Removal of Inorganic Salts in Municipal Solid Waste Incineration Fly Ash Using a Washing Ejector and Its Application for CO2 Capture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Properties of Municipal Solid Waste IFA
2.2. Experimental Equipment and Conditions
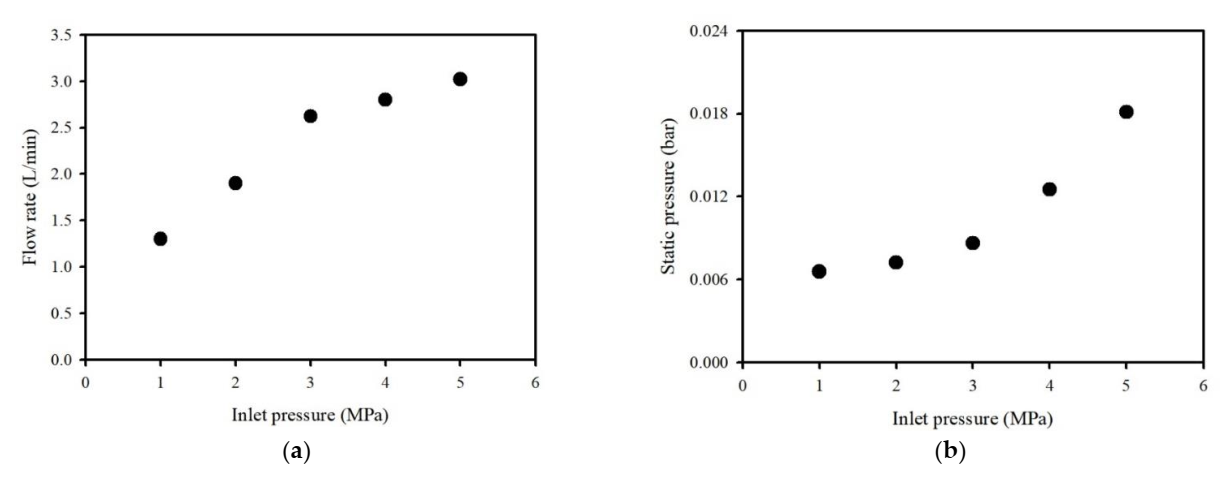
2.2.1. Washing Ejector
2.2.2. Characteristics of the Washed IFA Residue
2.3. Carbonation Reaction for the CO2 Capture from the Washing Effluent
2.4. Analytical Methods
3. Results
3.1. Removal of Inorganic Salts of the IFA using a Washing Ejector
3.2. Characteristics of the Effluent after Washing
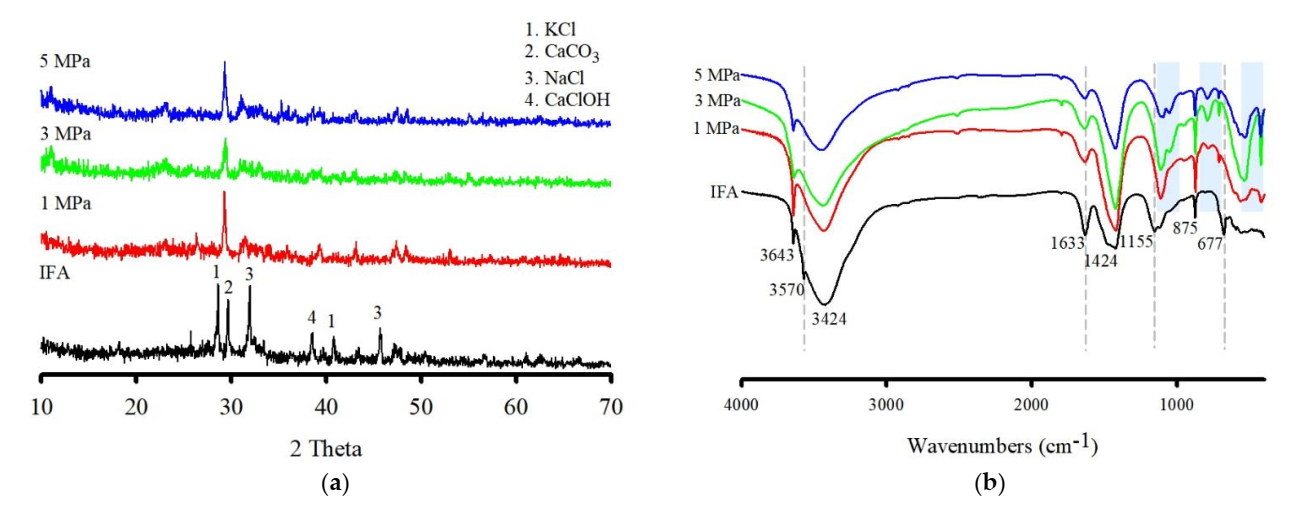
3.3. Characteristics of the Washed IFA Residue
3.3.1. TGA
3.3.2. The pH Leaching Characteristics
3.3.3. SEM
4. Discussion
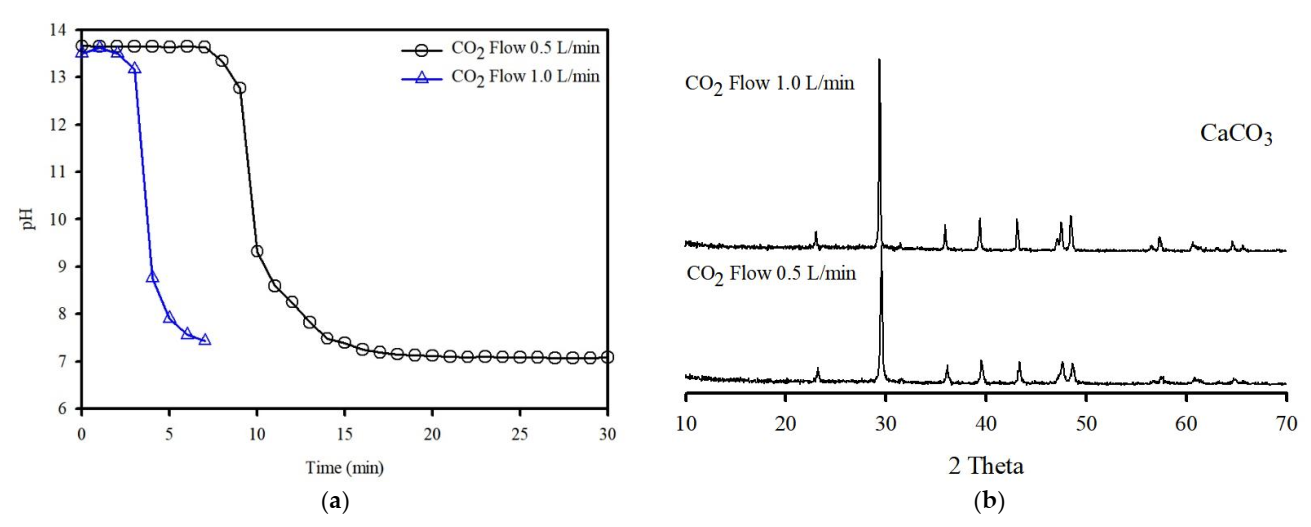
4.1. Carbonation for CO2 Capture on the Washing Effluent
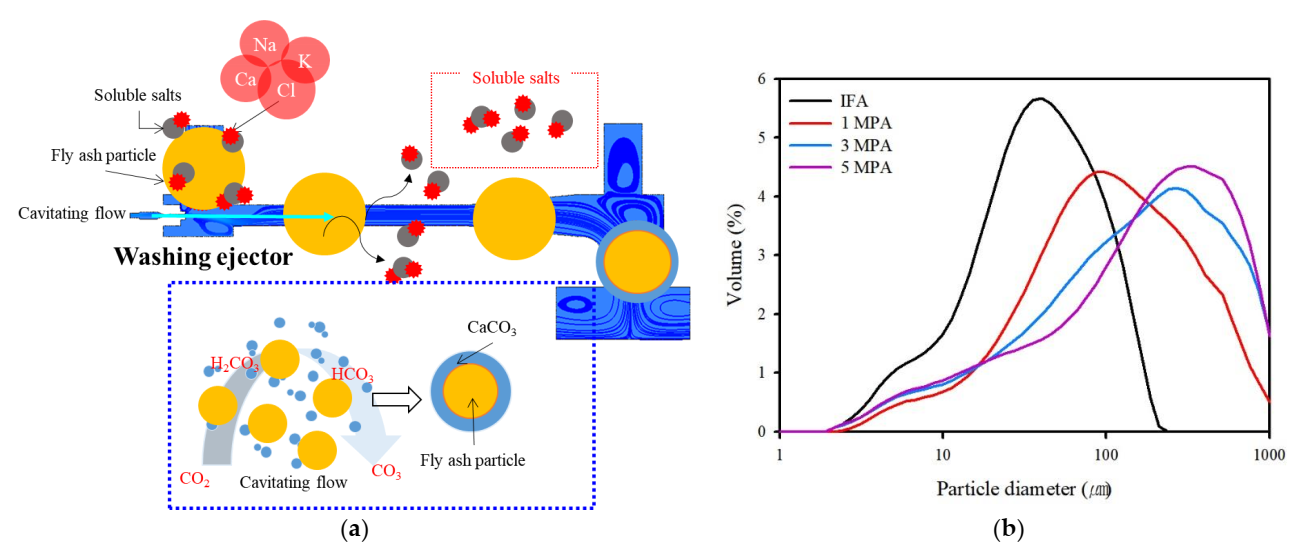
4.2. Effect on the IFA by Cavitation Bubbles and Prospects
5. Conclusions
- When a washing ejector is used for the removal of inorganic salts from the IFA, the contribution to the removal of inorganic salts is even high at fluid pressure, and a larger water-to-solid ratio can promote contact between the solids and water.
- The cavitation bubble influences the particles, and it can produce clean IFA surfaces. In addition, HCO3 can be formed while the cavitation flow in the washing ejector process reacts with CO2 in ambient air. As such, HCO3 can react with free Ca in the solution to form amorphous CaCO3.
- The washing effluent plays a major role in CO2 sequestration, thereby providing an abundant quantity of Ca and may be sufficiently reactive for use as a medium in the formation of CaCO3.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Park, J.; Tae, S.; Kim, T. Life cycle CO2 assessment of concrete by compressive strength on construction site in Korea. Renew. Sustain. Energy Rev. 2012, 16, 2940–2946. [Google Scholar] [CrossRef]
- Wee, J.-H. A review on carbon dioxide capture and storage technology using coal fly ash. Appl. Energy 2013, 106, 143–151. [Google Scholar] [CrossRef]
