Abstract
Chlamydiaceae are obligatory intracellular bacteria causing acute and chronic diseases in animals and humans worldwide, with recently discovered species with a still unclear pathogenic potential (i.e., C. gallinacea). In Italy, Chlamydiaceae infections are underestimated both in animals and humans. To estimate the prevalence of Chlamydiaceae species in poultry and occupationally exposed workers on farm, a cross-sectional study was carried out in north-western Italy. A total of 2063 samples from 83 commercial and 31 backyard poultry farms were analysed using real-time PCRs for Chlamydiaceae screening and species typing. Chlamydiaceae were detected in 23 farms, with a herd prevalence of 20.2% (95%CI: 13.2–28.7), higher in backyard farms (38.7%; 95%CI: 21.8–57.8) compared to commercial ones (13.3%; 95%CI: 6.8–22.5). C. gallinacea was found in 18 chicken farms, both commercial and backyard, and C. psittaci only in 3 backyard farms. Exposure to wild birds and factors related to biosecurity resulted the main risk factors associated with Chlamydia positivity. Out of the 113 sputum samples collected from farmers, 16 tested positive to Chlamydiaceae, with a prevalence of 14.2% (95%CI: 8, 3–22). To the best of our knowledge, for the first time at international level, C. gallinacea was detected in humans with farmer positivity associated with farm infectious status, suggesting a bird-to-human transmission.
1. Introduction
The obligate intracellular bacteria Chlamydia (spp.) are the aetiological agents of chlamydiosis in wild and domestic birds, mammals, and humans [1]. Due to their intrinsic high genetic diversity, the taxonomic classification within the family Chlamydiaceae in the Chlamydiales order is constantly evolving following the identification of new chlamydial strains, mainly of avian origin. According to very recent findings in flamingos, the family Chlamydiaceae, so far composed by the single genus Chlamydia, including 14 recognised species, appears to be enriched with a new proposed genus Chlamydiifrater gen. nov., including two new species named Chlamydiifrater phoenicopteri sp. nov. and Chlamydiifrater volucris sp. nov. [2,3]. As for the genus Chlamydia, in addition to the old ones (i.e., C. trachomatis, C. suis, C. muridarum, C. pneumoniae, C. abortus, C. caviae, C. felis, C. pecorum, and C. psittaci), five new species, characterised through whole-genome sequencing (WGS), have been introduced in the last decade. Specifically, C. avium, C. gallinacean, and C. buteonis were identified in birds while C. serpentis and C. poikilothermis in snakes [4,5,6]. Additionally, new candidate species and taxa have been lately described in fishes and reptiles [2,7,8].
C. psittaci, long considered to be the only pathogenic species in birds and aetiological agent of avian chlamydiosis and human psittacosis, is common in poultry farms worldwide [9,10]. The disease severity in birds varies according to host species, age, and immune status as well as to the virulence of the bacterial strain [10,11]. In most cases, C. psittaci outbreaks in poultry may be characterized by mild respiratory symptoms and latent infections with intermittent and recurrent shedding of the pathogen, often leading to chronic clinical forms [11,12]. Since high load of C. psittaci is shed via faeces and nasal discharges, aerosol dissemination and sometimes ingestion of contaminated material are the main routes of chlamydial transmission [13]. Other ways of transmission include sharing of contaminated water sources and bloodsucking ectoparasites’ bites; moreover, vertical transmission has been proven in poultry and some wild bird species [14,15]. Thus, birds act as carriers and important reservoirs of infection, posing a potential threat to both other animal species and humans [11,16,17,18].
Psittacosis, defined as the human disease caused by C. psittaci zoonotic infection, is an occupational disease, affecting mostly bird handlers, veterinarians, poultry workers, and slaughterhouse workers who are exposed to the highest risk of infection by manipulating or having contact with infected birds and fomites [10]. Symptoms in humans range from asymptomatic infection to severe and/or systemic disease affecting multiple organ systems with fever, headache, respiratory disease, and other manifestations (including endocarditis, myocarditis, hepatitis, arthritis, conjunctivitis, encephalitis) [11,19,20].
Nevertheless, a much more complex epidemiology for avian chlamydiosis has been disclosed, suggesting that species other than C. psittaci may also be involved in the aetiology of the disease in birds, including C. gallinacea, C. avium, C. abortus, C. pecorum, and C. trachomatis [4,21,22]. C. gallinacea appears to be widely distributed worldwide and has been reported as the predominant agent of chlamydiosis in poultry in Argentina, China, the Netherlands, Poland, the USA, Australia, and Mexico [4,23,24,25,26,27,28,29]. Since 2008, this new species has also been randomly detected in asymptomatic poultry in northern Italy (including Piedmont and Liguria regions) [30,31]. Moreover, a study conducted in 2018 on 160 rural free-range chicken farms recorded a PCR prevalence of 15% for C. gallinacea in several regions of Italy, with the isolation of eight strains [32]. By molecular typing on at least 25 strains, C. gallinacea appears as a high diverse species accounting for at least 13 ompA types and 15 sequence types (ST) [23,33].
While the zoonotic potential of C. gallinacea was first evoked after its identification following atypical cases of pneumonia in a French slaughterhouse [34], no case has been confirmed yet. More recently, a serological study conducted in Poland showed that almost 20% of exposed individuals, all farmers or farm workers, were seropositive for Chlamydiaceae. Unfortunately, due to the lack of species-specific serological methods, the study did not allow the identification of the humoral immune response to C. gallinacea specifically [26]. In the Netherlands, an investigation set up on throat swabs from farmers working in C. gallinacea-positive poultry farms could not detect any human infections by real-time PCR [27]. Therefore, pathogenicity and possible zoonotic potential of C. gallinacea have yet to be systematically investigated.
Chlamydial infections continue to be underestimated and underreported in both poultry and human sectors worldwide [35,36]. To date, the infection is not routinely investigated as part of the diagnosis panel in case of respiratory diseases and pneumonia in humans [36]. In Italy, avian chlamydiosis due to C. psittaci infection is included in the animal notifiable diseases list, and psittacosis is included among notifiable occupational diseases.
The Italian poultry sector is a completely self-sufficient system, producing more than what Italy consumes, with a self-supply rate of about 108% [37]. Poultry farming is practiced throughout the country, but it is particularly concentrated in northern regions. The Italian poultry farming scenario is made up of 15,300 farms in production, of which over 6000 are professional, employing almost 40,000 workers, and 1600 slaughtering, cutting, or egg processing plants, accounting for 25,500 employees. In Europe, Italy is the sixth largest poultry meat producer and the third largest eggs producer. Almost all of the Italian poultry production consists of an integrated chain, of which almost 90% is managed by big companies [38].
To date, studies on chlamydial agents are strategic due to their potential health and economic impact on poultry and humans. The project was undertaken with the aim of (i) investigating prevalence and diversity of Chlamydia spp. through a cross-sectional study, (ii) exploring potential risk factors in commercial and backyard poultry farms, and (iii) exploring potential risk factors in professionally exposed workers in the study area. Moreover, the study aimed at promoting more effective monitoring and reporting activities applying a One Health approach, as recommended at international level.
2. Materials and Methods
2.1. Study Design and Sampling Strategy
From May 2018 to January 2021, a cross-sectional study was performed on poultry farms in Cuneo province (Figure 1). This is the area with the highest poultry farm density in Piedmont region, accounting for approximately 420 commercial and 100 backyard farms, according to the National Data Bank (BDN) of livestock register (National Data Bank of the Veterinary Information System, Ministry of Health, March 2018) [39]. Due to the sharing of some risk factors, whenever possible, samples collection was carried out as part of the Avian Flu and Salmonella surveillance programmes in poultry farms.
