systematic review and meta-analysis. Int. Urogynecol. J. 2021, 32, 1633–1652. [Google Scholar] [CrossRef]
- Moossdorff-Steinhauser, H.F.A.; Berghmans, B.C.M.; Spaanderman, M.E.A.; Bols, E.M.J. Urinary incontinence during pregnancy: Prevalence, experience of bother, beliefs, and help-seeking behavior. Int. Urogynecol. J. 2020, 32, 695–701. [Google Scholar] [CrossRef]
- Maeda, N.; Urabe, Y.; Suzuki, Y.; Hirado, D.; Morikawa, M.; Komiya, M.; Mizuta, R.; Naito, K.; Shirakawa, T. Cross-Sectional Study of the Prevalence and Symptoms of Urinary Incontinence among Japanese Older Adults: Associations with Physical Activity, Health-Related Quality of Life, and Well-Being. Int. J. Environ. Res. Public Health 2021, 18, 360. [Google Scholar] [CrossRef]
- Al Kiyumi, M.H.; Al Belushi, Z.I.; Jaju, S.; Al Mahrezi, A.M. Urinary Incontinence Among Omani Women: Prevalence, risk factors and impact on quality of life. Sultan Qaboos Univ. Med. J. 2020, 20, e45–e53. [Google Scholar] [CrossRef] [Green Version]
- Woodley, S.J.; Hay-Smith, E.J.C. Narrative review of pelvic floor muscle training for childbearing women—why, when, what, and how. Int. Urogynecol. J. 2021, 32, 1977–1988. [Google Scholar] [CrossRef]
- Terry, R.; Jarvie, R.; Hay-Smith, J.; Salmon, V.; Pearson, M.; Boddy, K.; MacArthur, C.; Dean, S. “Are you doing your pelvic floor?” An ethnographic exploration of the interaction between women and midwives about pelvic floor muscle exercises (PFME) during pregnancy. Midwifery 2020, 83, 102647. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Jin, Y.; Feng, S. Knowledge, attitude and practice of pelvic floor dysfunction among obstetrical healthcare workers in China: A cross-sectional study. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102068. [Google Scholar] [CrossRef]
- Salmon, V.; Hay-Smith, E.J.C.; Jarvie, R.; Dean, S.; Terry, R.; Frawley, H.; Oborn, E.; Bayliss, S.E.; Bick, D.; Davenport, C.; et al. Implementing pelvic floor muscle training in women’s childbearing years: A critical interpretive synthesis of individual, professional, and service issues. Neurourol. Urodyn. 2020, 39, 863–870. [Google Scholar] [CrossRef] [Green Version]
- Tanahashi, T. Health service coverage and its evaluation. Bull. World Health Organ. 1978, 56, 295–303. [Google Scholar]
- Iyawa, G.E.; Dansharif, A.R.; Khan, A. Mobile apps for self-management in pregnancy: A systematic review. Health Technol. 2021, 11, 283–294. [Google Scholar] [CrossRef]
- Rygh, P.; Asklund, I.; Samuelsson, E. Real-world effectiveness of app-based treatment for urinary incontinence: A cohort study. BMJ Open 2021, 11, e040819. [Google Scholar] [CrossRef]
- Jaffar, A.; Mohd-Sidik, S.; Chai Nien, F.; Admodisastro, N.; Abdul Salam, S.N.; Ismail, N.D. Improving Pelvic Floor Muscle Training Adherence Among Pregnant Women: Validation Study. JMIR Hum. Factors 2022, 9, e30989. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef] [Green Version]