- Dindi, A.; Quang, D.V.; Vega, L.F.; Nashef, E.; Abu-Zahra, M.R. Applications of fly ash for CO2 capture, utilization, and storage. J. CO2 Util. 2019, 29, 82–102. [Google Scholar] [CrossRef]
- Choi, D.; Jung, S.; Jeon, Y.J.; Moon, D.H.; Kwon, E.E. Study on carbon rearrangements of CO2 co-feeding pyrolysis of corn stover and oak wood. J. CO2 Util. 2020, 42, 101320. [Google Scholar] [CrossRef]
- Dananjayan, R.R.T.; Kandasamy, P.; Andimuthu, R. Direct mineral carbonation of coal fly ash for CO2 sequestration. J. Clean. Prod. 2016, 112, 4173–4182. [Google Scholar] [CrossRef]
- Lee, T.; Jung, S.; Park, Y.-K.; Kim, T.; Wang, H.; Moon, D.H.; Kwon, E.E. Catalytic pyrolysis of polystyrene over steel slag under CO2 environment. J. Hazard. Mater. 2020, 395, 122576. [Google Scholar] [CrossRef]
- Allegrini, E.; Vadenbo, C.; Boldrin, A.; Astrup, T.F. Life cycle assessment of resource recovery from municipal solid waste incineration bottom ash. J. Environ. Manag. 2015, 151, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Yi, S.; Lim, H.S. Evaluation of the eco-efficiency of waste treatment facilities in Korea. J. Hazard. Mater. 2021, 411, 125040. [Google Scholar] [CrossRef]
- Hemalatha, T.; Ramaswamy, A. A review on fly ash characteristics–Towards promoting high volume utilization in developing sustainable concrete. J. Clean. Prod. 2017, 147, 546–559. [Google Scholar] [CrossRef]
- Gu, Q.; Wang, T.; Wu, W.; Wang, D.; Jin, B. Influence of pretreatments on accelerated dry carbonation of MSWI fly ash under medium temperatures. Chem. Eng. J. 2021, 414, 128756. [Google Scholar] [CrossRef]
- Li, J.; Dong, Z.; Yang, E.-H. Strain hardening cementitious composites incorporating high volumes of municipal solid waste incineration fly ash. Constr. Build. Mater. 2017, 146, 183–191. [Google Scholar] [CrossRef]
- Tang, P.; Chen, W.; Xuan, D.; Cheng, H.; Poon, C.S.; Tsang, D.C. Immobilization of hazardous municipal solid waste incineration fly ash by novel alternative binders derived from cementitious waste. J. Hazard. Mater. 2020, 393, 122386. [Google Scholar] [CrossRef]
- Yakubu, Y.; Zhou, J.; Shu, Z.; Zhang, Y.; Wang, W.; Mbululo, Y. Potential application of pre-treated municipal solid waste incineration fly ash as cement supplement. Environ. Sci. Pollut. Res. 2018, 25, 16167–16176. [Google Scholar] [CrossRef]