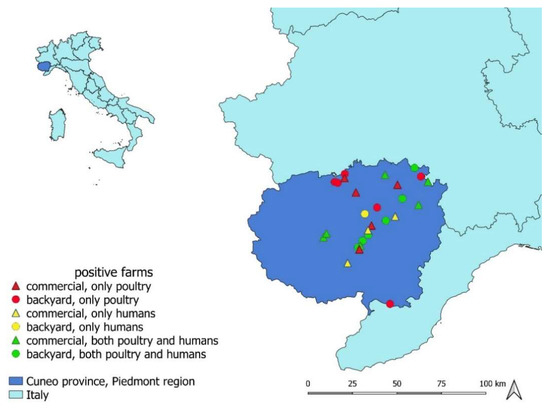
Figure 1.
Map of the Chlamydiaceae-positive farms plotted on the map of Cuneo province (Piedmont region, north-western Italy), where the current cross-sectional study was conducted. Farms in which only poultry, only humans, or both poultry and humans tested positive to Chlamydiaceae PCR screening are indicated with different colours and symbols.
In the target population of domestic poultry as present in BDN, the study involved a two-stage sampling plan. First of all, farms (primary sample units, PSU) were appropriately stratified according to: (i) holding size (large-scale commercial with >250 animals raised or backyard farms with <250 animals raised) and, only for large-scale commercial, (ii) species reared (chicken, duck, turkey, geese, or mixed poultry) and (iii) type of farming (weaners, layers, broilers, breeders). At the first stage, to calculate the PSU sample size aimed at estimating inter-herd Chlamydia prevalence, an expected prevalence (P) of 0.26 [40], an error of 0.10, a confidence level of 0.95, and the single-layer population size were considered. In each layer, farms were randomly selected; where this was not possible because of the smallness of the layer, all the farms present in it were considered. At the second stage, within each PSU a simple random sampling of animals (minimum sample size identified: n = 16 for commercial farms; n = 15 for backyard farms) was carried out, aimed at detecting a within-herd prevalence of 0.20 (design prevalence), assuming an animal-level sensitivity of 0.95, a specificity of 1.00, and a confidence level of 0.95. All the appropriate sample sizes were identified by means of Epitools (Copyright © 2022 AusVet) [41].
To define the role of potential risk factors associated with the presence of Chlamydia in poultry farms, data on structural characteristics, management, and farm location were collected through an epidemiological questionnaire, containing 33 closed-ended questions and filled in by a collecting veterinarian.
With the aim of studying potential zoonotic transmission of new Chlamydia species associated with occupational exposure to poultry, farmers and farm workers as well as veterinary officers were asked to voluntarily take part in the study by submitting and self-sampling their sputum. A self-administered questionnaire to collect individual anamnesis of the enrolled subjects was set up. Questions were related to biographical, anamnestic, and clinical information at the time of sampling as well as to the work activity. A cover letter presenting the objectives of the study was attached to the questionnaire together with a form to allow the processing of personal data in accordance with privacy regulations and instructions for sputum self-collection.
The study involved the collection of different types of samples: two dry cloacal swabs were taken from each live animal, placed in the same sterile tube, and kept at a 4 °C until the delivery to the laboratory. Sputum was collected from humans in a sterile container and kept at a −80 °C until analyses.
2.2. Laboratory Analyses
2.2.1. Nucleic Acid Extraction
DNA isolation from samples was performed as follows:
For cloacal swabs: upon arrival at the laboratory (within 48 h after collection), 2 mL of SPG buffer (liquid medium capable of preserving Chlamydiaceae viability) were added to the tube [9]. After centrifugation (1000× g/10′), total nucleic acids in the supernatant were purified using Maxwell® RCS Viral Total Nucleic Acid Purification Kit (AS1330), in Maxwell® RCS 48 instrument, according to the manufacturer’s instructions. Elution volume was set at 100 µL.
For human sputum: upon arrival at the microbiology laboratory, samples were immediately stored at −80 °C for subsequent analysis. After thawing, each sputum sample was dissolved with Sputasol buffer (ThermoFisher Scientific, Waltham, MA, USA) at a 1:1 ratio. Subsequently, extraction of bacterial DNA was performed on platform QIAsymphony SP/AS (Qiagen, Hilden, DE) using the DSP Virus/Pathogen Kit (Qiagen, Hilden, DE). The input volume was related to the starting volume of the sample (from 500 µL to 1 mL), while the elution volume was 110 µL.
2.2.2. Screening and Typing of Chlamydia Species
DNA samples were firstly screened for the ribosomal 23S gene (highly conserved within the family Chlamydiaceae) by a Chlamydiaceae-specific real-time PCR [42]. All positive DNA samples were further analysed with species-specific real-time PCR assays (i.e., species-typing) targeting the ompA gene for C. psittaci and C. abortus [21] and the enoA gene for C. avium [43] and C. gallinacea [17].
The amplification of the genomic material was performed using GoTaq® Probe qPCR Master Mix by Promega (Madison, WI, USA); for animal samples, the thermal cycle used on CFX96 Instrument (BIORAD, Hercules, CA, USA) for the species-typing was the following: 95 °C for 2 min, 95 °C for 15 s, and 60 °C for 1 min × 45 cycles, while for human samples, qPCR reactions were performed on the ABI7500 Instrument (Applied Biosystems, Waltham, MA, USA) using the above-mentioned thermal profile. Samples with a cycle threshold (Ct) values ≥ 40 were considered negative for each real-time PCR assays.
2.2.3. Data and Statistical Analyses
Veterinary and human data obtained as a result of laboratory investigations as well as information collected through the epidemiological questionnaires were entered in two ad hoc databases (provided as supplementary material 1) in which anamnestic, epidemiological, and laboratory data relating to each enrolled farm and to the samples collected (animal/human) were recorded. In the first database (veterinary data), the elementary epidemiological unit was the farm; in the second database (human data: farmers and farm workers, veterinary officers), it was the single sampled individual. Since they took part in the research on a voluntary basis, in 24 of the selected farms, no human samples were collected; on the other hand, in the other farms, from 1 to 8 individuals were sampled; therefore, in the second dataset, some farm codes are repeated. Animal and human data were matched, whenever possible (veterinary officers’ data, given that they work on the territory and cannot be linked to a single specific farm code, were analysed separately and only in relation to individual risk factors), using the unique key defined by the farm code.
A farm was considered positive when at least one animal tested positive. The data collected allowed to calculate point and interval (exact binomial) prevalence estimates. Inter-herd prevalence estimates both crude and by holding size category were produced and compared using the chi-square test, while within-herd prevalence estimated as the median value of the percentage of tested positive animals (and the values of the first and third quartile Q1–Q3) was calculated only for descriptive purposes. Contingency tables (bivariate analyses) have been used for the calculation of relative risks, in terms of prevalence ratios (PRs) of exposed to non-exposed, to identify putative risk factors potentially associated with the positivity of the farm. A PR value was considered statistically significant if its 95%CI did not overlap 1. Risk factors statistically associated to the risk or close to the statistical significance in the bivariate analysis were included in a multivariate Poisson regression model, using the farm as the epidemiological unit.
