Associations between Biomarkers of Metal Exposure and Dry Eye Metrics in Shipyard Welders: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Collection Procedures
2.4. Personal Information and Dry Eye Questionnaires
2.5. Environmental Factor Monitoring
2.6. Biochemistry Test
2.7. Toenail and Urinary Metal Concentration Measurements
2.8. Ocular Surface Analyzes (OSA)
2.9. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Characteristics of Metal Biomarkers
3.3. Correlations among Environmental Factors and Dry Eye Metrics
3.4. Correlations among Metal Biomarkers and Dry Eye Metrics
3.5. Associations between Metal Biomarkers and Dry Eye Metrics
3.6. Associations between Urinary Cd and Ocular Surface Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guha, N.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Vilahur, N.; Muller, K.; Straif, K. Carcinogenicity of welding, molybdenum trioxide, and indium tin oxide. Lancet Oncol. 2017, 18, 581–582. [Google Scholar] [CrossRef]
- Lai, C.-H.; Ho, S.-C.; Pan, C.-H.; Chen, W.-L.; Wang, C.-C.; Liang, C.-W.; Chien, C.-Y.; Riediker, M.; Chuang, K.-J.; Chuang, H.-C. Chronic exposure to metal fume PM2. 5 on inflammation and stress hormone cortisol in shipyard workers: A repeat measurement study. Ecotoxicol. Environ. Saf. 2021, 215, 112144. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-H.; Chou, C.-C.; Chuang, H.-C.; Lin, G.-J.; Pan, C.-H.; Chen, W.-L. Receptor for advanced glycation end products in relation to exposure to metal fumes and polycyclic aromatic hydrocarbon in shipyard welders. Ecotoxicol. Environ. Saf. 2020, 202, 110920. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-Y.; Lai, C.-H.; Chuang, H.-C.; Pan, C.-H.; Yen, C.-C.; Lin, W.-Y.; Chen, J.-K.; Lin, L.-Y.; Chuang, K.-J. Physicochemistry and cardiovascular toxicity of metal fume PM 2.5: A study of human coronary artery endothelial cells and welding workers. Sci. Rep. 2016, 6, 33515. [Google Scholar] [CrossRef] [Green Version]
- Clayton, J.A. Dry Eye. N. Engl. J. Med. 2018, 378, 2212–2223. [Google Scholar] [CrossRef]
- Farrand, K.F.; Fridman, M.; Stillman, I.Ö.; Schaumberg, D.A. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am. J. Ophthalmol. 2017, 182, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Asche, C.V.; Fairchild, C.J. The economic burden of dry eye disease in the United States: A decision tree analysis. Cornea 2011, 30, 379–387. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J. Tfos dews ii epidemiology report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Galor, A.; Kumar, N.; Feuer, W.; Lee, D.J. Environmental factors affect the risk of dry eye syndrome in a United States veteran population. Ophthalmology 2014, 121, 972–973.e971. [Google Scholar] [CrossRef]
- Pamphlett, R.; Cherepanoff, S.; Too, L.K.; Kum Jew, S.; Doble, P.A.; Bishop, D.P. The distribution of toxic metals in the human retina and optic nerve head: Implications for age-related macular degeneration. PLoS ONE 2020, 15, e0241054. [Google Scholar] [CrossRef]
- Cañadas, P.; Lantigua, Y.; Enríquez-de-Salamanca, A.; Fernandez, I.; Pastor-Idoate, S.; Sobas, E.M.; Dueñas-Laita, A.; Pérez-Castrillón, J.L.; Pastor Jimeno, J.C.; Calonge, M. Ocular Surface Pathology in Patients Suffering from Mercury Intoxication. Diagnostics 2021, 11, 1326. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.J.; Lee, S.H. Association between three heavy metals and dry eye disease in Korean adults: Results of the Korean national health and nutrition examination survey. Korean J. Ophthalmol. 2019, 33, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.-H.; Myong, J.-P. Are higher blood mercury levels associated with dry eye symptoms in adult Koreans? A population-based cross-sectional study. BMJ Open 2016, 6, e010985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Blackie, C.A.; Solomon, J.D.; Scaffidi, R.C.; Greiner, J.V.; Lemp, M.A.; Korb, D.R. The relationship between dry eye symptoms and lipid layer thickness. Cornea 2009, 28, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Asiedu, K.; Kyei, S.; Boampong, F.; Ocansey, S. Symptomatic dry eye and its associated factors: A study of university undergraduate students in Ghana. Eye Contact Lens 2017, 43, 262–266. [Google Scholar] [CrossRef]