- Zou, D.; Fan, W.; Xu, J.; Drioli, E.; Chen, X.; Qiu, M.; Fan, Y. One-step engineering of low-cost kaolin/fly ash ceramic membranes for efficient separation of oil-water emulsions. J. Membr. Sci. 2021, 621, 118954. [Google Scholar] [CrossRef]
- Nowak, B.; Rocha, S.F.; Aschenbrenner, P.; Rechberger, H.; Winter, F. Heavy metal removal from MSW fly ash by means of chlorination and thermal treatment: Influence of the chloride type. Chem. Eng. J. 2012, 179, 178–185. [Google Scholar] [CrossRef]
- Dou, X.; Ren, F.; Nguyen, M.Q.; Ahamed, A.; Yin, K.; Chan, W.P.; Chang, V.W.-C. Review of MSWI bottom ash utilization from perspectives of collective characterization, treatment and existing application. Renew. Sustain. Energy Rev. 2017, 79, 24–38. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, S.; Ji, R.; Liu, L.; Wang, X.; Zhang, Z. Effect of water-washing on the co-removal of chlorine and heavy metals in air pollution control residue from MSW incineration. Waste Manag. 2017, 68, 221–231. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, L.; Li, X.; Wang, W.; Xue, Y.; Zhu, X.; Hu, H.; Luo, G.; Naruse, I.; Bai, Z. Na&Ca removal from Zhundong coal by a novel CO2-water leaching method and the ashing behavior of the leached coal. Fuel 2017, 210, 8–14. [Google Scholar]
- Ding, L.; Gao, Y.; Li, X.; Wang, W.; Xue, Y.; Zhu, X.; Xu, K.; Hu, H.; Luo, G.; Naruse, I. A novel CO2-water leaching method for AAEM removal from Zhundong coal. Fuel 2019, 237, 786–792. [Google Scholar] [CrossRef]
- Dontriros, S.; Likitlersuang, S.; Janjaroen, D. Mechanisms of chloride and sulfate removal from municipal-solid-waste-incineration fly ash (MSWI FA): Effect of acid-base solutions. Waste Manag. 2020, 101, 44–53. [Google Scholar] [CrossRef]
- Wang, X.; Gao, M.; Wang, M.; Wu, C.; Wang, Q.; Wang, Y. Chloride removal from municipal solid waste incineration fly ash using lactic acid fermentation broth. Waste Manag. 2021, 130, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Zhang, K.; Yang, T.; Deng, D.; Qiao, J.; Wang, P.; You, Y.; Du, L.; Chen, G.; Kołodyńska, D. The influence of a washing pretreatment containing phosphate anions on single-mode microwave-based detoxification of fly ash from municipal solid waste incinerators. Chem. Eng. J. 2020, 387, 124053. [Google Scholar] [CrossRef]
