Physical Inactivity and Possible Sarcopenia in Rural Community Daycare Stations of Taiwan: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
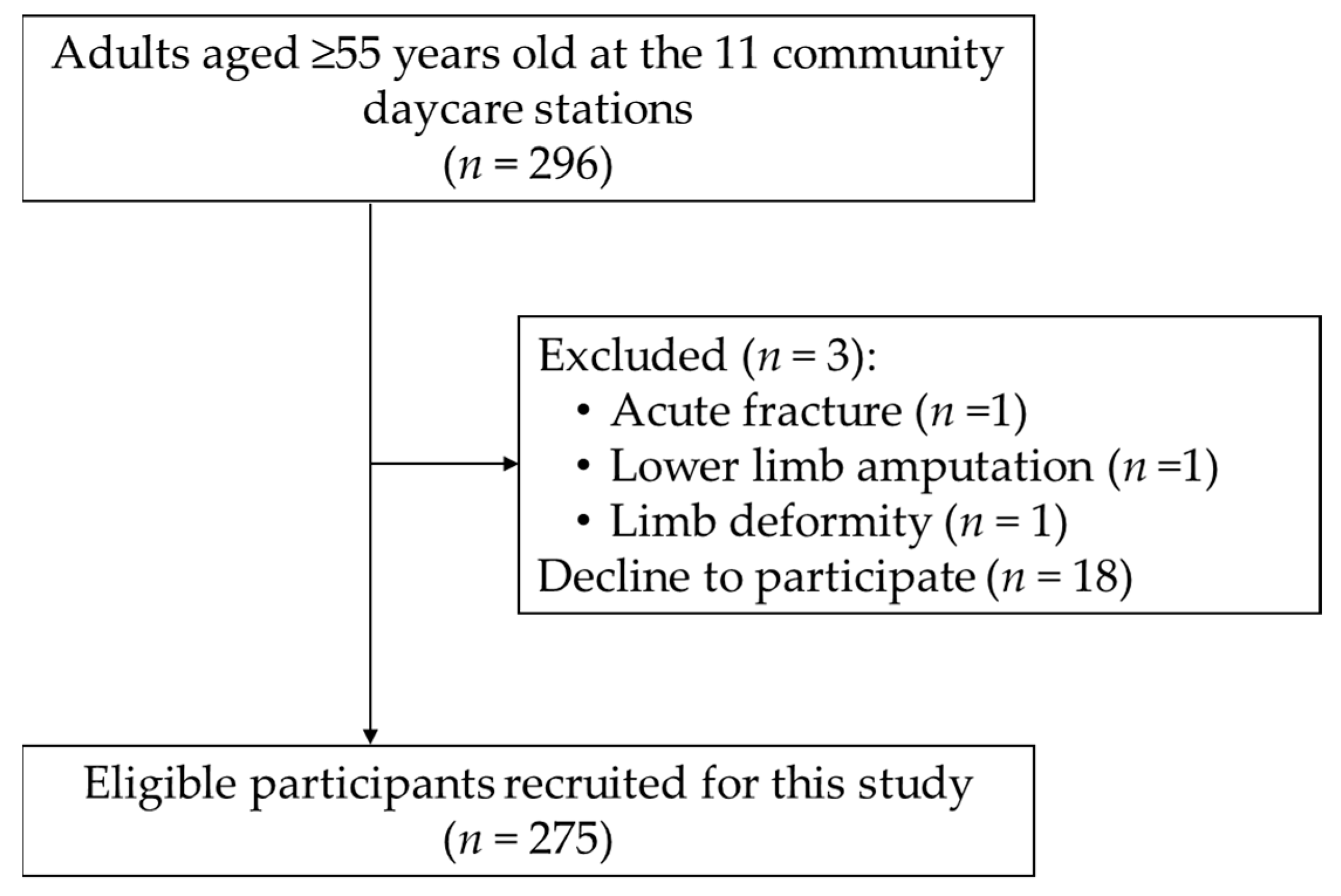
2.1. Study Design, Setting, and Participants
2.2. Assessment of Possible Sarcopenia
2.3. Assessment of Physical Inactivity
2.4. Assessment of Other Covariates
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Associated Factors of Possible Sarcopenia
3.3. Associated Factors of Physical Inactivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Development Council. Population Projections for the R.O.C. (Taiwan): 2018–2065. Available online: https://pop-proj.ndc.gov.tw/main_en/dataSearch.aspx?uid=78&pid=78 (accessed on 14 September 2021).
