Proposed Standard Test Protocols and Outcome Measures for Quantitative Comparison of Emissions from Electronic Nicotine Delivery Systems
Abstract
:1. Introduction
1.1. Context of Prior Work
1.2. Study Objectives
2. Materials and Methods
2.1. Experimental Materials, Methods, and Data Set
2.2. Emissions Outcome Measures
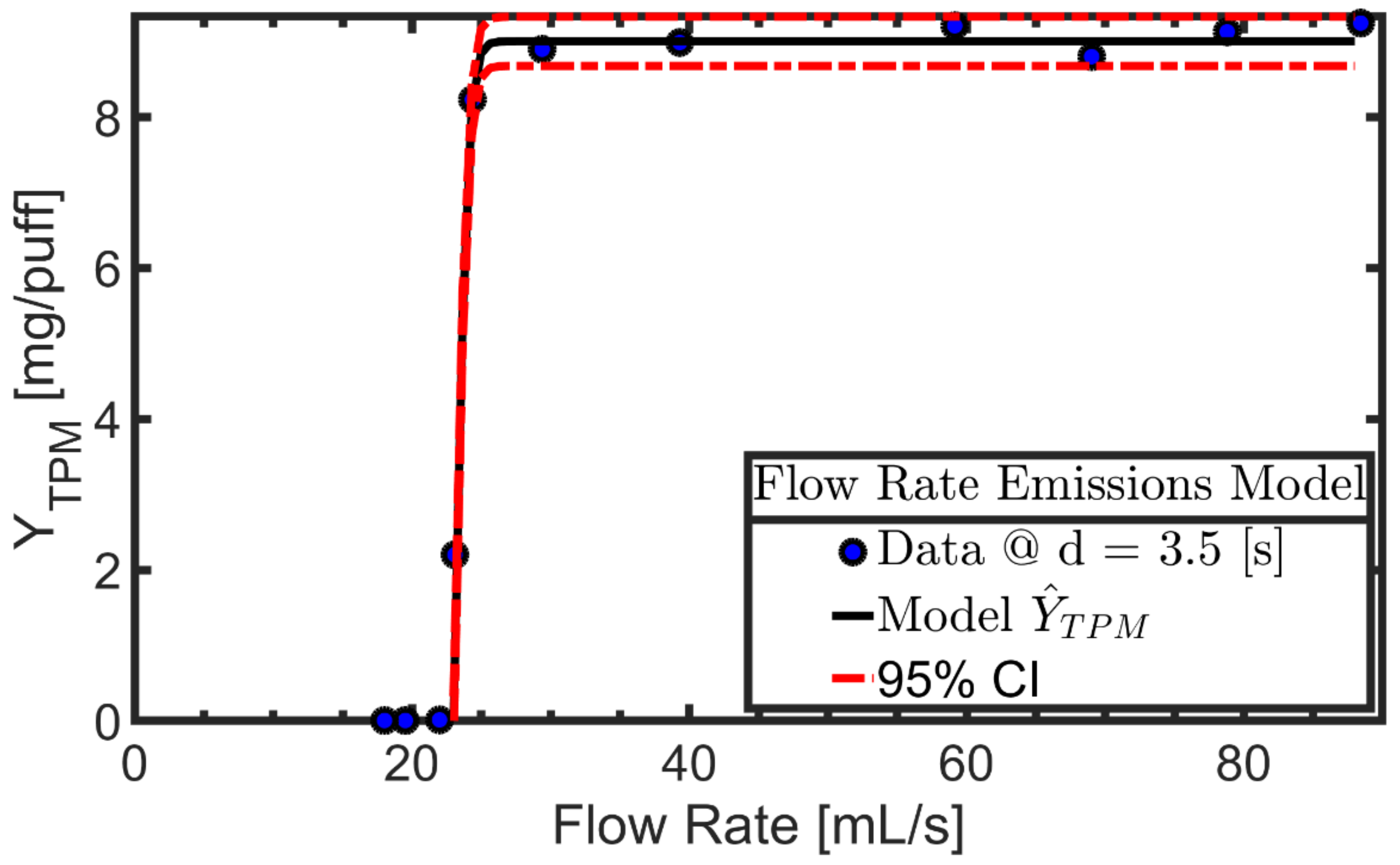
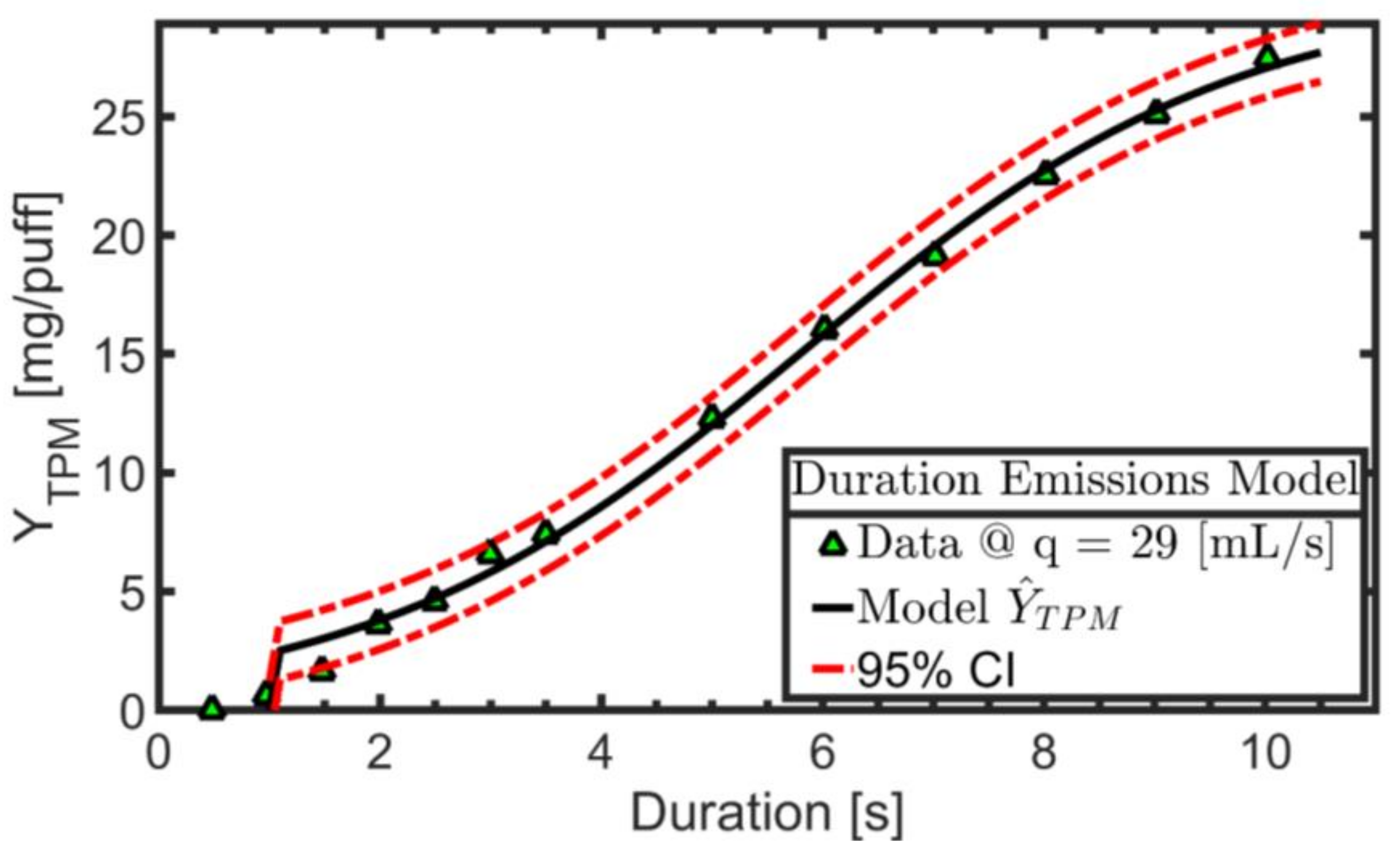