- Zeev, M.S.-B.; Miller, D.D.; Latkany, R. Diagnosis of dry eye disease and emerging technologies. Clin. Ophthalmol. (Auckl. NZ) 2014, 8, 581. [Google Scholar]
- Su, T.-Y.; Jeng, H.A.; Hsu, Y.-T.; Lai, C.-H.; Pan, C.-H. Impact of Heavy Metals in Ambient Air on Insulin Resistance of Shipyard Welders in Northern Taiwan. Sustainability 2021, 13, 13924. [Google Scholar] [CrossRef]
- Chun, Y.H.; Kim, H.R.; Han, K.; Park, Y.-G.; Song, H.J.; Na, K.-S. Total cholesterol and lipoprotein composition are associated with dry eye disease in Korean women. Lipids Health Dis. 2013, 12, 84. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.J.; Wang, I.J.; Hu, C.C.; Lin, H.C. Comorbidities of dry eye disease: A nationwide population-based study. Acta Ophthalmol. 2012, 90, 663–668. [Google Scholar] [CrossRef]
- Butovich, I.A.; Millar, T.J.; Ham, B.M. Understanding and analyzing meibomian lipids—A review. Curr. Eye Res. 2008, 33, 405–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Paiva, C.S. Effects of aging in dry eye. Int. Ophthalmol. Clin. 2017, 57, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyer, R.A.; Clarkson, T.W. Toxic effects of metals. In Casarett & Doull’s Toxicology: The Basic Science of Poisons, 5th ed.; Klaassen, C.D., Ed.; McGraw-Hill Health Professions Division: New York, NY, USA, 1996; Volume 23, pp. 813–858. [Google Scholar]
- Adair, B.M.; Hudgens, E.E.; Schmitt, M.T.; Calderon, R.L.; Thomas, D.J. Total arsenic concentrations in toenails quantified by two techniques provide a useful biomarker of chronic arsenic exposure in drinking water. Environ. Res. 2006, 101, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, A. Human nails as a biomarker of element exposure. Rev. Environ. Contam. Toxicol. 2006, 185, 141–177. [Google Scholar]
- Salcedo-Bellido, I.; Gutiérrez-González, E.; García-Esquinas, E.; de Larrea-Baz, N.F.; Navas-Acien, A.; Téllez-Plaza, M.; Pastor-Barriuso, R.; Lope, V.; Gómez-Ariza, J.L.; García-Barrera, T. Toxic metals in toenails as biomarkers of exposure: A review. Environ. Res. 2021, 197, 111028. [Google Scholar] [CrossRef]
- Järup, L.; Åkesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Thomson, R.M.; Parry, G.J. Neuropathies associated with excessive exposure to lead. Muscle Nerve: Off. J. Am. Assoc. Electrodiagn. Med. 2006, 33, 732–741. [Google Scholar] [CrossRef]
- Fox, D.; Campbell, M.; Blocker, Y. Functional alterations and apoptotic cell death in the retina following developmental or adult lead exposure. Neurotoxicology 1997, 18, 645–664. [Google Scholar]
- Mulak, M. The effect of heavy metals (lead, cadmium, and manganese) on the function of the visual system. Med. Pr. 1998, 49, 603–607. [Google Scholar]
- Erie, J.C.; Butz, J.A.; Good, J.A.; Erie, E.A.; Burritt, M.F.; Cameron, J.D. Heavy metal concentrations in human eyes. Am. J. Ophthalmol. 2005, 139, 888–893. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Guo, Y.L. Antioxidant nutrients and lead toxicity. Toxicology 2002, 180, 33–44. [Google Scholar] [CrossRef]
- Council, N.R. Potential Health Risks to DOD Firing-Range Personnel from Recurrent Lead Exposure. Available online: https://www.ncbi.nlm.nih.gov/books/NBK206971/ (accessed on 20 September 2021).