- Harby, K.; Chiva, S.; Muñoz-Cobo, J.-L. An experimental study on bubble entrainment and flow characteristics of vertical plunging water jets. Exp. Therm. Fluid Sci. 2014, 57, 207–220. [Google Scholar] [CrossRef]
- Li, M.; Bussonnière, A.; Bronson, M.; Xu, Z.; Liu, Q. Study of Venturi tube geometry on the hydrodynamic cavitation for the generation of microbubbles. Miner. Eng. 2019, 132, 268–274. [Google Scholar] [CrossRef]
- Shi, H.; Li, M.; Nikrityuk, P.; Liu, Q. Experimental and numerical study of cavitation flows in venturi tubes: From CFD to an empirical model. Chem. Eng. Sci. 2019, 207, 672–687. [Google Scholar] [CrossRef]
- Wang, B.; He, R.; Chen, M.; Pi, S.; Zhang, F.; Zhou, A.; Zhang, Z. Intensification on mass transfer between gas and liquid in fine bubble jet reactor. J. Environ. Chem. Eng. 2021, 9, 104718. [Google Scholar] [CrossRef]
- Song, L.; Yang, J.; Yu, S.; Xu, M.; Liang, Y.; Pan, X.; Yao, L. Ultra-high efficient hydrodynamic cavitation enhanced oxidation of nitric oxide with chlorine dioxide. Chem. Eng. J. 2019, 373, 767–779. [Google Scholar] [CrossRef]
- Cho, K.; Kim, H.; Purev, O.; Choi, N.; Lee, J. Physical Separation of Contaminated Soil Using a Washing Ejector Based on Hydrodynamic Cavitation. Sustainability 2022, 14, 252. [Google Scholar] [CrossRef]
- Kim, H.; Cho, K.; Purev, O.; Choi, N.; Lee, J. Remediation of Toxic Heavy Metal Contaminated Soil by Combining a Washing Ejector Based on Hydrodynamic Cavitation and Soil Washing Process. Int. J. Environ. Res. Public Health 2022, 19, 786. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Extra CO2 capture and storage by carbonation of biomass ashes. Energy Convers. Manag. 2020, 204, 112331. [Google Scholar] [CrossRef]
- Shi, D.; Hu, C.; Zhang, J.; Li, P.; Zhang, C.; Wang, X.; Ma, H. Silicon-aluminum additives assisted hydrothermal process for stabilization of heavy metals in fly ash from MSW incineration. Fuel Process. Technol. 2017, 165, 44–53. [Google Scholar] [CrossRef]