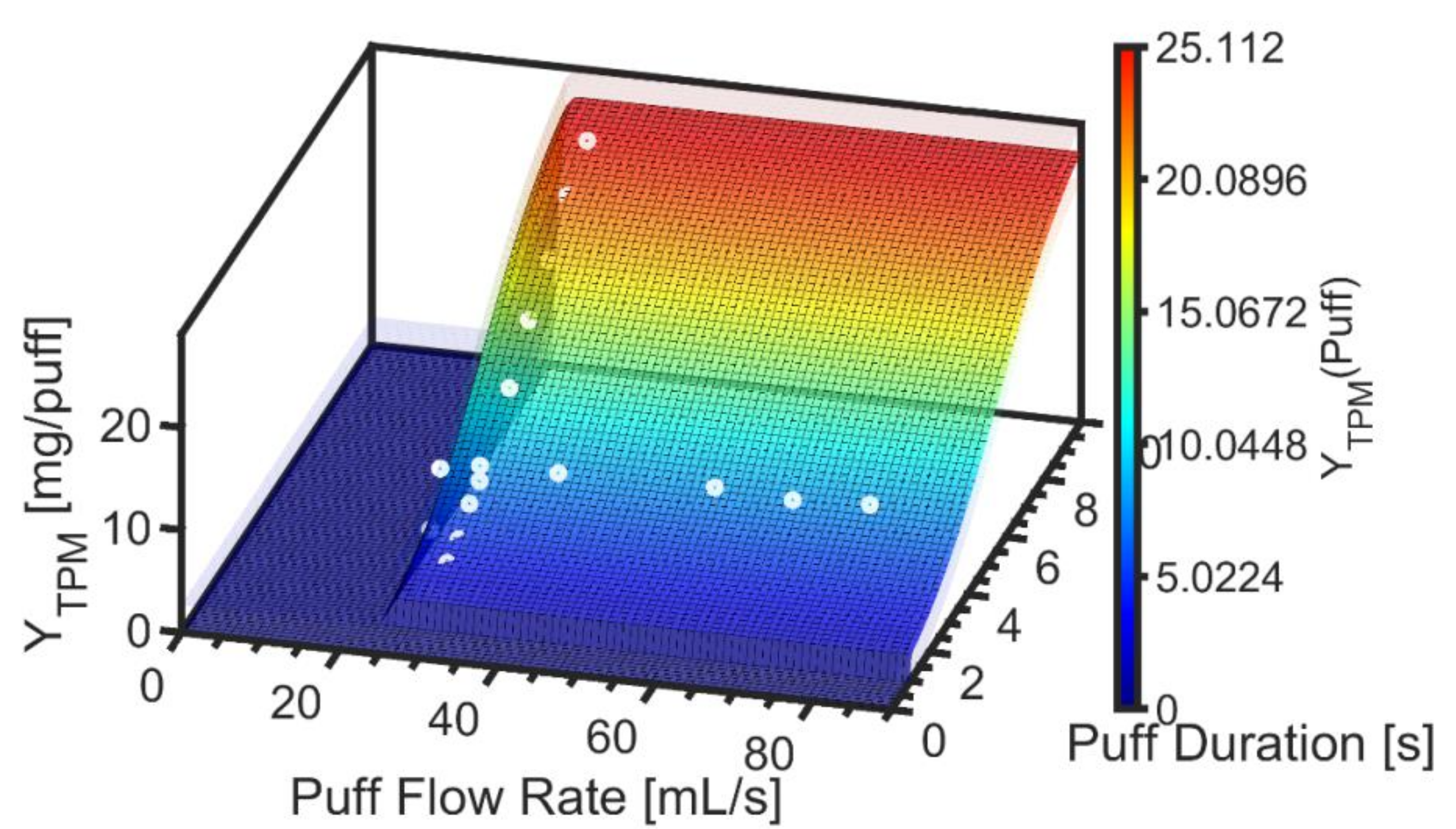
2.3. Empirical TPM Yield Emission Model
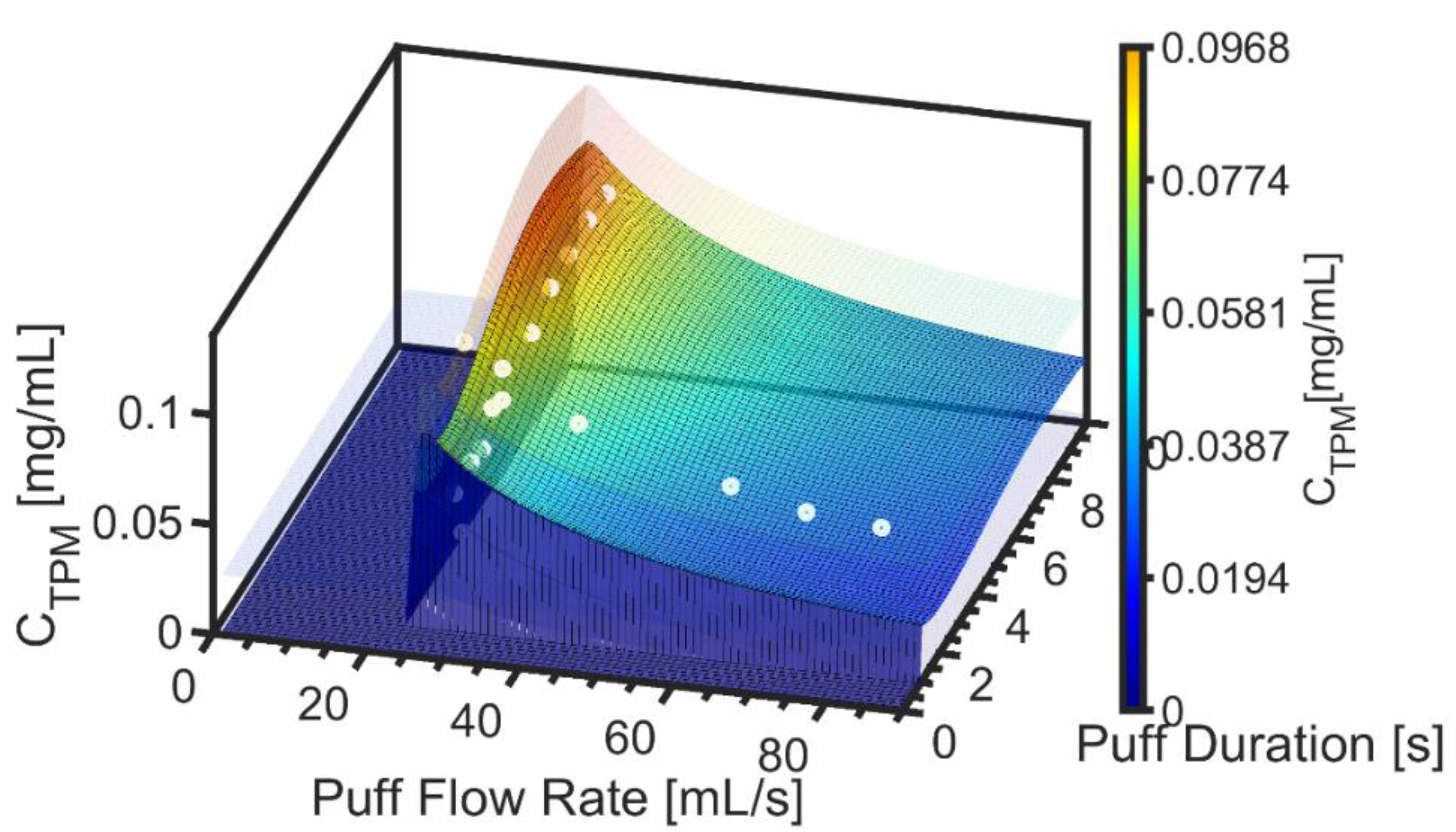
2.4. Empirical TPM Concentration Emission Model
2.5. Empirical Nicotine Mass Fraction Model
2.6. Empirical Nicotine Yield Model
2.7. Confidence Intervals on the Model Estimates
2.8. Presentation of Results
2.9. Investigation of Association between Product Characteristics and Emissions Characteristics