- Liang, C.C.; Change, Q.X.; Hung, Y.C.; Chen, C.C.; Lin, C.H.; Wei, Y.C.; Chen, J.C. Effects of a community care station program with structured exercise intervention on physical performance and balance in community-dwelling older adults: A prospective 2-year observational study. J. Aging Phys. Act. 2017, 25, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Z.; Liu, H.W.; Liu, P.P.S.; Peng, L.N.; Lin, S.Z.; Loh, C.H. Age-stratified differences of physical capacity in rural community-dwelling Taiwanese older women: A cross-sectional study. Arch. Gerontol. Geriatr. 2020, 90, 104123. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Dos Santos, L.; Cyrino, E.S.; Antunes, M.; Santos, D.A.; Sardinha, L.B. Sarcopenia and physical independence in older adults: The independent and synergic role of muscle mass and muscle function. J. Cachexia Sarcopenia Muscle 2017, 8, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Zepeda, M.U.; Sgaravatti, A.; Dent, E. Sarcopenia and post-hospital outcomes in older adults: A longitudinal study. Arch. Gerontol. Geriatr. 2017, 69, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.C.; Lin, C.C.; Hsiung, C.A.; Wang, C.Y.; Wu, C.H.; Chan, D.C.; Li, T.C.; Lin, W.Y.; Huang, K.C.; Chen, C.Y.; et al. Epidemiology of sarcopenia among community-dwelling older adults in Taiwan: A pooled analysis for a broader adoption of sarcopenia assessments. Geriatr. Gerontol. Int. 2014, 14 (Suppl. S1), 52–60. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef]
- Chen, Z.; Ho, M.; Chau, P.H. Prevalence, Incidence, and Associated Factors of Possible Sarcopenia in Community-Dwelling Chinese Older Adults: A Population-Based Longitudinal Study. Front. Med. 2021, 8, 769708. [Google Scholar] [CrossRef]
- Kim, M.; Won, C.W. Sarcopenia in Korean community-dwelling adults aged 70 years and older: Application of screening and diagnostic tools from the Asian Working Group for Sarcopenia 2019 update. J. Am. Med. Dir. Assoc. 2020, 21, 752–758. [Google Scholar] [CrossRef]
- Ko, Y.C.; Chie, W.C.; Wu, T.Y.; Ho, C.Y.; Yu, W.R. A cross-sectional study about the relationship between physical activity and sarcopenia in Taiwanese older adults. Sci. Rep. 2021, 11, 11488. [Google Scholar] [CrossRef]
- Makizako, H.; Nakai, Y.; Tomioka, K.; Taniguchi, Y. Prevalence of sarcopenia defined using the Asia Working Group for Sarcopenia criteria in Japanese community-dwelling older adults: A systematic review and meta-analysis. Phys. Ther. Res. 2019, 22, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.W.J.; Wee, S.L.; Lau, L.K.; Jabbar, K.A.; Seah, W.T.; Ng, D.H.M.; Ling Tan, Q.L.; Chen, K.K.; Jagadish, M.U.; Ng, T.P. Prevalence and associated factors of sarcopenia in Singaporean adults-The Yishun study. J. Am. Med. Dir. Assoc. 2021, 22, 885.e1–885.e10. [Google Scholar] [CrossRef] [PubMed]
- Bruyere, O.; Beaudart, C.; Ethgen, O.; Reginster, J.Y.; Locquet, M. The health economics burden of sarcopenia: A systematic review. Maturitas 2019, 119, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, J.; Maeda, K.; Shimizu, A.; Inoue, T.; Murotani, K.; Mori, N.; Satake, S.; Matsui, Y.; Arai, H. Diagnostic accuracy of sarcopenia by “possible sarcopenia” premiered by the Asian Working Group for Sarcopenia 2019 definition. Arch. Gerontol. Geriatr. 2021, 97, 104484. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, X.; Xu, M.; Zhang, Z.; He, L.; Li, Y. Sarcopenia prevalence and associated factors among older Chinese population: Findings from the China Health and Retirement Longitudinal Study. PLoS ONE 2021, 16, e0247617. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Sakaguchi, K.; Ogawa, W.; Tamori, Y. Clinical features of 65-year-old individuals in Japan diagnosed with possible sarcopenia based on the Asian Working Group for Sarcopenia 2019 criteria. Geriatr. Gerontol. Int. 2021, 21, 689–696. [Google Scholar] [CrossRef]