- Thabane, L.; Hopewell, S.; Lancaster, G.A.; Bond, C.M.; Coleman, C.L.; Campbell, M.J.; Eldridge, S.M. Methods and processes for development of a CONSORT extension for reporting pilot randomized controlled trials. Pilot Feasibility Stud. 2016, 2, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaffar, A.; Sidik, S.M.; Foo, C.; Muhammad, N.; Manaf, R.A.; Ismail, S.F.; Suhaili, N. Protocol of a Single-Blind Two-Arm (Waitlist Control) Parallel-Group Randomised Controlled Pilot Feasibility Study for mHealth App among Incontinent Pregnant Women. Int. J. Environ. Res. Public Health 2021, 18, 4792. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Dunn, L. The Declaration of Helsinki on Medical Research involving Human Subjects: A Review of Seventh Revision. J. Nepal Health Res. Counc. 2020, 17, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Avery, K.; Donovan, J.; Peters, T.J.; Shaw, C.; Gotoh, M.; Abrams, P. ICIQ: A brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol. Urodyn. 2004, 23, 322–330. [Google Scholar] [CrossRef]
- Lim, R.; Liong, M.L.; Lau, Y.K.; Yuen, K.H. Validity, reliability, and responsiveness of the ICIQ-UI SF and ICIQ-LUTSqol in the Malaysian population. Neurourol. Urodyn. 2017, 36, 438–442. [Google Scholar] [CrossRef]
- Jaffar, A.; Sidik, S.M.; Admodisastro, N.; Mansor, E.I.; Fong, L.C. Expert’s Usability Evaluation of the Pelvic Floor Muscle Training mHealth App for Pregnant Women. Int. J. Adv. Comput. Sci. Appl. 2021, 12, 165–173. [Google Scholar] [CrossRef]
- Abbott, J.H. The Distinction Between Randomized Clinical Trials (RCTs) and Preliminary Feasibility and Pilot Studies: What They Are and Are Not. J. Orthop. Sports Phys. Ther. 2014, 44, 555–558. [Google Scholar] [CrossRef]
- Hutchesson, M.J.; Taylor, R.; Shrewsbury, V.A.; Vincze, L.; Campbell, L.E.; Callister, R.; Park, F.; Schumacher, T.L.; Collins, C.E. Be healthe for your heart: A pilot randomized controlled trial evaluating a web-based behavioral intervention to improve the cardiovascular health of women with a history of preeclampsia. Int. J. Environ. Res. Public Health 2020, 17, 5779. [Google Scholar] [CrossRef]
- Newman-Beinart, N.A.; Norton, S.; Dowling, D.; Gavriloff, D.; Vari, C.; Weinman, J.A.; Godfrey, E.L. The development and initial psychometric evaluation of a measure assessing adherence to prescribed exercise: The Exercise Adherence Rating Scale (EARS). Physiotherapy 2017, 103, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Rosediani, M.; Juliawati, M.; Norwati, D. Knowledge, attitude and practice towards pelvic floor muscle exercise among pregnant women attending antenatal clinic in Universiti Sains Malaysia Hospital, Malaysia. Int. Med. J. 2012, 19, 37–38. [Google Scholar]
- Sacomori, C.; Cardoso, F.L.; Porto, I.P.; Negri, N.B. The development and psychometric evaluation of a self-efficacy scale for practicing pelvic floor exercises. Braz. J. Phys. Ther. 2013, 17, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Leon, A.C.; Davis, L.L.; Kraemer, H.C. The role and interpretation of pilot studies in clinical research. J. Psychiatr. Res. 2011, 45, 626–629. [Google Scholar] [CrossRef] [Green Version]
- Billingham, S.A.; Whitehead, A.L.; Julious, S.A. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med. Res. Methodol. 2013, 13, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Day, S.J.; Altman, D.G. Statistics notes: Blinding in clinical trials and other studies. BMJ 2000, 321, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, G.A.; Barrett, K.C.; Leech, N.L.; Gloeckner, G.W. IBM SPSS for Introductory Statistics: Use and Interpretation; Routledge: London, UK, 2019. [Google Scholar]