3. Results
3.1. Exemplary Results for One Product
3.2. Summative Results for Quantitative Comparison of All 13 Products
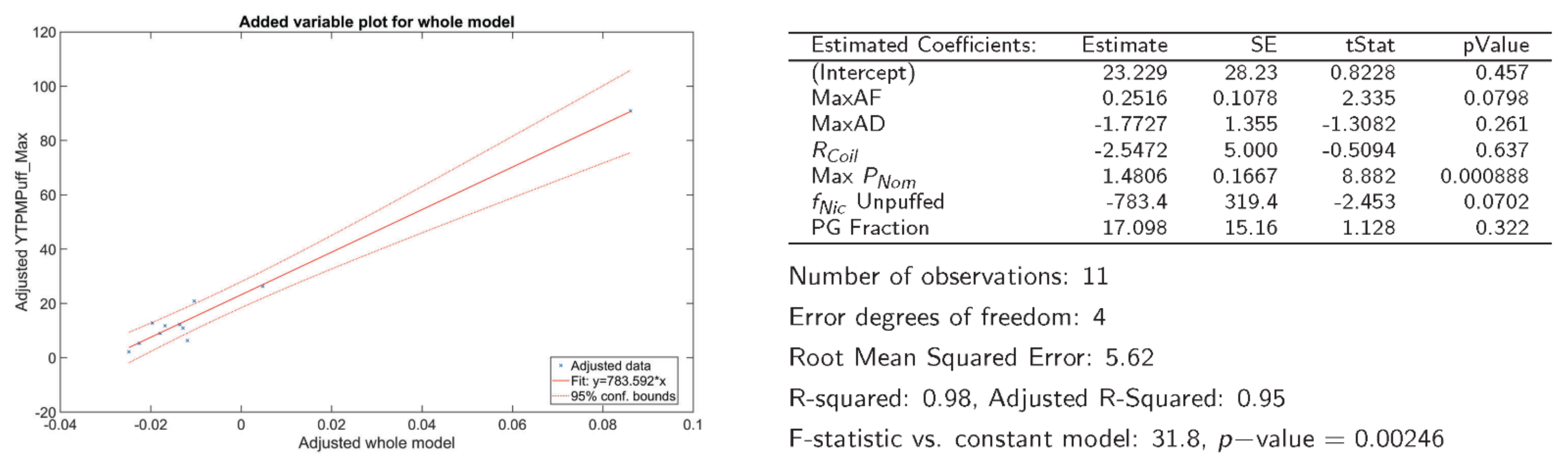
3.3. Association between ENDS Emissions and Product Characteristics
4. Discussion
4.1. Key Findings of Regulatory Significance
4.2. Limitations and Scope
4.3. Gaps Which Need to Be Addressed
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Son, Y.; Bhattarai, C.; Samburova, V.; Khlystov, A. Carbonyls and Carbon Monoxide Emissions from Electronic Cigarettes Affected by Device Type and Use Patterns. Int. J. Environ. Res. Public Health 2020, 17, 2767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hensel, E.C.; Eddingsaas, N.C.; Saleh, Q.M.; Jayasekera, S.; Sarles, S.E.; Thomas, M.; Myers, B.T.; DiFrancesco, A.G.; Robinson, R.J. Nominal Operating Envelope of Pod and Pen Style Electronic Cigarettes. Front. Public Health 2021, 9, 1201. [Google Scholar] [CrossRef] [PubMed]
- ISO/TC-126/SC-3; ISO 20768:2018 Vapour product—Routine analytical vaping machine—definitions and standard conditions. International Organization for Standardization: Geneva, Switzerland, 2018.