- Levin, S.M.; Goldberg, M. Clinical evaluation and management of lead-exposed construction workers. Am. J. Ind. Med. 2000, 37, 23–43. [Google Scholar] [CrossRef]
- Huang, A.; Janecki, J.; Galor, A.; Rock, S.; Menendez, D.; Hackam, A.S.; Jeng, B.H.; Kumar, N. Association of the indoor environment with dry eye metrics. JAMA Ophthalmol. 2020, 138, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Seen, S.; Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchino, Y.; Kawakita, T.; Miyazawa, M.; Ishii, T.; Onouchi, H.; Yasuda, K.; Ogawa, Y.; Shimmura, S.; Ishii, N.; Tsubota, K. Oxidative stress induced inflammation initiates functional decline of tear production. PLoS ONE 2012, 10, e0127720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cejková, J.; Ardan, T.; Simonová, Z.; Cejka, C.; Malec, J.; Dotrelová, D.; Brunová, B. Decreased expression of antioxidant enzymes in the conjunctival epithelium of dry eye (Sjögren’s syndrome) and its possible contribution to the development of ocular surface oxidative injuries. Histol Histopathol. 2008, 23, 1477–1483. [Google Scholar] [CrossRef]
- Chuang, H.-C.; Su, T.-Y.; Chuang, K.-J.; Hsiao, T.-C.; Lin, H.-L.; Hsu, Y.-T.; Pan, C.-H.; Lee, K.-Y.; Ho, S.-C.; Lai, C.-H. Pulmonary exposure to metal fume particulate matter cause sleep disturbances in shipyard welders. Environ. Pollut. 2018, 232, 523–532. [Google Scholar] [CrossRef]
- Lekhanont, K.; Rojanaporn, D.; Chuck, R.S.; Vongthongsri, A. Prevalence of dry eye in Bangkok, Thailand. Cornea 2006, 25, 1162–1167. [Google Scholar] [CrossRef]
- Bazeer, S.; Jansonius, N.; Snieder, H.; Hammond, C.; Vehof, J. The relationship between occupation and dry eye. Ocul. Surf. 2019, 17, 484–490. [Google Scholar] [CrossRef]
- Supiyaphun, C.; Jongkhajornpong, P.; Rattanasiri, S.; Lekhanont, K. Prevalence and risk factors of dry eye disease among University Students in Bangkok, Thailand. PLoS ONE 2021, 16, e0258217. [Google Scholar] [CrossRef]
- Mandell, J.T.; Idarraga, M.; Kumar, N.; Galor, A. Impact of air pollution and weather on dry eye. J. Clin. Med. 2020, 9, 3740. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total (n = 84) | Exposed Group (n = 59) | Control Group (n = 25) | p Value |
|---|---|---|---|---|
| Continuous variable (Mean ± SD) | ||||