- Chang, C.F.; Yeh, Y.L.; Chang, H.Y.; Tsai, S.H.; Wang, J.Y. Prevalence and risk factors of sarcopenia among older adults aged ≥ 65 years admitted to daycare centers of Taiwan: Using AWGS 2019 guidelines. Int. J. Environ. Res. Public Health 2021, 18, 8299. [Google Scholar] [CrossRef]
- Thomas, E.; Battaglia, G.; Patti, A.; Brusa, J.; Leonardi, V.; Palma, A.; Bellafiore, M. Physical activity programs for balance and fall prevention in elderly: A systematic review. Medicine 2019, 98, e16218. [Google Scholar] [CrossRef]
- Cesari, M.; Vellas, B.; Hsu, F.C.; Newman, A.B.; Doss, H.; King, A.C.; Manini, T.M.; Church, T.; Gill, T.M.; Miller, M.E.; et al. A physical activity intervention to treat the frailty syndrome in older persons-results from the LIFE-P study. J. Gerontol. Biol. Sci. Med. Sci. 2015, 70, 216–222. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Norman, I.J.; While, A.E. Physical activity in older people: A systematic review. BMC Public Health 2013, 13, 449. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Dawson, A.; Shaw, S.C.; Harvey, N.C.; Kanis, J.A.; Binkley, N.; Reginster, J.Y.; Chapurlat, R.; Chan, D.C.; Bruyere, O.; et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: Systematic review. Osteoporos. Int. 2017, 28, 1817–1833. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Z.; Lin, J.Y.; Wu, P.L.; Kuo, Y.F. Effects of a hybrid intervention combining exergaming and physical therapy among older adults in a long-term care facility. Geriatr. Gerontol. Int. 2019, 19, 147–152. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zuniga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Office of Internal Affairs, Health, Welfare and Labor. Promote the 10-Year Long-Term Care Project 2.0. Available online: https://www.ey.gov.tw/Page/448DE008087A1971/aa69f5ba-4fc4-4825-9ba8-c93588dcbc86 (accessed on 30 September 2021).
- Bennell, K.; Dobson, F.; Hinman, R. Measures of physical performance assessments: Self-Paced Walk Test (SPWT), Stair Climb Test (SCT), Six-Minute Walk Test (6MWT), Chair Stand Test (CST), Timed Up & Go (TUG), Sock Test, Lift and Carry Test (LCT), and Car Task. Arthritis Care Res. 2011, 63 (Suppl. S11), S350–S370. [Google Scholar]
- Chou, C.Y.; Chiu, C.J.; Chang, C.M.; Wu, C.H.; Lu, F.H.; Wu, J.S.; Yang, Y.C. Disease-related disability burden: A comparison of seven chronic conditions in middle-aged and older adults. BMC Geriatr. 2021, 21, 201. [Google Scholar] [CrossRef]
- Lusardi, M.M.; Fritz, S.; Middleton, A.; Allison, L.; Wingood, M.; Phillips, E.; Criss, M.; Verma, S.; Osborne, J.; Chui, K.K. Determining risk of falls in community dwelling older adults: A systematic review and meta-analysis using posttest probability. J. Geriatr. Phys. Ther. 2017, 40, 1–36. [Google Scholar] [CrossRef]
- Takeshima, N.; Rogers, M.E.; Islam, M.M.; Yamauchi, T.; Watanabe, E.; Okada, A. Effect of concurrent aerobic and resistance circuit exercise training on fitness in older adults. Eur. J. Appl. Physiol. 2004, 93, 173–182. [Google Scholar] [CrossRef]