For human data, bivariate PRs were calculated to identify candidate factors associated with the risk of positivity in occupational-exposed workers: the host- and farm-level putative risk factors were included in a preliminary mixed-effects Poisson regression model to account for the potential random effect associated to farm. After confirming its absence, a final multivariate Poisson regression model was used.
The Stata SE (version 16.1) software was used for statistical processing of both veterinary and human data (College Station, TX: StataCorp LLC).
2.2.4. MLST Typing
Genotyping by MLST was carried out according to the scheme developed by Pannekoek and colleagues [44], targeting seven housekeeping genes: gatA, oppA, hflX, gidA, enoA, hemN, and fumC. Target genes were amplified and sequenced using primers and conditions described for C. gallinacea by Guo and colleagues [33]. A dendrogram was constructed with the software MEGA7 using the Neighbor-Joining method. New allele sequences are accessible via the Chlamydiales MLST web-site [45] (http://pubmlst.org/chlamydiales/ (accessed on 16 September 2021)).
3. Results
In the study period, 114 farms were visited: 31 backyard farms and 83 large-scale commercial farms. Sample collection for Chlamydiaceae presence investigation involved a total of 2063 domestic birds and 145 occupational-exposed workers, including farmers, farm workers, and veterinary officers.
3.1. Poultry Samples
A total of 2063 cloacal samples were collected from domestic poultry, 1518 of which were from 83 commercial farms and 545 from 31 backyard farms, representing 73 and 27% of the total number of sampled farms, respectively. The farms were characterized by reared species and type of farming, as detailed in Table 1.

Table 1.
Number of farms and samples by holding size, reared species, and laboratory results.
Out of the 114 sampled farms, Chlamydiaceae DNA was detected in 23 of them, corresponding to an overall herd prevalence of 20.2% (95%CI: 13.2–28.7). In detail, the prevalence in large commercial farms with 11 positive farms out of 83 (13.3%; 95%CI: 6.8–22.5) was statistically lower (chi-square test, p = 0.0026) than that in backyard farms (12 out of 31 tested, 38.7%; 95%CI: 21.8–57.8). All commercial farms that tested positive reared chickens, while positive backyard farms reared all the species involved in the study, i.e., chickens, mixed species, or pigeons. The median within-herd prevalence value in the 23 positive farms was 24% (Q1 = 9.4; Q3 = 52). Chlamydia species typing performed by PCR on 155 positive samples showed the circulation both of C. gallinacea and C. psittaci. The former was detected in 132 samples collected in 21 positive farms (10 commercial and 11 backyard farms). The latter was detected in seven samples collected in three backyard farms: in two of them, both C. gallinacea and C. psittaci were detected. In detail, C. gallinacea was found only in poultry and mixed-species farms, while C. psittaci was found in two mixed-species farms (including ducks and chicken) and in one pigeon farm, with an inter-herd prevalence of 9.68% (3/31) in backyard farms (Figure 2 and Figure 3). More details are shown in Table 1.
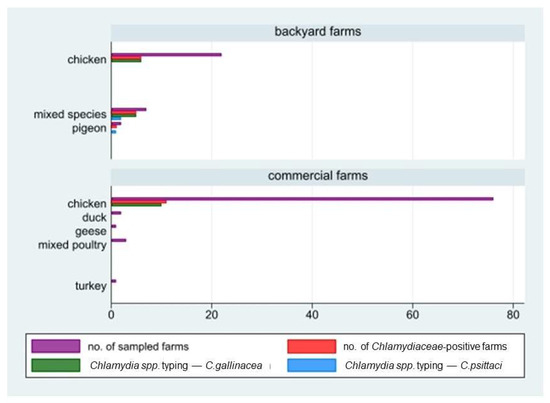
Figure 2.
Number of farms in study by holding size (backyard/commercial farms), reared species, and laboratory results (positivity to Chlamydiaceae screening and species typing; C. gallinacea and C. psittaci).
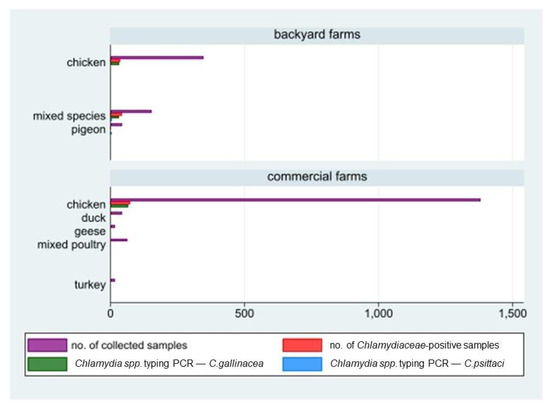
Figure 3.
Number of animal samples collected by holding size (backyard/commercial farms), reared species, and laboratory results (positivity to Chlamydiaceae screening and species typing: C. gallinacean and C. psittaci).
3.2. Risk Factors Analysis for Poultry Farms
Based on the bivariate analysis of the questionnaires data, eight factors showed an association with the risk of Chlamydiaceae presence in a farm, i.e., holding size (backyard vs. commercial); presence of free-range sheds (yes vs. no); presence of nets to prevent birds entry (no vs. yes); full/empty cycles (no vs. yes); litter use (no/only in free range groups vs. yes); and presence of feathers, faeces, or bushes in the surroundings of the holdings (yes vs. no) (Table 2 and Figure 4). These factors were selected as candidate covariates to fit a multivariate model.

Table 2.
Risk factors potentially associated with Chlamydiaceae presence in a farm. N, number of farms in the category; Pos, number of positive farms in the category; %, Pos/N; PR, prevalence ratio of exposed to non-exposed. Only the factors statistically significant are shown (i.e., 1 not included in the 95%CI).
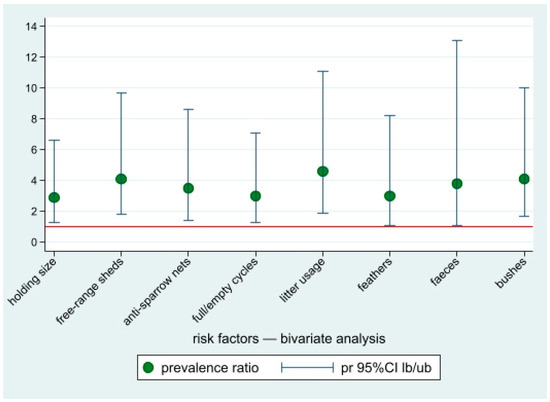
Figure 4.
Prevalence ratios of the risk factors potentially associated with Chlamydiaceae positivity for occupational-exposed workers, bivariate analysis. Point estimates and lower and upper 95%CI bounds. Reference line: pr = 1.
In the multivariate Poisson regression model, only two factors were still statistically associated with the risk of farm positivity: farms where litter was only used in free-range flocks (or not regularly used) (PR = 3.6 (95%CI: 1.5–8.9)) and farms with bushes in the surroundings (PR = 3.4 (95%CI 1.4–8.5)) compared to farms with free surroundings.
3.3. Human Samples
During the study, a total of 145 human samples were collected, including 113 samples from farmers or farm workers and 32 from veterinary officers and veterinary healthcare professionals. Sixteen out of the 113 farm workers (p = 14.2%, 95%CI: 8.3–22) tested positive to the Chlamydiaceae PCR screening, whereas none of the veterinary officers or healthcare professionals tested positive (Figure 5).