- Saleh, Q.M.; Hensel, E.C.; Robinson, R.J. Method for Quantifying Variation in the Resistance of Electronic Cigarette Coils. Int. J. Environ. Res. Public Health 2020, 17, 7779. [Google Scholar] [CrossRef] [PubMed]
- Saleh, Q.; Hensel, E.C.; Robinson, R. Coil Resistance Testing Apparatus for VUSE ALTO. 2020. Available online: https://dx.doi.org/10.17504/protocols.io.bibnkame (accessed on 7 November 2021).
- Robinson, R.J.; Eddingsaas, N.C.; DiFrancesco, A.G.; Jayasekera, S.; Hensel, E.C., Jr. A framework to investigate the impact of topography and product characteristics on electronic cigarette emissions. PLoS ONE 2018, 13, e0206341. [Google Scholar] [CrossRef] [PubMed]
- Hensel, E.C.; Eddingsaas, N.C.; DiFrancesco, A.G.; Jayasekera, S.; O’Dea, S.; Robinson, R.J. Framework to Estimate Total Particulate Mass and Nicotine Delivered to E-cig Users from Natural Environment Monitoring Data. Sci. Rep. 2019, 9, 8752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seber, G.A.F.; Wild, C.J. Nonlinear Regression; John Wiley & Sons: Hoboken NJ, USA, 2003; Volume 62, p. 63. [Google Scholar]
- The MathWorks Inc. Matlab; 2021A; The MathWorks, Inc.: Natick, MA, USA, 2021; Available online: https://www.mathworks.com/ (accessed on 7 November 2021).
- Lane, D.M. Online Statistics Education: A Multimedia Course of Study. Available online: http://onlinestatbook.com/ (accessed on 7 November 2021).
- Huber, P.J. Robust Statistics; John Wiley & Sons: Hoboken NJ, USA, 1981. [Google Scholar]
- Dumouchel, W.; O’Brien, F. Integrating a Robust Option into a Multiple Regression Computing Environment. In Computing and Graphics in Statistics; Springer: Berlin/Heidelberg, Germany, 1992; pp. 41–48. [Google Scholar]
- Holland, P.W.; Welsch, R.E. Robust regression using iteratively reweighted least-squares. Commun. Stat.-Theory Methods 1977, 6, 813–827. [Google Scholar] [CrossRef]
- Street, J.O.; Carroll, R.J.; Ruppert, D. A note on computing robust regression estimates via iteratively reweighted least squares. Am. Stat. 1988, 42, 152–154. [Google Scholar]
- Lovell, M.C. A Simple Proof of the FWL Theorem. J. Econ. Educ. 2008, 39, 88–91. [Google Scholar] [CrossRef]
- Dawkins, L.; Cox, S.; Goniewicz, M.; McRobbie, H.; Kimber, C.; Doig, M.; Kośmider, L. ‘Real-World’ Compensatory Behaviour with Low Nicotine Concentration E-Liquid: Subjective Effects and Nicotine, Acrolein and Formaldehyde Exposure. Addiction 2018, 113, 1874–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawkins, L.E.; Kimber, C.F.; Doig, M.; Feyerabend, C.; Corcoran, O. Self-titration by experienced e-cigarette users: Blood nicotine delivery and subjective effects. Psychopharmacology 2016, 233, 2933–2941. [Google Scholar] [CrossRef] [PubMed]