- Willey, J.Z.; Moon, Y.P.; Kulick, E.R.; Cheung, Y.K.; Wright, C.B.; Sacco, R.L.; Elkind, M.S.V. Physical inactivity predicts slow gait speed in an elderly multi-ethnic cohort study: The Northern Manhattan Study. Neuroepidemiology 2017, 49, 24–30. [Google Scholar] [CrossRef]
- VanSwearingen, J.M.; Studenski, S.A. Aging, motor skill, and the energy cost of walking: Implications for the prevention and treatment of mobility decline in older persons. J. Gerontol. Biol. Sci. Med. Sci. 2014, 69, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Bowden Davies, K.A.; Pickles, S.; Sprung, V.S.; Kemp, G.J.; Alam, U.; Moore, D.R.; Tahrani, A.A.; Cuthbertson, D.J. Reduced physical activity in young and older adults: Metabolic and musculoskeletal implications. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819888824. [Google Scholar] [CrossRef] [PubMed]
- Beavers, K.M.; Beavers, D.P.; Houston, D.K.; Harris, T.B.; Hue, T.F.; Koster, A.; Newman, A.B.; Simonsick, E.M.; Studenski, S.A.; Nicklas, B.J.; et al. Associations between body composition and gait-speed decline: Results from the Health, Aging, and Body Composition study. Am. J. Clin. Nutr. 2013, 97, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Bertschi, D.; Kiss, C.M.; Beerli, N.; Kressig, R.W. Sarcopenia in hospitalized geriatric patients: Insights into prevalence and associated parameters using new EWGSOP2 guidelines. Eur. J. Clin. Nutr. 2021, 75, 653–660. [Google Scholar] [CrossRef]
- Therakomen, V.; Petchlorlian, A.; Lakananurak, N. Prevalence and risk factors of primary sarcopenia in community-dwelling outpatient elderly: A cross-sectional study. Sci. Rep. 2020, 10, 19551. [Google Scholar] [CrossRef]
- Yee, X.S.; Ng, Y.S.; Allen, J.C.; Latib, A.; Tay, E.L.; Abu Bakar, H.M.; Ho, C.Y.J.; Koh, W.C.C.; Kwek, H.H.T.; Tay, L. Performance on sit-to-stand tests in relation to measures of functional fitness and sarcopenia diagnosis in community-dwelling older adults. Eur. Rev. Aging Phys. Act. 2021, 18, 1. [Google Scholar] [CrossRef]
- Muntner, P.; Gu, D.; Wildman, R.P.; Chen, J.; Qan, W.; Whelton, P.K.; He, J. Prevalence of physical activity among Chinese adults: Results from the International Collaborative Study of Cardiovascular Disease in Asia. Am. J. Public Health 2005, 95, 163–1636. [Google Scholar] [CrossRef]
- Hallal, P.C.; Andersen, L.B.; Bull, F.C.; Guthold, R.; Haskell, W.; Ekelund, U.; Lancet Physical Activity Series Working, G. Global physical activity levels: Surveillance progress, pitfalls, and prospects. Lancet 2012, 380, 247–257. [Google Scholar] [CrossRef]
- Trayers, T.; Lawlor, D.A.; Fox, K.R.; Coulson, J.; Davis, M.; Stathi, A.; Peters, T. Associations of objectively measured physical activity with lower limb function in older men and women: Findings from the Older People and Active Living (OPAL) study. J. Aging Phys. Act. 2014, 22, 34–43. [Google Scholar] [CrossRef]
- Wearing, J.; Konings, P.; de Bie, R.A.; Stokes, M.; de Bruin, E.D. Prevalence of probable sarcopenia in community-dwelling older Swiss people—A cross-sectional study. BMC Geriatr. 2020, 20, 307. [Google Scholar] [CrossRef]
- Akosile, C.O.; Agu, C.U.; Adegoke, B.O.A.; Okoye, E.C.; Okeke, I.A.; Emeahara, G. Physical activity fear of falling and falls in Nigerian older adults. Int. J. Aging Soc. 2014, 3, 25–35. [Google Scholar] [CrossRef]
- Low, S.T.; Balaraman, T. Physical activity level and fall risk among community-dwelling older adults. J. Phys. Ther. Sci. 2017, 29, 1121–1124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shaw, L.; Kiegaldie, D.; Farlie, M.K. Education interventions for health professionals on falls prevention in health care settings: A 10-year scoping review. BMC Geriatr. 2020, 20, 460. [Google Scholar] [CrossRef]