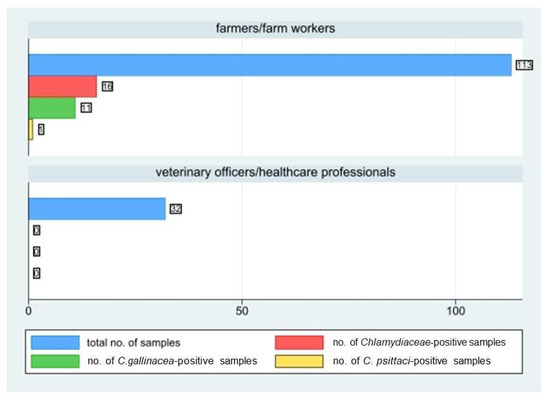
Figure 5.
Number of human samples collected by farmers/farm workers and veterinary officers/healthcare professionals, number of positive ones to Chlamydiaceae screening, and to C. gallinacea and C. psittaci species-typing.
The prevalence in commercial farms (9 workers out of 87; 10.3%; 95%CI: 4.8–18.7) was significantly lower (chi-square test: p = 0.03) than in in backyard farms (7 out 26; 26.9%, 95%CI: 11.6–47.8).
In 12 farms (6 commercial and 6 backyard farms) both animals and farm workers tested positive to Chlamydiaceae PCR (Table 3). We did not detect more than one person positive in the same farm. Chlamydia species-typing revealed C. gallinacea in 11 out of 16 human positive samples and C. psittaci in only 1 human sputum. In 8 out of these 11 positive human cases, C. gallinacea was confirmed also at farm level. In 3 out of the 11 human cases positive for C. gallinacea, the farm in which the positive subject was working resulted negative for the presence of Chlamydiaceae.

Table 3.
Matching between animal and human positivity to Chlamydiaceae within the same farm.
No association between positivity and clinical signs in humans emerged in our study. For what concerns the personal protective equipment (PPE), 31% (n = 27) of farmers/farm workers operating in commercial farms declared not to use it compared to the 88% (n = 23) of those who work in backyard farms.
3.4. Risk Factors Analysis for Occupational-Exposed Humans
Potential risk factors associated with the risk of Chlamydiaceae positivity for occupational-exposed workers by means of the bivariate PRs calculation (Figure 6) are shown in Table 4. These factors were subsequently submitted to the multivariate analysis. In the final Poisson regression model, only one factor was associated to the risk of human positivity, i.e., working in a positive farm (PR = 9.5; 95%CI: 2.7–33.7).
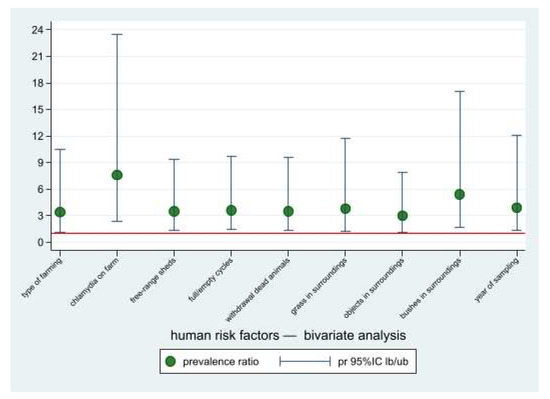
Figure 6.
Prevalence ratios of the risk factors potentially associated with chlamydia positivity for occupational-exposed workers, bivariate analysis. Point estimates and lower and upper 95%CI bounds. Reference line: pr = 1.

Table 4.
Risk factors potentially associated with Chlamydiaceae positivity for occupational-exposed workers (bivariate PRs calculation), n = 113. N, number of workers in the category; Pos, number of positive workers in the category (%, Pos/N); PR, prevalence ratio of exposed to non-exposed. Table shows only the factors statistically significant (i.e., 1 not included in the 95%CI).
3.5. MLST Typing Results
Of the 16 human DNA samples analysed, the MLST genotyping on all seven housekeeping genes was possible only for two of them due to quality of the DNA extract. Due to the scarce quantity and quality of the DNA extracted from poultry samples, only a few DNA samples chosen within the four most affected chickens (in terms of number of positive samples and DNA concentration) were analysed by MLST typing, with a total of five MLST profiles obtained. Identical sequences were obtained each time for samples from the same farm. Except for farm 44638, for which a new sequence of the enoA allele was obtained, all the other gene sequences matched with sequences already present in the database, and an allele number could be assigned to each of them (Table 5). However, interestingly, the combination of alleles was new, generating new STs for these four farms. Of the 16 DNAs from human samples tested, MLST typing of 44638h_89 yielded a new ST identical to that detected on the same farm in poultry, while a new ST (again corresponding to a novel combination of alleles already described within C. gallinacea) was observed for 71895h_22 (no animal samples from this farm were analysed). The phylogenetic tree constructed from the concatenated MLST sequences shows a distribution of these STs among the STs identified so far from Asia and Europe, without a specific distribution that could correspond to Italy (Figure 7).

Table 5.
MLST typing results on samples from farm 41645, 44638, 60260, and 67320 and two human samples. Samples in bold are presented in the phylogenetic tree in Figure 7.
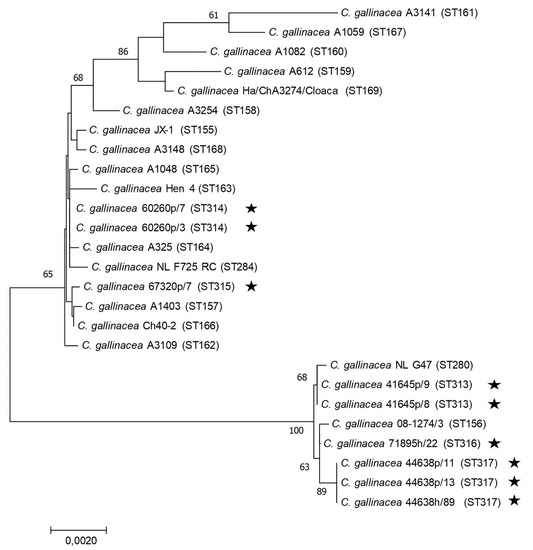
Figure 7.
Concatenated MLST-based phylogeny of C. gallinacea using the Neighbor-joining method: concatenated sequences (3098 nt) were aligned and analysed in MEGA7. Phylogenetic trees were constructed using the Neighbour-Joining method and the Maximum Composite Likelihood model on all available C. gallinacea sequence type (ST) at [45]. Bootstrap tests were for 500 replicates. Numbers on the nodes indicate bootstrap values over 50% of the main branches. Horizontal line scale is for genetic distances. MLST sequence Type (ST) are indicated between parentheses. The star symbol represents the C. gallinacea samples analysed in this study.
4. Discussion
This One Health study aimed to investigate prevalence and diversity of Chlamydiaceae in domestic poultry and in professionally exposed workers in Piedmont as well as risk factors linked to animal and human positivity.
In about one in five poultry farms in the study, Chlamydiaceae presence was confirmed by real-time PCR with a prevalence three times higher in backyard flocks than in commercial ones. Circulation of both C. gallinacea and C. psittaci was observed, but the former was largely more represented. Chlamydiaceae prevalence in poultry farms in Europe is highly variable, ranging from 6.9% in Slovakia [45] and 15.9% in Poland [26] to 47% in the Netherlands [27]. The higher prevalence in backyard than in commercial farms was expected, as previously described by Ornelas-Eusebio and coll. [29].
Chlamydia species-typing confirmed the circulation of C. gallinacea in all the backyard and commercial chicken farms resulted positive to the Chlamydiaceae screening but not in any mallard, turkey, geese, and mixed-poultry commercial farms. Our result is in line with recent findings describing this new chlamydial species as widespread and predominant in poultry, especially in chicken, worldwide [23,27,28,29,32].