- Abu-Bader, S.H. Using Statistical Methods in Social Science Research: With a Complete SPSS Guide; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Ma, Y.; Mazumdar, M.; Memtsoudis, S.G. Beyond repeated-measures analysis of variance: Advanced statistical methods for the analysis of longitudinal data in anesthesia research. Reg. Anesth. Pain Med. 2012, 37, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Firet, L.; Teunissen, T.A.; Kool, R.B.; van Doorn, L.; Aourag, M.; Lagro-Janssen, A.L.; Assendelft, W.J. Women’s adoption of a web-based intervention for stress urinary incontinence: A qualitative study. BMC Health Serv. Res. 2021, 21, 574. [Google Scholar] [CrossRef]
- Hay-Smith, J.; Dean, S.; Burgio, K.; McClurg, D.; Frawley, H.; Dumoulin, C. Pelvic-floor-muscle-training adherence “modifiers”: A review of primary qualitative studies-2011 ICS State-of-the-Science Seminar research paper III of IV. Neurourol. Urodyn. 2015, 34, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, A.L.; Julious, S.A.; Cooper, C.L.; Campbell, M.J. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat. Methods Med. Res. 2016, 25, 1057–1073. [Google Scholar] [CrossRef]
- Ali, S.H.; Foreman, J.; Capasso, A.; Jones, A.M.; Tozan, Y.; DiClemente, R.J. Social media as a recruitment platform for a nationwide online survey of COVID-19 knowledge, beliefs, and practices in the United States: Methodology and feasibility analysis. BMC Med. Res. Methodol. 2020, 20, 116. [Google Scholar] [CrossRef]
- Cooper, C.L.; Whitehead, A.; Pottrill, E.; Julious, S.A.; Walters, S.J. Are pilot trials useful for predicting randomisation and attrition rates in definitive studies: A review of publicly funded trials. Clin. Trials 2018, 15, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.R.; Salisbury, A.L.; Uebelacker, L.A.; Abrantes, A.M.; Battle, C.L. Stress, coping and silver linings: How depressed perinatal women experienced the COVID-19 pandemic. J. Affect. Disord. 2022, 298 Pt A, 329–336. [Google Scholar] [CrossRef]
- Robinson, K.A.; Dinglas, V.D.; Sukrithan, V.; Yalamanchilli, R.; Mendez-Tellez, P.A.; Dennison-Himmelfarb, C.; Needham, D.M. Updated systematic review identifies substantial number of retention strategies: Using more strategies retains more study participants. J. Clin. Epidemiol. 2015, 68, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xu, X.; Luo, J.; Chen, Z.; Feng, S. Effect of app-based audio guidance pelvic floor muscle training on treatment of stress urinary incontinence in primiparas: A randomized controlled trial. Int. J. Nurs. Stud. 2020, 104, 103527. [Google Scholar] [CrossRef] [PubMed]
- Bø, K. Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World J. Urol. 2012, 30, 437–443. [Google Scholar] [CrossRef]
- Zubieta, M.; Carr, R.L.; Drake, M.J.; Bø, K. Influence of voluntary pelvic floor muscle contraction and pelvic floor muscle training on urethral closure pressures: A systematic literature review. Int. Urogynecol. J. 2016, 27, 687–696. [Google Scholar] [CrossRef]