| Age (years) | 44.27 ± 12.75 | 41.15 ± 11.76 | 51.64 ± 12.16 | 0.002 * |
| BMI (kg/m2) | 26.36 ± 3.93 | 26.14 ± 3.77 | 26.89 ± 4.32 | 0.463 |
| FPG (mg/dL) | 94.57 ± 18.83 | 94.83 ± 21.41 | 93.96 ± 10.86 | 0.573 |
| Categorical variables (n (%)) | ||||
| Smoking | 25 (29.8) | 19 (32.2) | 6 (24.0) | 0.455 |
| Drinking | 21 (25.0) | 14 (23.7) | 7 (28.0) | 0.681 |
| Cholesterol (mg/dL) | 196.04 ± 50.36 | 202.05 ± 56.69 | 181.84 ± 26.54 | 0.027 * |
| HbA1c (%) | 5.57 ± 0.80 | 5.61 ± 0.91 | 5.49 ± 0.42 | 0.616 |
| PM2.5 (μg/m3) | 286.05 ± 69.44 | 288.35 ± 73.54 | 280.63 ± 59.68 | 0.758 |
| Temperature (°C) | 25.54 ± 1.27 | 25.39 ± 1.26 | 25.91 ± 1.25 | 0.098 |
| Humidity (%) | 73.57 ± 16.52 | 75.61 ± 17.08 | 68.76 ± 14.32 | 0.092 |
| Dry eye metrics (Mean ± SD) a | ||||
| OSDI score b | 7.20 ± 5.85 | 6.98 ± 5.85 | 7.72 ± 5.95 | 0.596 |
| SPEED score c | 4.75 ± 4.51 | 4.76 ± 4.90 | 4.72 ± 3.53 | 0.639 |
| Schirmer’s Test (mm) d | 10.12 ± 7.94 | 10.27 ± 7.49 | 9.76 ± 9.08 | 0.277 |
| Tear Meniscus Height (mm) e | 0.14 ± 0.07 | 0.14 ± 0.07 | 0.15 ± 0.08 | 0.825 |
| Dry eye metrics (n (%)) | ||||
| OSDI score ≥ 13 b | 18 (21.4) | 12 (20.3) | 6 (24.0) | 0.710 |
| SPEED score > 6 c | 27 (32.1) | 17 (28.8) | 10 (40.0) | 0.318 |
| OSDI score ≥ 13 and SPEED score > 6 | 15 (17.8) | 10 (16.9) | 5 (20.0) | 0.740 |
| Schirmer’s Test ≤ 10 mm d | 54 (64.2) | 37 (63.7) | 17 (68.0) | 0.646 |
| Tear Meniscus Height < 0.2 mm e | 71 (84.5) | 50 (84.7) | 21 (84.0) | 0.932 |
| Variable | Total (n = 84) | Exposed Group (n = 59) | Control Group (n = 25) | p Value |
|---|---|---|---|---|
| Urinary creatinine (Mean ± SD) | ||||
| (mg/dL) | 0.95 ± 0.24 | 0.93 ± 0.13 | 1.00 ± 0.039 | 0.747 |
| Urinary metal level (Mean ± SD) | ||||
| V (μg/L) | 0.49 ± 0.23 | 0.52 ± 0.22 | 0.42 ± 0.25 | 0.014 * |
| Cr (μg/L) | 1.05 ± 0.66 | 1.14 ± 0.75 | 0.83 ± 0.26 | 0.022 * |
| Mn (μg/L) | 0.81 ± 0.87 | 0.77 ± 0.79 | 0.88 ± 1.05 | 0.926 |
| Fe (μg/L) | 48.62 ± 26.33 | 48.86 ± 23.22 | 48.06 ± 33.06 | 0.335 |
| Ni (μg/L) | 1.55 ± 3.29 | 1.14 ± 2.01 | 2.53 ± 5.13 | 0.437 |
| Co (μg/L) | 0.24 ± 0.49 | 0.18 ± 0.49 | 0.38 ± 0.49 | 0.179 |
| Cu (μg/L) | 1.31 ± 3.07 | 1.36 ± 3.02 | 1.20 ± 3.24 | 0.114 |
| Zn (μg/L) | 886.28 ± 532.32 | 952.07 ± 545.23 | 731.01 ± 475.17 | 0.081 |