C. psittaci was found only in the backyard sector, in a pigeon farm, and in two mixed-poultry farms rearing various species, including chicken and ducks. In the case of C. psittaci, which is able to infect more than 500 bird species from 30 different orders [16,46] and is probably ubiquitous in both domestic/companion as well as wild/free-living bird populations [47], the primary host is represented by the orders Psittaciformes and Columbiformes [12]. Nowadays, C. psittaci is regarded as the dominant species in pigeon and mixed-species farms and no longer represents the endemic chlamydial species in chicken (Gallus gallus), being replaced by C. gallinacea, as described before.
In our study, two out of the three C. psittaci rural farms were also positive for C. gallinacea. Mixed infection with different chlamydial species is well known and documented [4,17,24,48,49].
C. avium was not detected in any poultry farm in study. In Europe, this chlamydial species has been primarily described in wild pigeons and psittacines [12,50].
In the current study, the detection of either C. gallinacea or C. psittaci was not associated with any evident sign of disease in animals [26,27,33]. This feature, already described for C. psittaci [10,16], has also been observed for C. gallinacea, suggesting a moderate pathogenicity since it did not cause any symptoms in experimentally infected chickens other than a slowdown in weight gain [23]. Consistently with that, Chlamydia can survive as commensal organism in the gastrointestinal tract for extended periods of time before eventually eliciting symptoms [12,51,52]. More insights on C. gallinacea pathogenicity have been recently reported by experimental infections in chickens, showing that infection with the NL_G47 strain does not lead to acute clinical disease after oral inoculation, and the bacteria mainly reside in the epithelium of the gut [53,54]. Nevertheless, asymptomatic infected birds may play an important role in the shedding of Chlamydiaceae via respiratory excretions and faeces, causing a persistent environmental contamination [10,55,56].
Almost one in ten of the human samples tested positive for Chlamydiaceae (one in seven if considering only farmers), with about 70% infected by C. gallinacea. Noteworthily, they were all poultry farm workers and farmers, while none of the veterinary officers or healthcare professionals tested positive. None of the positive farmers reported previous or ongoing signs or symptoms of respiratory illness or pneumonia. Prevalence in humans from the present study is consistent with that observed in animals from the same sampled farms. The risk was therefore associated with the status of the farm: 12 of the 16 positive farmers were working in farms where Chlamydiaceae had been detected. Indeed, a strong Chlamydia-species correspondence between animals and farmers has been highlighted although in four positive human cases, Chlamydiaceae presence was not confirmed in the farm where they declared to work. It should be considered that operating on more than one farm is common for farm workers, and this makes them a potential bridge for Chlamydiaceae spread among farms. These findings highlighted a clear link between occupational exposure and infection in humans and strongly suggest the zoonotical potential of C. gallinacea, hitherto only assumed [25,34,49,57]. To the best of our knowledge, this is the first description of C. gallinacea in occupationally exposed human specimens. In contrast to a similar Dutch study [27], which failed to detect C. gallinacea in human samples, our study included sputum samples instead of throat swabs. Sputum represents the most widely accepted specimen for the diagnosis of bacterial respiratory infections, and it is most likely to be (as demonstrated for C. pneumoniae) a better source of bacterial DNA than throat epithelium due to a higher concentration of bacteria in the deep-sited pneumonic infiltrates [58]. However, it should also be noted that in the past, primarily prior to the widespread use of molecular assays, diagnosis of psittacosis included only the targeted testing for C. psittaci, effectively limiting data on other Chlamydia species [32]. Although no association between Chlamydia positivity and clinical signs in humans was found in our study, the detection of C. gallinacea DNA in sputum from poultry workers requires attention. It must be considered that people working with Chlamydia-excreting poultry are likely to breathe in infectious particles, highlighting the importance of using PPE during routine activities in the farm.
Intra-species genetic diversity and phylogenetic relationships have been also investigated on a limited number of poultry and human samples. The MLST analysis confirms the genetic diversity of C. gallinacea by describing five new STs from different farms. Interestingly, identical STs are circulating within the same farm and not only in poultry, as demonstrated by the case of human/chicken ST identity found in the same farm. A high genetic diversity has been already described in C. gallinacea strains from China [33] and the Netherlands [53], but whether this feature might lead to differences in the pathogenic potential between strains is still to be determined.
While no human cases related to C. gallinacea have been reported in the literature since the first description of this species more than a decade ago [34], and considering the limited impact of C. gallinacea on poultry production [23], our results call for caution, as the consequences of a C. gallinacea infection in particularly vulnerable individuals (children, elderly), the immunocompromised, or clinically critical patients remain unknown.
As mentioned before, Chlamydiaceae prevalence appeared to be higher in backyard farms than in commercial ones, probably due to the less confined rearing conditions in backyard systems leading to an increased exposure of domestic poultry to wild birds and to the external environment that can act as source of Chlamydiaceae infection. In Italy, biosecurity and preventive measures have been implemented in recent years, especially in commercial farms, to counteract the entry and spread of pathogens, i.e., avian flu. Strict biosecurity measures, such as cleaning and disinfection of equipment and barn bedding, as well as feed management and preventive medicine principles represent protective factors towards pathogens introduction into the farms [29,32,55,59,60,61]. Backyard farmers are likely less aware of how to contain the risk of entry and spread of infectious diseases in their herds, mainly for the looser biosecurity regulations imposed to backyard flocks compared to commercial ones by law [61]. Moreover, in our study, they seemed less aware of the importance of PPE use in daily routine: a high percentage of backyard farmers declared not to use them in daily activities, almost three times higher than commercial ones. Because of its features, rural breeding should indeed be considered a risk factor itself for Chlamydiaceae presence. Since backyard chicken industry is one of the fastest growing industries in many countries of the world, the set-up of specific biosecurity rules is imperative.
In our study, the risk factor analysis showed that farms in which litter is not regularly used have a risk of positivity almost four times higher than farms in which litter is normally used. This finding is not in line with other studies in which the use of bedding material- and litter-removing practice are instead recognized as risk factors. The role played by the litter in introducing and maintaining Chlamydiaceae seems related to the large movements of infected dust during litter removal. Dust can represent a vehicle of bacteria, viruses, and toxins that may adhere to poultry feathers, representing a source of Chlamydia infection [4,29,62]. Nevertheless, You and colleagues [15] demonstrated that C. gallinacea can be efficiently transmitted by faecal-oral route but not via aerosol. Based on these recent observations and in relation to our findings, we can hypothesize that Chlamydia may be removed from the farm premises more easily and regularly if it is settled on the litter. This practice is particularly relevant in case of full/empty cycles, when stringent cleaning and disinfection are done at the end of each cycle, provided that farm workers always wear personal protective equipment.
Our study also found that farms with bushes in the surroundings had a risk of Chlamydia infection 3.4 times higher than those with no bushes. This may be due to a higher exposure of poultry to contact with wild birds, which may hide or nest there. Wild birds have shown to be often infected by the same strains circulating in domestic flocks, representing a source of infection for poultry, especially in cases of poor confinement of reared animals, i.e., in backyard and free-range farms as well as in commercial farms where biosecurity measures are poorly or not properly implemented [10,61].