- Braekken, I.H.; Majida, M.; Engh, M.E.; Bø, K.; Brækken, I.H. Test-retest reliability of pelvic floor muscle contraction measured by 4D ultrasound. Neurourol. Urodyn. 2009, 28, 68–73. [Google Scholar] [CrossRef]
- Sacomori, C.; Zomkowski, K.; Porto, I.D.P.; Cardoso, F.L.; Sperandio, F.F. Adherence and effectiveness of a single instruction of pelvic floor exercises: A randomized clinical trial. Int. Urogynecol. J. 2019, 31, 951–959. [Google Scholar] [CrossRef]
- Pt, C.S.; Berghmans, B.; De Bie, R.; Mesters, I.; Cardoso, F.L. Predictors for adherence to a home-based pelvic floor muscle exercise program for treating female urinary incontinence in Brazil. Physiother. Theory Pract. 2018, 36, 186–195. [Google Scholar]
- Araujo, C.C.; Marques, A.D.A.; Juliato, C.R. The Adherence of Home Pelvic Floor Muscles Training Using a Mobile Device Application for Women with Urinary Incontinence: A Randomized Controlled Trial. Female Pelvic Med. Reconstr. Surg. 2020, 26, 697–703. [Google Scholar] [CrossRef]
- Sidik, S.M.; Jaffar, A.; Foo, C.N.; Muhammad, N.A.; Manaf, R.A.; Ismail, S.I.F.; Alagirisamy, P.; Fazlah, A.F.A.; Suli, Z.; Goodyear-Smith, F. KEPT-app trial: A pragmatic, single-blind, parallel, cluster-randomised effectiveness study of pelvic floor muscle training among incontinent pregnant women: Study protocol. BMJ Open 2021, 11, e039076. [Google Scholar] [CrossRef] [PubMed]
- Jaffar, A.; Mohd-Sidik, S.; Abd Manaf, R.; Foo, C.N.; Gan, Q.F.; Saad, H. Quality of life among pregnant women with urinary incontinence: A cross-sectional study in a Malaysian primary care clinic. PLoS ONE 2021, 16, e0250714. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Malaysian citizen | Non-Malaysian citizen (due to Non-Malay speaking) |
| Mobile phone (Android) and internet access | Mobile phone (iPhone) |
| Pregnant woman | Planning to be pregnant or post-partum woman |
| Age more than 18 years | Age less than 18 years (Teenage pregnancy) |
| Any parity at 26–27 weeks gestation | Chronic medical problem(s) before pregnancy |
| Stress UI or Mixed UI (International Consultation on Incontinence Questionnaire-UI-Short Form [24,25]) | Urge UI Complicated pregnancy (not advisable to perform PFMT) |
| Outcome | Description |
|---|---|
| Primary outcome: | |
| PFMT Adherence | Increasing PFMT adherence from lowest score (0) to maximum score (24) of Exercise Adherence Rating Scale (EARS) [29]. |
| Secondary outcomes: | |
| Urinary incontinence | Severity urinary incontinence symptoms using the International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-UI SF) [24,25]. |
| Quality of Life | To assess the quality of life among pregnant women with UI at baseline, one-month, and two-month post-intervention. International Consultation on Incontinence Questionnaire Urinary Incontinence-Lower Urinary Tract Symptom quality of life (ICIQ-LUTSqol) [24,25]. |
| PFMT Knowledge, Attitude, and Practice | To assess the knowledge, attitude, and practices towards PFMT at baseline, one-month, and two-month post-intervention. Knowledge, attitude, and practice towards pelvic floor muscle training [30] |
| PFMT Self-efficacy | To measure the self-efficacy score at baseline, one-month, and two-month post-intervention. Self-Efficacy Scale For Practicing Pelvic Floor Exercise Questionnaire (SESPPFE) [31] |