- Oh, C.; Rhee, S.; Oh, M.; Park, J. Removal characteristics of As (III) and As (V) from acidic aqueous solution by steel making slag. J. Hazard. Mater. 2012, 213, 147–155. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; Yin, H.; Chen, J.; Zhang, N. Intermediate-calcium based cementitious materials prepared by MSWI fly ash and other solid wastes: Hydration characteristics and heavy metals solidification behavior. J. Hazard. Mater. 2018, 349, 262–271. [Google Scholar] [CrossRef]
- Pratiwi, W.D.; Ekaputri, J.J.; Fansuri, H. Combination of precipitated-calcium carbonate substitution and dilute-alkali fly ash treatment in a very high-volume fly ash cement paste. Constr. Build. Mater. 2020, 234, 117273. [Google Scholar] [CrossRef]
- Mozgawa, W.; Król, M.; Dyczek, J.; Deja, J. Investigation of the coal fly ashes using IR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 889–894. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Jarvis, M.; Hitchins, T. Kinetics and mechanism of mineral carbonation of olivine for CO2 sequestration. Miner. Eng. 2019, 131, 185–197. [Google Scholar] [CrossRef]
- Gu, S.; Fu, B.; Ahn, J.-W.; Fang, B. Mechanism for phosphorus removal from wastewater with fly ash of municipal solid waste incineration, Seoul, Korea. J. Clean. Prod. 2021, 280, 124430. [Google Scholar] [CrossRef]
- Gilbert, K.; Bennett, P.C.; Wolfe, W.; Zhang, T.; Romanak, K.D. CO2 solubility in aqueous solutions containing Na+, Ca2+, Cl−, SO42− and HCO3-: The effects of electrostricted water and ion hydration thermodynamics. Appl. Geochem. 2016, 67, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Sun, W.; Hu, Y.; Gao, Z.; Liu, R.; Zhang, Q.; Liu, H.; Meng, X. The utilization of waste by-products for removing silicate from mineral processing wastewater via chemical precipitation. Water Res. 2017, 125, 318–324. [Google Scholar] [CrossRef]




| Ca | Cl | Na | K | Si | S | Mg |
|---|---|---|---|---|---|---|
| 31.2 | 22.9 | 7.6 | 4.3 | 2.1 | 2.0 | 1.1 |
| Pb * | Cd * | As * | Cu * | Zn * | Cr * | Fe * |
| 9313.3 | 1795.5 | 167.9 | 8213.3 | 88,609.2 | 2456.6 | 204,518.4 |