The current study suggests and underlines the role played by human activities in infectious diseases entry and spread on farms. This role may be mitigated by applying strict biosecurity measures along the whole production chain. The focus must be on changing and improving people’s behaviour in such a way that the risk of disease entry, spread, and transmission could be decreased consequently. The implementation of biosecurity measures, paying particular attention to vehicles and personnel movement in and out of farms and efficient and complete cleaning and disinfection operations, should go along with a strict surveillance on animal clinical status by recognizing signs and symptoms of infectious disease timely. Moreover, the use of suitable PPE remains essential not only to protect the health of farmers but also to reduce the risk of disease spread on farms. A great percentage of farmers/farm workers in the study declared not to use PPE in daily routine, and this represents a weak point that requires attention. In this sense, specific training activities to the staff of poultry farms organized by health authorities, veterinarians, professional associations, etc., would be strategic in order to give instructions and suggestions and to educate personnel on the importance of biosecurity measures aimed at reducing the risk of introduction and maintenance of these agents on farm environment [63].
Despite the limitations intrinsic to cross-sectional studies, we think that the external validity of the risk factor analysis of our study was not put at risk by the way the data were collected. Our dataset was not based on convenience sampling, as the recruited herds were obtained through stratified random sampling. Moreover, even if the presence of risk factors and outcomes were determined simultaneously by design, it is unlikely that the right temporal sequence (i.e., whether the exposure to the considered risk factors or the infection came first) can be misinterpreted.
However, the available sample size may have limited the achievable precision of the risk estimates, therefore preventing to statistically confirm the association with part of the candidate risk factors included in our bivariate analysis. New, larger studies in the future should further investigate their potential role.
5. Conclusions
C. gallinacea was confirmed to be the endemic chlamydial species in chickens in our territories, whereas C. psittaci was found only in backyard farms, in pigeons, and mixed-species farms.
The high prevalence of Chlamydia spp. in backyard flocks we detected paired with the fast-growing backyard chicken industries in many countries worldwide highlights the urgent need of specific legislation regarding strict biosecurity rules in this sector.
The significative Chlamydia-species correspondence proven in this study between animals and workers in the same farm, particularly strong for C. gallinacea, raises new questions that need to be addressed, especially related to the possible zoonotic potential of this species.
The results of the study yielded baseline information to address future epidemiologic, farm management, and public health policies for the prevention of chlamydial infection inside and outside Italy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19042174/s1, Supplementary Material 1.
Author Contributions
M.L.M. conceived the study idea and methodology, acquired the funding, participated in study design, advised the questionnaires, supervised the project, and critically revised the article. M.M. managed data, wrote the original draft, and edited the revisions. F.R. participated in study design, data management, lab analyses, and revised the article. S.B. coordinated the field sampling and the collection of epidemiological data. G.R. and P.B. (Paola Barzanti) conceived the study design, advised the questionnaires, managed the epidemiological data, and revised the article. O.A.S. collected and managed raw study data and analysed animal samples. N.V. and S.R. performed the confirmatory assays, and N.V. participated in the definition of the study methodology. P.B. (Paolo Bottino) conceived the laboratory human study and analysed human samples. K.L., R.A. and F.V. conceived and performed MLST characterization of positive samples and corresponded data sets analysis, and K.L. critically revised the article. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Ministry of Health in the context of Ricerca Sanitaria Corrente 2016 funding (grant code IZSPLV 10/16 RC).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Human Research Ethics Committee of the S. Croce and Carle” Hospital-via Monte Zovetto 18, 12100 Cuneo, Italy (Parere Unico n.04-18 del 28 March 2018).
Informed Consent Statements
Informed consent was obtained from all human subjects involved in the study.
Acknowledgments
The authors are very grateful for the support of official public veterinarians of the Local Veterinary Health Unit of Cuneo 1 (Giancarlo Barbero, Elio Boetti, Piercarlo Curti, Giacomo Piumetti, Valentina Vottero) and Cuneo 2 (Moshe David, Giovanni Olivieri, Barbara Remonda), who took actively part in field sampling and in the epidemiological data collection. Similarly, we thank Pier Giuseppe Biolatti (IZSPLV, Cuneo Unit) for the logistics support in the samples collection in Cuneo province. The authors also wish to thank Rossana Cavallo, Antonio Curtoni, Francesca Sidoti, Cristina Costa, and Elisa Zanotto (A.O.U. “Città della Salute e della Scienza di Torino”, S.C. Microbiology and Virology U., Turin, Italy) for supporting the project, for their suggestions, and their cooperation on human samples analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Droogenbroeck, C.; Beeckman, D.S.; Verminnen, K.; Marien, M.; Nauwynck, H.; Boesinghe, L.D.T.D.; Vanrompay, D. Simultaneous zoonotic transmission of Chlamydophila psittaci genotypes D, F and E/B to a veterinary scientist. Vet. Microbiol. 2009, 135, 78–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laroucau, K.; Ortega, N.; Vorimore, F.; Aaziz, R.; Mitura, A.; Szymanska-Czerwinska, M.; Cicerol, M.; Salinas, J.; Sachse, K.; Caro, M. Detection of a novel Chlamydia species in captive spur-thighed tortoises (Testudo graeca) in southeastern Spain and proposal of Candidatus Chlamydia testudinis. Syst. Appl. Microbiol. 2020, 43, 126071. [Google Scholar] [CrossRef] [PubMed]
- Vorimore, F.; Hölzer, M.; Liebler-Tenorio, E.; Barf, L.-M.; Delannoy, S.; Vittecoq, M.; Wedlarski, R.; Lécu, A.; Scharf, S.; Blanchard, Y.; et al. Evidence for the existence of a new genus Chlamydiifrater gen. nov. inside the family Chlamydiaceae with two new species isolated from flamingo (Phoenicopterus roseus): Chlamydiifrater phoenicopteri sp. nov. and Chlamydiifrater volucris sp. nov. Syst. Appl. Microbiol. 2021, 44, 126200. [Google Scholar] [CrossRef]
- Sachse, K.; Laroucau, K.; Riege, K.; Wehner, S.; Dilcher, M.; Creasy, H.H.; Weidmann, M.; Myers, G.; Vorimore, F.; Vicari, N.; et al. Evidence for the existence of two new members of the family Chlamydiaceae and proposal of Chlamydia avium sp. nov. and Chlamydia gallinacea sp. nov. Syst. Appl. Microbiol. 2014, 37, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Laroucau, K.; Vorimore, F.; Aaziz, R.; Solmonson, L.; Hsia, R.; Bavoil, P.; Fach, P.; Hölzer, M.; Wuenschmann, A.; Sachse, K. Chlamydia buteonis, a new Chlamydia species isolated from a red-shouldered hawk. Syst. Appl. Microbiol. 2019, 42, 125997. [Google Scholar] [CrossRef]
- Staub, E.; Marti, H.; Biondi, R.; Levi, A.; Donati, M.; Leonard, C.A.; Ley, S.D.; Pillonel, T.; Greub, G.; Seth-Smith, H.M.B.; et al. Novel Chlamydia species isolated from snakes are temperature-sensitive and exhibit decreased susceptibility to azithromycin. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Borel, N.; Greub, G. International Committee on Systematics of Prokaryotes (ICSP) Subcommittee on the taxonomy of Chlamydiae. Minutes of the closed meeting, 5 July 2018, Woudschoten, Zeist, The Netherlands. Int. J. Syst. Evol. Microbiol. 2019, 69, 2606–2608. [Google Scholar] [CrossRef]
- Chaiwattanarungruengpaisan, S.; Thongdee, M.; Anuntakarun, S.; Payungporn, S.; Arya, N.; Punchukrang, A.; Ramasoota, P.; Singhakaew, S.; Atithep, T.; Sariya, L. A new species of Chlamydia isolated from Siamese crocodiles (Crocodylus siamensis). PLoS ONE 2021, 16, e0252081. [Google Scholar] [CrossRef]
- Avian Chlamydiosis; OIE—World Organisation for Animal Health: Paris, France.