| Characteristics | Overall (n = 26) | Baseline Comparison | Test | p-Value | |
|---|---|---|---|---|---|
| Intervention Group (n = 16) | Control Group (n = 10) | ||||
| Age (year), M ± SD | 29.5 ± 4.8 | 29.7 ± 3.9 | 29.1 ± 6.2 | t | 0.772 |
| BMI (kg m2), M ± SD | 28.2 ± 3.9 | 28.7 ± 4.1 | 27.4 ± 3.7 | t | 0.428 |
| Ethnicity, % (n) | |||||
| Malay | 92.3 (24) | 93.8 (15) | 90.0 (9) | ||
| Non-Malay | 7.7 (2) | 6.2 (1) | 10.0 (1) | FET | 1.0 |
| Education, % (n) | |||||
| Primary and Secondary | 30.8 (8) | 31.3 (5) | 30.0 (3) | ||
| College/University | 69.2 (18) | 68.7 (11) | 70.0 (7) | FET | 1.0 |
| Occupational, % (n) | |||||
| Unemployed | 30.8 (8) | 25.0 (4) | 40.0 (4) | ||
| Employed | 69.2 (18) | 75.0 (12) | 60.0 (6) | FET | 0.664 |
| Parity, % (n) | |||||
| Nulliparous | 34.6 (9) | 37.5 (6) | 30.0 (3) | ||
| Multiparous ≥ 1 | 65.4 (17) | 62.5 (10) | 70.0 (7) | FET | 1.0 |
| Type of UI, % (n) | |||||
| SUI | 57.7 (15) | 62.5 (10) | 50.0 (5) | ||
| MUI | 42.3 (11) | 37.5 (6) | 50.0 (5) | FET | 0.689 |
| Outcome Measures | Overall (n = 26) | Baseline Comparison | Test | p-Value | |
|---|---|---|---|---|---|
| Intervention Group (n = 85) | Control Group (n = 85) | ||||
| UI Severity Score | |||||
| Median (IQR) | 10.00 (5.0) | 7.50 (4.0) | 11.50 (6.0) | MWT | 0.031 * |
| M ± SD | 9.77 ± 5.76 | 7.88 ± 2.34 | 12.80 ± 8.15 | ||
| Quality of life | |||||
| Median (IQR) | 30.50 (12.00) | 29.50 (6.5) | 36.50 (16.75) | MWT | 0.135 |
| M ± SD | 32.85 ± 8.71 | 30.25 ± 5.95 | 37.00 ± 10.97 | ||
| PFMT Knowledge Score | |||||
| Median (IQR) | 7.50 (6.75) | 8.00 (8.25) | 7.50 (6.0) | t | 0.497 |
| M ± SD | 7.62 ±4.46 | 7.125 ± 4.43 | 8.40 ± 4.65 | ||
| PFMT Attitude Score | |||||
| Median (IQR) | 31.00 (5.5) | 31.0 (6.0) | 31.5 (9.25) | MWT | 0.391 |
| M ± SD | 29.01 ± 6.23 | 30.45 ± 5.20 | 30.60 ± 4.97 | ||
| PFMT Practice Score | |||||
| Median (IQR) | 8.00 (5.00) | 8.0 (5.0) | 8.0 (4.5) | MWT | 0.698 |
| M ± SD | 7.77 ± 2.82 | 7.94 ± 3.07 | 7.40 ± 2.50 | ||
| PFMT Self-Efficacy Score | |||||
| Median (IQR) | 53.53 (43.68) | 51.18 (47.94) | 60.88 (42.65) | t | 0.475 |
| M ± SD | 51.47± 26.21 | 48.49 ± 27.51 | 56.24 ± 24.62 | ||
| PFMT Adherence | |||||
| Median (IQR) | 15.00 (4.50) | 14.50 (6.8) | 15.00 (3.00) | t | 0.832 |
| M ± SD | 13.81 ± 4.23 | 13.25 ± 5.12 | 14.70 ± 2.50 | ||
| Outcome Measures | β | SE | 95%CI | p |
|---|---|---|---|---|
| PFMT Adherence | ||||
| Group effect a | 0.442 | 2.4276 | −4.316 to 5.200 | 0.052 |
| Time 2 | 2.369 | 1.0113 | −4.442 to 5.126 | 0.889 |
| Time 3 | 0.033 | 1.0113 | 0.387 to 4.351 | 0.019 * |
| Group*time 2 b | 1.154 | 2.7036 | −4.145 to 6.453 | 0.670 |
| Group*time 3 b | −2.910 | 2.8799 | −8.554 to 2.735 | 0.312 |
| Outcome Measures | β | SE | 95%CI | p |
|---|---|---|---|---|
| PFMT knowledge | ||||
| Group effect a | −1.343 | 1.6716 | −4.619 to 1.933 | 0.422 |
| Time 2 | −0.473 | 0.6801 | −1.806 to 0.860 | 0.487 |
| Time 3 | 9.218 | 5.5097 | −1.5812 to 0.017 | 0.094 |
| Group*time 2 b | 2.968 | 1.1630 | 0.688 to 5.247 | 0.011 * |
| Group*time 3 b | −3.708 | 5.8277 | −15.130 to 7.714 | 0.525 |
| PFMT attitude | ||||
| Group effect a | −2.642 | 2.2175 | −6.988 to 1.704 | 0.233 |