- The Food and Drug Administration (FDA). 83 FR 11818 Tobacco Product Standard for Nicotine Level of Combusted Cigarettes; Office of the Federal Register, National Archives and Records Administration: College Park, MD, USA, 2018; Volume 83, pp. 11818–11843. [Google Scholar]
- Primack, B.A.; Shensa, A.; Sidani, J.E.; Hoffman, B.L.; Soneji, S.; Sargent, J.D.; Hoffman, R.M.; Fine, M.J. Initiation of Traditional Cigarette Smoking after Electronic Cigarette Use Among Tobacco-Naïve US Young Adults. Am. J. Med. 2018, 131, 443.e441–443.e449. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, K.; Baiocchi, M.; Halpern-Felsher, B. Adolescents’ and Young Adults’ Use and Perceptions of Pod-Based Electronic Cigarettes. JAMA Netw. Open 2018, 1, e183535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute of Medicine. Ending the Tobacco Problem: A Blueprint for the Nation; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar] [CrossRef]





| Nominal Puff Flow Rate [mL/s] | Nominal Puff Duration [s] | Nominal Puff Volume [mL] | TPM Yield [mg/puff] | St Dev [mg/puff] |
|---|---|---|---|---|
| 25 | 2 | 50 | 2.00 | 0.20 |
| 25 | 3 | 75 | 2.10 | 0.20 |
| 25 | 4 | 100 | 2.80 | 0.20 |
| 25 | 5 | 125 | 3.30 | 0.30 |
| 33 | 4 | 132 | 2.80 | 0.20 |
| Product ID | Device Manufacturer | Device Model | Consumable Manufacturer | Consumable Labeled Flavor | Unpuffed E-Liquid fNic [−] | PG Fraction [−] | Mean Rcoil [Ω] | Max PNom (1) [W] | MaxAF [mL/s] | MaxAD [s] |
|---|---|---|---|---|---|---|---|---|---|---|
| EC07-02 | JUUL LABS | Juul | JUUL LABS | Virginia Tobacco | 0.052 | 0.33 | 1.633 | 6.7 | 85 | 6.5 |
| EC10-01 | VUSE | Alto | VUSE | Original | 0.052 | N/R | 1.063 | 10.2 | 50 | 5 |
| EC12-01 | SMOK | Novo 2 | MAD HATTER JUICE | Classic Tobacco | 0.039 | 0.44 | 1.463 | 7.4 | 58 | 8 |
| EC14-01 | BLU | myblu | BLU | Classic Tobacco | 0.02 | 0.42 | 1.416 | 7.7 | 88 | 10 |
| EC15-01 | NJOY | Ace | NJOY | Classic Tobacco | 0.05 | 0.48 | 1.034 | 10.5 | 58 | 5.5 |
| EC16-01 | UWELL | Caliburn | MAD HATTER JUICE | Classic Tobacco | 0.039 | 0.44 | 1.405 | 7.8 | 88 | 10 |
| EC17-01 | ASPIRE | Breeze 2 | MAD HATTER JUICE | Classic Tobacco | 0.039 | 0.44 | 0.631 | 17.3 | 30 | 10.5 |
| EC18-01 | VAPOR4LIFE | V4L Titan | VAPOR4LIFE | Wowbacco | 0.023 | 0.73 | 2.258 | 4.8 | 50 | 10 |
| EC19-01 | LOGIC VAPES | Logic Pro | LOGIC VAPES | Tobacco | 0.016 | 0.77 | 2.443 | 4.5 | 48 | 12 |
| EC20-01 | LOONTECH | Hyde Original | LOONTECH | Spearmint | 0.061 | N/R | 1.61 | 6.8 | 86 | 10 |
| EC22-01 | VUSE | Vibe | VUSE | Original Tobacco | 0.03 | 0.23 | 2.693 | 4.0 | 68 | 6 |
| EC23-01 | SMOK | Stick Prince | MAD HATTER JUICE | Classic Tobacco | 0.052 | 0.44 | 0.174 | 62.6 | 90 | 8 |
| EC24-01 | PUFF BAR | Puff Bar | PUFF BAR | Tobacco | 0.049 | 0.54 | 1.688 | 6.5 | 50 | 3.6 |