| Composition | Untreated | Water-to-Solid Ratio | ||||
|---|---|---|---|---|---|---|
| IFA | 1:1 | 1:1 | 1:1 | 1.5:1 | 2:1 | |
| 1 MPa | 3 MPa | 5 MPa | 5 MPa | 5 MPa | ||
| Cl | 22.9 | 5.01 | 3.47 | 3.06 | 2.72 | 1.52 |
| Na2O | 10.3 | 2.03 | 1.93 | 1.54 | 1.37 | 1.22 |
| K2O | 5.20 | 1.68 | 1.23 | 1.13 | 1.01 | 0.81 |
| CaO | 43.6 | 57.7 | 52.1 | 51.6 | 53.3 | 52.7 |
| SiO2 | 4.55 | 8.66 | 8.68 | 9.1 | 8.96 | 9.80 |
| Al2O3 | 1.79 | 4.24 | 9.85 | 9.9 | 10.5 | 10.1 |
| MgO | 1.82 | 3.91 | 3.14 | 3.0 | 3.31 | 3.18 |
| Fe2O3 | 1.34 | 2.85 | 4.62 | 5.9 | 4.45 | 6.86 |
| TiO2 | 1.31 | 3.31 | 4.37 | 4.5 | 4.39 | 4.64 |
| SO3 | 5.02 | 6.67 | 6.16 | 5.7 | 5.83 | 5.61 |
| pH | EC (S/m) | Cl (g/L) | Br (g/L) | SO4 (g/L) | Ca (g/L) | Na (g/L) | K (g/L) |
|---|---|---|---|---|---|---|---|
| 13.8 | 3.3 | 19.84 | 0.45 | 1.29 | 5.24 | 3.10 | 2.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Purev, O.; Cho, K.; Choi, N.; Lee, J.; Yoon, S. Removal of Inorganic Salts in Municipal Solid Waste Incineration Fly Ash Using a Washing Ejector and Its Application for CO2 Capture. Int. J. Environ. Res. Public Health 2022, 19, 2306. https://doi.org/10.3390/ijerph19042306
Kim H, Purev O, Cho K, Choi N, Lee J, Yoon S. Removal of Inorganic Salts in Municipal Solid Waste Incineration Fly Ash Using a Washing Ejector and Its Application for CO2 Capture. International Journal of Environmental Research and Public Health. 2022; 19(4):2306. https://doi.org/10.3390/ijerph19042306
Chicago/Turabian StyleKim, Hyunsoo, Oyunbileg Purev, Kanghee Cho, Nagchoul Choi, Jaewon Lee, and Seongjin Yoon. 2022. "Removal of Inorganic Salts in Municipal Solid Waste Incineration Fly Ash Using a Washing Ejector and Its Application for CO2 Capture" International Journal of Environmental Research and Public Health 19, no. 4: 2306. https://doi.org/10.3390/ijerph19042306
APA StyleKim, H., Purev, O., Cho, K., Choi, N., Lee, J., & Yoon, S. (2022). Removal of Inorganic Salts in Municipal Solid Waste Incineration Fly Ash Using a Washing Ejector and Its Application for CO2 Capture. International Journal of Environmental Research and Public Health, 19(4), 2306. https://doi.org/10.3390/ijerph19042306