- Harper, A.; Wilkinson, I. Falls in older adults: Causes, assessment and management. Medicine 2021, 49, 32–37. [Google Scholar] [CrossRef]
- Gale, C.R.; Cooper, C.; Aihie Sayer, A. Prevalence and risk factors for falls in older men and women: The English Longitudinal Study of Ageing. Age Ageing 2016, 45, 789–794. [Google Scholar] [CrossRef]
- Ryu, M.; Jo, J.; Lee, Y.; Chung, Y.S.; Kim, K.M.; Baek, W.C. Association of physical activity with sarcopenia and sarcopenic obesity in community-dwelling older adults: The Fourth Korea National Health and Nutrition Examination Survey. Age Ageing 2013, 42, 734–740. [Google Scholar] [CrossRef]
- Flöter, A.; Nathorst-Böös, J.; Carlström, K.; Ohlsson, C.; Ringertz, H.; Schoultz, B. Effects of combined estrogen/testosterone therapy on bone and body composition in oophorectomized women. Gynecol. Endocrinol. 2005, 20, 155–160. [Google Scholar] [CrossRef]
- Chou, Y.C.; Kröger, T.; Pu, C.Y. Models of long-term care use among older people with disabilities in Taiwan: Institutional care, community care, live-in migrant care and family care. Eur. J. Ageing 2014, 12, 95–104. [Google Scholar] [CrossRef]
- Lim, J.Y.; Low, N.A.; Merchant, R.A. Prevalence of sarcopenia in pre-frail community dwelling older adult and utility of SARC-F, SARC-CalF and calf circumference in case finding. J. Frailty Sarcopenia Falls 2020, 5, 53–56. [Google Scholar] [CrossRef]
- Rolland, Y.; Lauwers-Cances, V.; Cournot, M.; Nourhashemi, F.; Reynish, W.; Riviere, D.; Vellas, B.; Grandjean, H. Sarcopenia, calf circumference, and physical function of elderly women: A cross-sectional study. J. Am. Geriatr. Soc. 2003, 51, 1120–1124. [Google Scholar] [CrossRef]
- Velazquez-Alva, M.C.; Irigoyen Camacho, M.E.; Lazarevich, I.; Delgadillo Velazquez, J.; Acosta Dominguez, P.; Zepeda Zepeda, M.A. Comparison of the prevalence of sarcopenia using skeletal muscle mass index and calf circumference applying the European consensus definition in elderly Mexican women. Geriatr. Gerontol. Int. 2017, 17, 161–170. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All (n = 275) | Men (n = 69, 25%) | Women (n = 206, 75%) | Regression Analysis with Advanced Age a | ||
|---|---|---|---|---|---|---|
| B | β or EXP (B) | p Value | ||||
| Age (years) | 71.9 (70.8–73.0) | 73.2 (70.8–75.5) | 71.5 (90.2–72.8) | |||
| BMI (kg/m2) | 26.9 (26.3–27.6) | 25.9 (24.7–27.1) | 27.3 (26.6–28.0) | −0.10 | −0.19 | 0.003 |
| Comorbidities, n (%) | ||||||
| Hypertension | 153 (55.4%) | 32 (46.4%) | 121 (58.7%) | −0.00 | 1.00 | 0.893 |
| Heart disease | 46 (16.7%) | 10 (14.5%) | 36 (17.5%) | 0.01 | 1.01 | 0.675 |
| Diabetes mellitus | 82 (29.7%) | 19 (27.5%) | 63 (30.6%) | −0.00 | 1.00 | 0.923 |
| Knee Osteoarthritis | 48 (17.5%) | 3 (4.3%) | 45 (21.8%) | 0.00 | 1.00 | 0.938 |
| Stroke | 20 (7.3%) | 6 (8.7%) | 14 (6.8%) | 0.00 | 1.00 | 0.946 |
| History of falls, n (%) | 86 (31.3%) | 22 (31.9%) | 64 (31.1%) | 0.01 | 1.01 | 0.655 |
| Physical inactivity, n (%) | 80 (29.1%) | 15 (21.7%) | 65 (31.6%) | 0.05 | 1.05 | 0.001 |
| Handgrip strength (kg) | 21.5 (20.7–22.4) | 27.2 (25.1–29.3) | 19.6 (18.9–20.3) * | −0.27 | −0.35 | <0.001 |
| 5xSTS (s) | 15.0 (13.9–16.0) | 16.5 (14.4–18.7) | 14.5 (13.3–15.7) | 0.36 | 0.38 | <0.001 |
| Gait speed (m/s) | 1.00 (0.96–1.04) | 0.92 (0.84–0.99) | 1.04 (0.99–1.08) * | −0.02 | −0.39 | <0.001 |
| TUG (s) | 13.3 (12.2–14.5) | 14.6 (12.7–16.4) | 12.9 (11.5–14.4) | 0.28 | 0.26 | <0.001 |