| As (μg/L) | 202.02 ± 361.28 | 215.27 ± 422.03 | 170.75 ± 139.72 | 0.788 |
| Se (μg/L) | 77.41 ± 33.57 | 81.44 ± 33.38 | 67.90 ± 32.73 | 0.670 |
| Cd (μg/L) | 1.08 ± 1.04 | 1.08 ± 1.14 | 1.08 ± 0.80 | 0.442 |
| Hg (μg/L) | 1.42 ± 0.98 | 1.40 ± 0.93 | 1.47 ± 1.11 | 0.872 |
| Pb (μg/L) | 0.12 ± 0.32 | 0.12 ± 0.33 | 0.12 ± 0.31 | 0.721 |
| Toenail metal level (Mean ± SD) | ||||
| V (μg/g) | 0.10 ± 0.60 | 0.13 ± 0.71 | 0.03 ± 0.04 | 0.002 * |
| Cr (μg/g) | 4.56 ± 14.80 | 5.15 ± 17.13 | 3.17 ± 6.75 | 0.015 * |
| Mn (μg/g) | 1.95 ± 4.70 | 2.46 ± 5.51 | 0.74 ± 0.91 | 0.000 * |
| Fe (μg/g) | 120.90 ±273.76 | 147.81 ± 319.78 | 57.40 ± 77.86 | 0.000 * |
| Ni (μg/g) | 0.04 ± 0.13 | 0.05 ± 0.15 | 0.02 ± 0.03 | 0.004 * |
| Co (μg/g) | 4.46 ± 9.14 | 5.04 ± 10.46 | 3.11 ± 4.64 | 0.149 |
| Cu (μg/g) | 4.15 ± 8.78 | 4.64 ± 10.04 | 2.98 ± 4.60 | 0.263 |
| Zn (μg/g) | 8.74 ± 17.16 | 9.49 ± 19.71 | 6.98 ± 8.64 | 0.025 * |
| As (μg/g) | 203.48 ± 494.20 | 229.76 ± 586.01 | 141.45 ± 95.12 | 0.035 * |
| Se (μg/g) | 201.58 ± 491.96 | 227.38 ± 583.40 | 140.69 ± 94.81 | 0.053 |
| Cd (μg/g) | 201.87 ± 491.85 | 227.96 ± 583.26 | 140.28 ± 94.25 | 0.043 * |
| Hg (μg/g) | 202.78 ± 495.73 | 229.00 ± 587.90 | 140.90 ± 94.49 | 0.060 |
| Pb (μg/g) | 0.29 ± 0.48 | 0.34 ± 0.56 | 0.19 ± 0.17 | 0.018 * |
| Variables | OSDI | SPEED | Schirmer’s Test | Tear Meniscus Height |
|---|---|---|---|---|
| PM2.5 (μg/m3) | 0.103 | 0.028 | 0.009 | −0.047 |
| Temperature (°C) | 0.069 | −0.001 | 0.056 | 0.185 |
| Humidity (%) | 0.003 | 0.034 | −0.096 | −0.336 * |
| Variables | OSDI | SPEED | Schirmer’s Test | Tear Meniscus Height |
|---|---|---|---|---|
| Urinary metal (μg/L) | ||||
| V | 0.093 | 0.055 | 0.187 | −0.032 |
| Cr | 0.089 | 0.028 | 0.112 | −0.104 |
| Mn | 0.056 | 0.095 | 0.094 | −0.011 |
| Fe | 0.058 | 0.034 | 0.102 | −0.102 |
| Ni | 0.027 | −0.017 | −0.094 | 0.055 |
| Co | 0.267 * | 0.226 * | 0.005 | 0.083 |
| Cu | 0.185 | 0.135 | 0.077 | −0.014 |
| Zn | 0.194 | 0.124 | 0.190 | −0.005 |
| As | 0.058 | 0.016 | −0.019 | −0.061 |
| Se | 0.276 * | 0.125 | 0.139 | 0.068 |
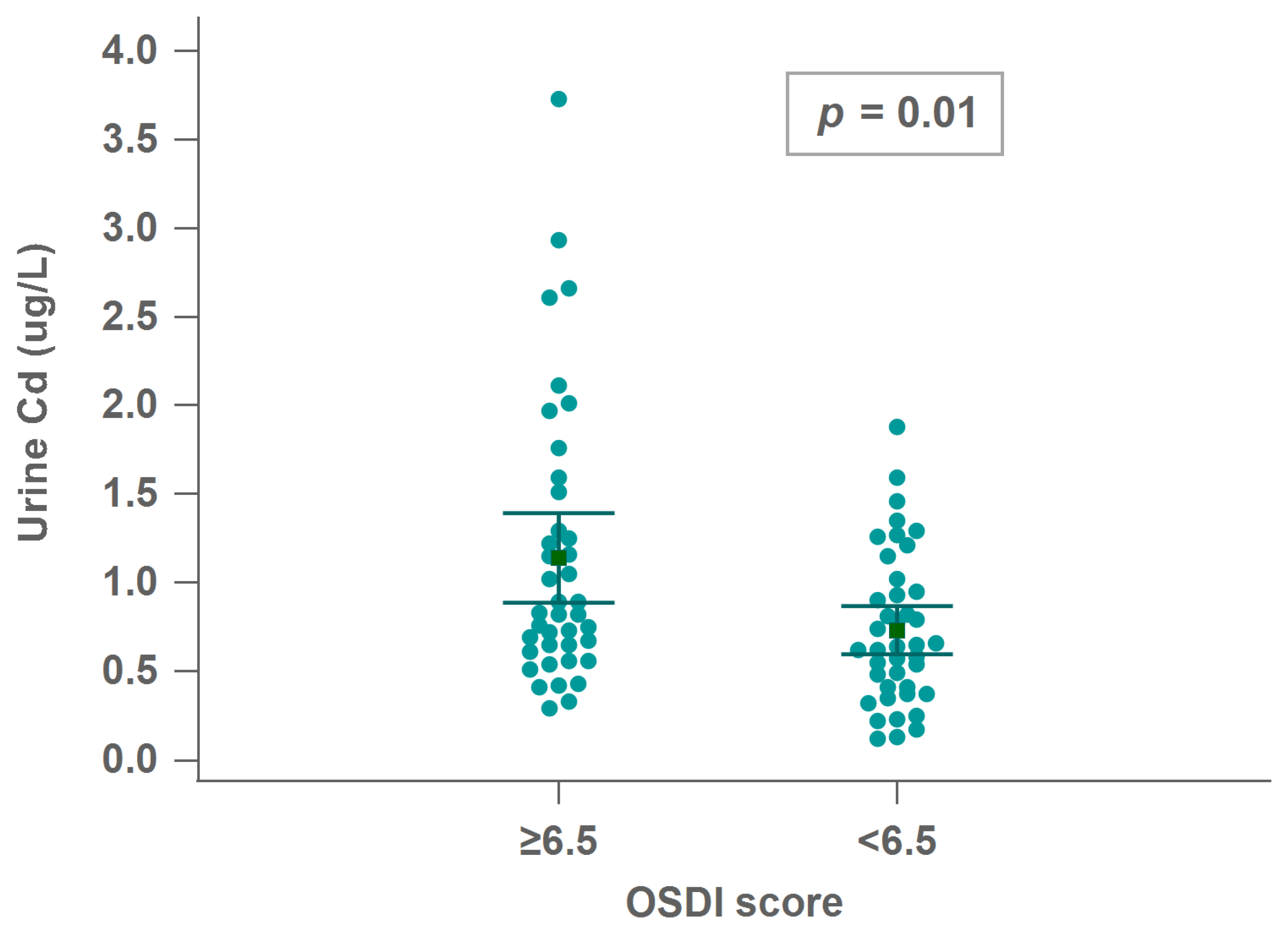
| Cd | 0.298 ** | 0.226 * | 0.098 | 0.046 |
| Hg | 0.093 | 0.011 | 0.088 | 0.003 |
| Pb | 0.167 | 0.149 | −0.043 | −0.153 |
| Toenail metal (μg/g) | ||||