- Andersen, A.A.; Vanrompay, D. Avian Chlamydiosis. Rev. Sci. Tech. Int. Off. Epizoot. 2000, 19, 396–404. [Google Scholar] [CrossRef]
- Ravichandran, K.; Anbazhagan, S.; Karthik, K.; Angappan, M.; Dhayananth, B. A comprehensive review on avian chlamydiosis: A neglected zoonotic disease. Trop. Anim. Health Prod. 2021, 53, 1–17. [Google Scholar] [CrossRef]
- Sachse, K.; Laroucau, K.; Vanrompay, D. Avian Chlamydiosis. Curr. Clin. Microbiol. Rep. 2015, 2, 10–21. [Google Scholar] [CrossRef]
- Vanrompay, D. Avian Chlamydiosis. In Diseases of Poultry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 1086–1107. [Google Scholar] [CrossRef]
- Lublin, A.; Shudari, G.; Mechani, S.; Weisman, Y. Egg transmission of Chlamydia psittaci in turkeys. Vet. Rec. 1996, 139, 300. [Google Scholar] [PubMed]
- You, J.; Wu, Y.; Zhang, X.; Wang, X.; Gong, J.; Zhao, Z.; Zhang, J.; Zhang, J.; Sun, Z.; Li, J.; et al. Efficient fecal-oral and possible vertical, but not respiratory, transmission of emerging Chlamydia gallinacea in broilers. Vet. Microbiol. 2019, 230, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Knittler, M.R.; Sachse, K. Chlamydia psittaci: Update on an underestimated zoonotic agent. Pathog. Dis. 2014, 73, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Laroucau, K.; Aaziz, R.; Meurice, L.; Servas, V.; Chossat, I.; Royer, H.; De Barbeyrac, B.; Vaillant, V.; Moyen, J.L.; Meziani, F.; et al. Outbreak of psittacosis in a group of women exposed to Chlamydia psittaci-infected chickens. Eurosurveillance 2015, 20, 21155. [Google Scholar] [CrossRef]
- Sachse, K.; Laroucau, K. Avian chlamydiosis: Two more bacterial players discovered. Vet. J. 2014, 200, 347–348. [Google Scholar] [CrossRef]
- El-Ghany, W.A.A. Avian Chlamydiosis: A World-wide Emerging and Public Health Threat. Adv. Anim. Vet. Sci. 2020, 8, 82–97. [Google Scholar] [CrossRef]
- Chu, J.; Yarrarapu, S.N.S.; Durrani, M.I. Psittacosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Pantchev, A.; Sting, R.; Bauerfeind, R.; Tyczka, J.; Sachse, K. New real-time PCR tests for species-specific detection of Chlamydophila psittaci and Chlamydophila abortus from tissue samples. Vet. J. 2009, 181, 145–150. [Google Scholar] [CrossRef]
- Stokes, H.; Berg, M.; Bennett, A. A Review of Chlamydial Infections in Wild Birds. Pathogens 2021, 10, 948. [Google Scholar] [CrossRef]
- Guo, W.; Li, J.; Kaltenboeck, B.; Gong, J.; Fan, W.; Wang, C. Chlamydia gallinacea, not C. psittaci, is the endemic chlamydial species in chicken (Gallus gallus). Sci. Rep. 2016, 6, 19638. [Google Scholar] [CrossRef]
- Li, J.; Guo, W.; Kaltenboeck, B.; Sachse, K.; Yang, Y.; Lu, G.; Zhang, J.; Luan, L.; You, J.; Huang, K.; et al. Chlamydia pecorum is the endemic intestinal species in cattle while C. gallinacea, C. psittaci and C. pneumoniae associate with sporadic systemic infection. Vet. Microbiol. 2016, 193, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luther, M.; Macklin, K.; Pugh, D.; Li, J.; Zhang, J.; Roberts, J.; Kaltenboeck, B.; Wang, C. Chlamydia gallinacea: A widespread emerging Chlamydia agent with zoonotic potential in backyard poultry. Epidemiol. Infect. 2017, 145, 2701–2703. [Google Scholar] [CrossRef] [PubMed]
- Szymańska-Czerwińska, M.; Mitura, A.; Zaręba, K.; Schnee, C.; Koncicki, A.; Niemczuk, K. Poultry in Poland as Chlamydiaceae carrier. J. Vet. Res. 2017, 61, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Heijne, M.; Van Der Goot, J.A.; Fijten, H.; Van Der Giessen, J.W.; Kuijt, E.; Maassen, C.B.M.; Van Roon, A.; Wit, B.; Koets, A.P.; Roest, H.I.J. A cross sectional study on Dutch layer farms to investigate the prevalence and potential risk factors for different Chlamydia species. PLoS ONE 2018, 13, e0190774. [Google Scholar] [CrossRef] [PubMed]
- Stokes, H.; Martens, J.; Chamings, A.; Walder, K.; Berg, M.; Segal, Y.; Bennett, A.T. Identification of Chlamydia gallinacea in a parrot and in free-range chickens in Australia. Aust. Vet. J. 2019, 97, 398–400. [Google Scholar] [CrossRef]
- Ornelas-Eusebio, E.; Garcia-Espinosa, G.; Vorimore, F.; Aaziz, R.; Durand, B.; Laroucau, K.; Zanella, G. Cross-sectional study on Chlamydiaceae prevalence and associated risk factors on commercial and backyard poultry farms in Mexico. Prev. Vet. Med. 2020, 176, 104922. [Google Scholar] [CrossRef]
- Vicari, N.; Mandola, M.L.; Centorbi, R.; Rizzo, F.; Andreoli, G.; Bellotti, M.; Magnino, S. Detection of Chlamydiaceae in Tissue and Swabs from Wild Birds Samples for Avian Influenza Surveillance in 2008–2009 in Piedmont, Italy. Available online: https://www.researchgate.net/publication/270283937 (accessed on 28 November 2021).
- Rizzo, F.; Vicari, N.; Robetto, S.; Manfredini, A.; Giammarino, M.; Orusa, R.; Mandola, M.L. Detection of a Novel Chlamydiaceae in Wild Birds in North Western Italy. Available online: https://www.researchgate.net/publication/235327414 (accessed on 28 November 2021).
- Donati, M.; Laroucau, K.; Guerrini, A.; Balboni, A.; Salvatore, D.; Catelli, E.; Lupini, C.; Levi, A.; Di Francesco, A. Chlamydiosis in Backyard Chickens (Gallus gallus) in Italy. Vector-Borne Zoonotic Dis. 2018, 18, 222–225. [Google Scholar] [CrossRef]
- Guo, W.; Jelocnik, M.; Li, J.; Sachse, K.; Polkinghorne, A.; Pannekoek, Y.; Kaltenboeck, B.; Gong, J.; You, J.; Wang, C. From genomes to genotypes: Molecular epidemiological analysis of Chlamydia gallinacea reveals a high level of genetic diversity for this newly emerging chlamydial pathogen. BMC Genom. 2017, 18, 949. [Google Scholar] [CrossRef]
- Laroucau, K.; Vorimore, F.; Aaziz, R.; Berndt, A.; Schubert, E.; Sachse, K. Isolation of a new chlamydial agent from infected domestic poultry coincided with cases of atypical pneumonia among slaughterhouse workers in France. Infect. Genet. Evol. 2009, 9, 1240–1247. [Google Scholar] [CrossRef]
- Rodolakis, A.; Mohamad, K.Y. Zoonotic potential of Chlamydophila. Vet. Microbiol. 2010, 140, 382–391. [Google Scholar] [CrossRef]
- Hogerwerf, L.; Roof, I.; De Jong, M.J.K.; Dijkstra, F.; Van Der Hoek, W. Animal sources for zoonotic transmission of psittacosis: A systematic review. BMC Infect. Dis. 2020, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Annata Avicola 2020 » Unaitalia. Available online: https://www.unaitalia.com/mercato/annata-avicola/ (accessed on 28 November 2021).