| Time 2 | −3.552 | 2.8517 | −9.141 to 2.037 | 0.213 |
| Time 3 | −0.309 | 1.9151 | −4.062 to 3.445 | 0.872 |
| Group*time 2 b | 6.246 | 4.0333 | −1.659 to 4.151 | 0.121 |
| Group*time 3 b | 5.884 | 2.7729 | 0.449 to 11.319 | 0.034 * |
| PFMT practice | ||||
| Group effect a | 0.530 | 1.0594 | −1.547 to 2.606 | 0.617 |
| Time 2 | 1.352 | 0.6657 | 0.048 to 2.657 | 0.042 |
| Time 3 | 2.668 | 1.1254 | 0.463 to 4.874 | 0.018 * |
| Group*time 2 b | 0.924 | 1.1624 | −1.355 to 3.202 | 0.427 |
| Group*time 3 b | 0.179 | 1.6070 | −2.971 to 3.329 | 0.911 |
| PFMT Self-efficacy | ||||
| Group effect a | −17.916 | 10.2310 | −37.968 to 2.137 | 0.080 |
| Time 2 | −19.178 | 14.2831 | −47.172 to 8.817 | 0.179 |
| Time 3 | −9.049 | 10.9787 | −30.567 to 12.469 | 0.410 |
| Group*time 2 b | 32.541 | 15.7129 | 1.745 to 63.338 | 0.038 * |
| Group*time 3 b | 16.939 | 14.4040 | −11.293 to 45.170 | 0.240 |
| Outcome Measures | β | SE | 95%CI | p |
|---|---|---|---|---|
| UI Symptom severity | ||||
| Group effect a | −4.989 | 2.4942 | −9.878 to −0.101 | 0.045 * |
| Time 2 | −4.748 | 2.4159 | −9.483 to −0.013 | 0.049 * |
| Time 3 | −5.389 | 3.0569 | −11.380 to 0.603 | 0.078 |
| Group*time 2 b | 4.172 | 2.4941 | −0.717 to 9.060 | 0.094 |
| Group*time 3 b | 3.498 | 3.1421 | −2.661 to 9.656 | 0.266 |
| Quality of Life | ||||
| Group effect a | −7.048 | 3.2752 | −13.467 to −0.628 | 0.031 * |
| Time 2 | 0.000 | 0.0002 | −0.001 to 0.000 | 0.328 |
| Time 3 | −2.000 | 2.2608 | 2.431 to 0.783 | 0.376 |
| Group*time 2 b | 0 | 0.0002 | 0.000 to 0.001 | 0.253 |
| Group*time 3 b | −1.000 | 2.6965 | −6.285 to 4.285 | 0.137 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaffar, A.; Mohd Sidik, S.; Foo, C.N.; Muhammad, N.A.; Abdul Manaf, R.; Suhaili, N. Preliminary Effectiveness of mHealth App-Based Pelvic Floor Muscle Training among Pregnant Women to Improve Their Exercise Adherence: A Pilot Randomised Control Trial. Int. J. Environ. Res. Public Health 2022, 19, 2332. https://doi.org/10.3390/ijerph19042332
Jaffar A, Mohd Sidik S, Foo CN, Muhammad NA, Abdul Manaf R, Suhaili N. Preliminary Effectiveness of mHealth App-Based Pelvic Floor Muscle Training among Pregnant Women to Improve Their Exercise Adherence: A Pilot Randomised Control Trial. International Journal of Environmental Research and Public Health. 2022; 19(4):2332. https://doi.org/10.3390/ijerph19042332
Chicago/Turabian StyleJaffar, Aida, Sherina Mohd Sidik, Chai Nien Foo, Noor Azimah Muhammad, Rosliza Abdul Manaf, and Nazhatussima Suhaili. 2022. "Preliminary Effectiveness of mHealth App-Based Pelvic Floor Muscle Training among Pregnant Women to Improve Their Exercise Adherence: A Pilot Randomised Control Trial" International Journal of Environmental Research and Public Health 19, no. 4: 2332. https://doi.org/10.3390/ijerph19042332
APA StyleJaffar, A., Mohd Sidik, S., Foo, C. N., Muhammad, N. A., Abdul Manaf, R., & Suhaili, N. (2022). Preliminary Effectiveness of mHealth App-Based Pelvic Floor Muscle Training among Pregnant Women to Improve Their Exercise Adherence: A Pilot Randomised Control Trial. International Journal of Environmental Research and Public Health, 19(4), 2332. https://doi.org/10.3390/ijerph19042332