| V | −0.012 | −0.020 | −0.040 | 0.249 * |
| Cr | −0.127 | −0.125 | 0.007 | 0.057 |
| Mn | −0.103 | −0.134 | 0.007 | 0.154 |
| Fe | −0.059 | −0.050 | −0.038 | 0.185 |
| Ni | −0.052 | −0.017 | 0.085 | 0.249 * |
| Co | 0.179 | 0.100 | 0.080 | 0.105 |
| Cu | 0.210 | 0.126 | 0.072 | 0.103 |
| Zn | −0.006 | 0.007 | 0.021 | 0.062 |
| As | 0.166 | 0.154 | −0.063 | −0.158 |
| Se | 0.166 | 0.136 | −0.080 | −0.122 |
| Cd | 0.172 | 0.146 | −0.065 | −0.139 |
| Hg | 0.175 | 0.158 | −0.101 | −0.114 |
| Pb | 0.239 * | 0.178 | −0.032 | 0.070 |
| Variable | Univariate | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Urine Cd | High OSDI (categorical variable) a | |||||||
| 2.260 (1.116, 4.578) | 0.024 * | 2.309 (1.099–4.850) | 0.027 * | 2.043 (0.953–4.376) | 0.067 | 2.016 (0.959–4.239) | 0.064 | |
| Low Schirmer’s Test (categorical variable) b | ||||||||
| 0.849 (0.551–1.308) | 0.849 | 0.655 (0.370–1.159) | 0.146 | 0.594 (0.322–1.094) | 0.095 | 0.597 (0.324–1.103) | 0.099 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liou, Y.-H.; Chen, Y.-J.; Chen, W.-L.; Li, K.-Y.; Chou, T.-Y.; Huang, Y.-C.; Wang, C.-C.; Lai, C.-H. Associations between Biomarkers of Metal Exposure and Dry Eye Metrics in Shipyard Welders: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 2264. https://doi.org/10.3390/ijerph19042264
Liou Y-H, Chen Y-J, Chen W-L, Li K-Y, Chou T-Y, Huang Y-C, Wang C-C, Lai C-H. Associations between Biomarkers of Metal Exposure and Dry Eye Metrics in Shipyard Welders: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2022; 19(4):2264. https://doi.org/10.3390/ijerph19042264
Chicago/Turabian StyleLiou, Ying-Hsi, Ying-Jen Chen, Wei-Liang Chen, Kuan-Ying Li, Ting-Yu Chou, Yung-Chi Huang, Chung-Ching Wang, and Ching-Huang Lai. 2022. "Associations between Biomarkers of Metal Exposure and Dry Eye Metrics in Shipyard Welders: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 19, no. 4: 2264. https://doi.org/10.3390/ijerph19042264
APA StyleLiou, Y.-H., Chen, Y.-J., Chen, W.-L., Li, K.-Y., Chou, T.-Y., Huang, Y.-C., Wang, C.-C., & Lai, C.-H. (2022). Associations between Biomarkers of Metal Exposure and Dry Eye Metrics in Shipyard Welders: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 19(4), 2264. https://doi.org/10.3390/ijerph19042264