| Low handgrip strength b, n (%) | 109 (39.6%) | 38 (55.1%) | 71 (34.5%) * | 0.11 | 1.11 | <0.001 |
| Poor 5xSTS performance c, n (%) | 164 (59.6%) | 46 (66.7%) | 118 (57.3%) | 0.08 | 1.09 | <0.001 |
| Slow gait speed d, n (%) | 114 (41.5%) | 35 (50.7%) | 79 (38.3%) | 0.09 | 1.09 | <0.001 |
| TUG deficits e, n (%) | 133 (48.4%) | 45 (65.2%) | 88 (42.7%) * | 0.10 | 1.11 | <0.001 |
| Possible sarcopenia, n (%) | 189 (68.7%) | 54 (78.3%) | 135 (65.5%) * | 0.11 | 1.12 | <0.001 |
| Variables | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| Crude OR | 95% CI | p-Value | Adjusted OR | 95% CI | p-Value | |
| Age (years) | 1.12 | 1.08–1.16 | <0.001 | 1.09 | 1.04–1.14 | <0.001 |
| Sex (with women as ref.) | 1.89 | 1.00–3.59 | 0.051 | |||
| BMI (kg/m2) | 0.98 | 0.93–1.03 | 0.392 | |||
| Number of comorbidities | 1.03 | 0.80–1.33 | 0.825 | |||
| Medical conditions | ||||||
| Hypertension | 0.85 | 0.50–1.43 | 0.846 | |||
| Heart disease | 1.23 | 0.61–2.47 | 0.569 | |||
| Diabetes mellitus | 1.41 | 0.79–2.51 | 0.244 | |||
| Osteoarthritis | 0.92 | 0.47–1.79 | 0.803 | |||
| Stroke | 0.86 | 0.33–2.23 | 0.751 | |||
| History of falls | 1.38 | 0.78–2.43 | 0.264 | |||
| Physical inactivity | 3.49 | 1.77–6.89 | <0.001 | 1.95 | 0.88–4.30 | 0.100 |
| Slow gait speed (<1.0 m/s) | 8.17 | 4.08–16.36 | <0.001 | 4.92 | 2.34–10.33 | <0.001 |
| TUG deficits (>11 s) | 11.49 | 5.83–22.67 | <0.001 | 6.65 | 3.20–13.84 | <0.001 |
| Variables | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| Crude OR | 95% CI | p-Value | Adjusted OR | 95% CI | p-Value | |
| Age (years) | 1.05 | 1.02–1.08 | 0.001 | 1.00 | 0.96–1.04 | 0.875 |
| Sex (with women as ref.) | 0.61 | 0.32–1.17 | 0.138 | |||
| BMI (kg/m2) | 1.04 | 0.99–1.10 | 0.147 | |||
| Number of comorbidities | 1.29 | 1.00–1.67 | 0.051 | |||
| Medical conditions | ||||||
| Hypertension | 1.14 | 0.67–1.96 | 0.624 | |||
| Heart disease | 1.51 | 0.77–2.94 | 0.277 | |||
| Diabetes mellitus | 1.16 | 0.66–2.04 | 0.605 | |||
| Osteoarthritis | 1.56 | 0.81–3.01 | 0.184 | |||
| Stroke | 1.02 | 0.38–2.77 | 0.963 | |||
| History of falls | 1.26 | 0.73–2.19 | 0.408 | |||
| Low handgrip strength | 3.27 | 1.91–5.61 | <0.001 | 2.31 | 1.12–4.74 | 0.023 |
| Poor 5xSTS performance (≥12 s) | 2.90 | 1.61–5.21 | <0.001 | 2.27 | 1.15–4.47 | 0.018 |
| Slow gait speed (<1.0 m/s) | 3.95 | 2.28–6.85 | <0.001 | 2.31 | 1.16–4.61 | 0.018 |
| TUG deficits (>11 s) | 4.09 | 2.32–7.23 | <0.001 | 2.87 | 1.44–5.73 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-Z.; Loh, C.-H.; Hsieh, J.-G.; Lin, S.-Z. Physical Inactivity and Possible Sarcopenia in Rural Community Daycare Stations of Taiwan: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 2182. https://doi.org/10.3390/ijerph19042182
Wu Y-Z, Loh C-H, Hsieh J-G, Lin S-Z. Physical Inactivity and Possible Sarcopenia in Rural Community Daycare Stations of Taiwan: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2022; 19(4):2182. https://doi.org/10.3390/ijerph19042182
Chicago/Turabian StyleWu, Yu-Zu, Ching-Hui Loh, Jyh-Gang Hsieh, and Shinn-Zong Lin. 2022. "Physical Inactivity and Possible Sarcopenia in Rural Community Daycare Stations of Taiwan: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 19, no. 4: 2182. https://doi.org/10.3390/ijerph19042182
APA StyleWu, Y.-Z., Loh, C.-H., Hsieh, J.-G., & Lin, S.-Z. (2022). Physical Inactivity and Possible Sarcopenia in Rural Community Daycare Stations of Taiwan: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 19(4), 2182. https://doi.org/10.3390/ijerph19042182