- Home. Available online: https://agrimpresaonline.it/ (accessed on 28 November 2021).
- Sistema Informativo Veterinario. Available online: https://www.vetinfo.it/ (accessed on 28 November 2021).
- Mandola, M.L.; Rizzo, F.; Vicari, N.; Braghin, S.; Ghia, C.; Belvedere, M.; David, M.; Magnino, S. Chlamydial Infections in Poultry: Preliminary Evidences in Piedmont, Northwestern Italy. Available online: https://www.researchgate.net/publication/320287975 (accessed on 28 November 2021).
- Epitools. Available online: http://epitools.ausvet.com.au (accessed on 28 November 2021).
- Ehricht, R.; Slickers, P.; Goellner, S.; Hotzel, H.; Sachse, K. Optimized DNA microarray assay allows detection and genotyping of single PCR-amplifiable target copies. Mol. Cell. Probes 2006, 20, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Zocevic, A.; Vorimore, F.; Vicari, N.; Gasparini, J.; Jacquin, L.; Sachse, K.; Magnino, S.; Laroucau, K. A Real-Time PCR Assay for the Detection of Atypical Strains of Chlamydiaceae from Pigeons. PLoS ONE 2013, 8, e58741. [Google Scholar] [CrossRef] [PubMed]
- Pannekoek, Y.; Dickx, V.; Beeckman, D.S.A.; Jolley, K.A.; Keijzers, W.C.; Vretou, E.; Maiden, M.C.J.; Vanrompay, D.; Van Der Ende, A. Multi Locus Sequence Typing of Chlamydia Reveals an Association between Chlamydia psittaci Genotypes and Host Species. PLoS ONE 2010, 5, e14179. [Google Scholar] [CrossRef] [PubMed]
- Chlamydiales spp. Available online: https://pubmlst.org/organisms/chlamydiales-spp (accessed on 16 September 2021).
- Kaleta, E.F.; Taday, E.M.A. Avian host range of Chlamydophila spp. based on isolation, antigen detection and serology. Avian Pathol. 2003, 32, 435–462. [Google Scholar] [CrossRef]
- Burnard, D.; Polkinghorne, A. Chlamydial infections in wildlife–conservation threats and/or reservoirs of ‘spill-over’ infections? Vet. Microbiol. 2016, 196, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Szymańska-Czerwińska, M.; Niemczuk, K.; Sachse, K.; Mitura, A.; Karpińska, T.A.; Reichert, M. Detection of a new Non-Classified Chlamydia Species in Hens in Poland. J. Vet. Res. 2013, 57, 25–28. [Google Scholar] [CrossRef][Green Version]
- Szymańska-Czerwińska, M.; Niemczuk, K. Avian Chlamydiosis Zoonotic Disease. Vector-Borne Zoonotic Dis. Larchmt. N 2016, 16, 1–3. [Google Scholar] [CrossRef]
- Popelin-Wedlarski, F.; Roux, A.; Aaziz, R.; Vorimore, F.; Lagourette, P.; Crispo, M.; Borel, N.; Laroucau, K. Captive Psittacines with Chlamydia avium Infection. Avian Dis. 2020, 64, 542–546. [Google Scholar] [CrossRef]
- Reinhold, P.; Sachse, K.; Kaltenboeck, B. Chlamydiaceae in cattle: Commensals, trigger organisms, or pathogens? Vet. J. 2011, 189, 257–267. [Google Scholar] [CrossRef]
- Rank, R.G.; Yeruva, L.; Batista, M.T.; Souza, R.D.; Paccez, J.D.; Luiz, W.B.; Ferreira, E.L.; Cavalcante, R.C.M.; Ferreira, R.C.C.; Ferreira, L.C.S.; et al. Hidden in Plain Sight: Chlamydial Gastrointestinal Infection and Its Relevance to Persistence in Human Genital Infection. Infect. Immun. 2014, 82, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Heijne, M.; van der Goot, J.; Buys, H.; Dinkla, A.; Roest, H.J.; van Keulen, L.; Koets, A. Pathogenicity of Chlamydia gallinacea in chickens after oral inoculation. Vet. Microbiol. 2021, 259, 109166. [Google Scholar] [CrossRef] [PubMed]
- Heijne, M.; Jelocnik, M.; Umanets, A.; Brouwer, M.S.M.; Dinkla, A.; Harders, F.; van Keulen, L.J.M.; Roest, H.J.; Schaafsma, F.; Velkers, F.C.; et al. Genetic and phenotypic analysis of the pathogenic potential of two novel Chlamydia gallinacea strains compared to Chlamydia psittaci. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Hulin, V.; Oger, S.; Vorimore, F.; Aaziz, R.; de Barbeyrac, B.; Berruchon, J.; Sachse, K.; Laroucau, K. Host preference and zoonotic potential of Chlamydia psittaci and C. gallinacea in poultry. Pathog. Dis. 2015, 73, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Krawiec, M.; Piasecki, T.; Wieliczko, A. Prevalence of Chlamydia psittaci and Other Chlamydia Species in Wild Birds in Poland. Vector-Borne Zoonotic Dis. Larchmt. N 2015, 15, 652–655. [Google Scholar] [CrossRef]
- Beeckman, D.S.A.; Vanrompay, D.C.G. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2009, 15, 11–17. [Google Scholar] [CrossRef]
- Saikku, P. Diagnosis of Chlamydia pneumoniae. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 1998, 4 (Suppl. 4), 4S7–4S13. [Google Scholar] [CrossRef]
- Sims, L.D. Risks Associated with Poultry Production Systems. In Proceedings of the International Conference Poultry in the Twenty-First Century, Bangkok, Thailand, 5–7 November 2008. [Google Scholar]
- Vorimore, F.; Thébault, A.; Poisson, S.; Cléva, D.; Robineau, J.; De Barbeyrac, B.; Durand, B.; Laroucau, K. Chlamydia psittaci in ducks: A hidden health risk for poultry workers. Pathog. Dis. 2015, 73, 1–9. [Google Scholar] [CrossRef]
- Ayala, A.J.; Yabsley, M.J.; Hernandez, S.M. A Review of Pathogen Transmission at the Backyard Chicken–Wild Bird Interface. Front. Vet. Sci. 2020, 7, 539925. [Google Scholar] [CrossRef]
- David, B.; Mejdell, C.M.; Michel, V.; Lund, V.; Moe, R.O. Air Quality in Alternative Housing Systems May Have an Impact on Laying Hen Welfare. Part II—Ammonia. Animals 2015, 5, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Biosecurity for Highly Pathogenic Avian Influenza: Issues and Options; Food and Agriculture Organization of the United Nations, Ed.; FAO Animal Production and Health Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).