| Product | Device | Device | Consumable | Consumable | Max YTPM | Max CTPM | Max fNic | Max YNic | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Manufacturer | Model | Manufacturer | Labeled | Value ± | 95% CI | Value ± | 95% CI | Value ± | 95% CI | Value ± | 95% CI |
| Code | Flavor | [mg TPM/puff] | [mg/mL] | [mg Nic/mg TPM] | [mg Nic/puff] | |||||||
| EC07-02 | Juul Labs | Juul | JUUL Labs | Virginia Tobacco | 2.210 | 2.251 | 0.028 | 0.024 | 0.046 | 0.013 | 0.101 | 0.104 |
| EC10-01 | VUSE | Alto | VUSE | Original | 11.433 | 7.230 | 0.061 | 0.085 | 0.046 | 0.007 | 0.531 | 0.305 |
| EC12-01 | SMOK | Novo 2 | Mad Hatter Juice | Classic Tobacco | 12.814 | 12.896 | 0.057 | 0.091 | 0.051 | 0.005 | 0.650 | 0.312 |
| EC14-01 | Blu | myblu | Blu | Classic Tobacco | 26.341 | 2.707 | 0.110 | 0.026 | 0.024 | 0.005 | 0.634 | 0.081 |
| EC15-01 | NJOY | Ace | NJOY | Classic Tobacco | 11.790 | 0.829 | 0.190 | 0.012 | 0.056 | 0.010 | 0.663 | 0.076 |
| EC16-01 | Uwell | Caliburn | Mad Hatter Juice | Classic Tobacco | 12.193 | 3.960 | 0.167 | 0.059 | 0.046 | 0.006 | 0.565 | 0.186 |
| EC17-01 | Aspire | Breeze 2 | Mad Hatter Juice | Classic Tobacco | 10.911 | 8.972 | 0.300 | 0.072 | 0.039 | 0.019 | 0.424 | 0.401 |
| EC18-01 | Vapor4Life | V4L Titan | Vapor4Life | Wowbacco | 6.365 | 1.664 | 0.094 | 0.019 | 0.030 | 0.006 | 0.188 | 0.061 |
| EC19-01 | Logic Vapes | Logic Pro | Logic | Tobacco | 20.911 | 1.445 | 0.276 | 0.036 | 0.020 | 0.013 | 0.412 | 0.054 |
| EC20-01 | Loontech | Hyde | Hyde | Spear Mint | 16.714 | 2.646 | 0.114 | 0.027 | 0.061 | 0.009 | 1.015 | 0.177 |
| EC22-01 | VUSE | Vibe | VUSE | Original | 8.998 | 1.211 | 0.077 | 0.010 | 0.031 | 0.008 | 0.276 | 0.048 |
| EC23-01 | SMOK | Stick Prince | Mad Hatter Juice | Classic Tobacco | 90.943 | 25.939 | 0.651 | 0.312 | 0.046 | 0.018 | 4.175 | 1.688 |
| EC24-01 | Puff Bar | Puff Bar | Puff Bar | Tobacco | 5.343 | 5.721 | 0.288 | 0.144 | 0.049 | 0.013 | 0.260 | 0.204 |
| Factor | Max YTPM [mg/puff] | Max CTPM [mg/mL] | Max fNic [mg Nic/mg Nic] | Max YNic [mg/puff] | ||||
|---|---|---|---|---|---|---|---|---|
| Level | Lower | Upper | Lower | Upper | Lower | Upper | Lower | Upper |
| 1 | 0.0 | 4.46 | 0.0 | 0.052 | 0.0 | 0.038 | 0.0 | 0.249 |
| 2 | 4.46 | 8.03 | 0.052 | 0.087 | 0.249 | 0.465 | ||
| 3 | 8.03 | 11.06 | 0.087 | 0.148 | 0.465 | 0.962 | ||
| 4 | 11.06 | 19.9 | 0.148 | 0.226 | 0.962 | 1.192 | ||
| 5 | 19.9 | inf | 0.226 | inf | 0.038 | inf | 1.192 | inf |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hensel, E.C.; Eddingsaas, N.C.; Saleh, Q.M.; Jayasekera, S.; Sarles, S.E.; DiFrancesco, A.G.; Robinson, R.J. Proposed Standard Test Protocols and Outcome Measures for Quantitative Comparison of Emissions from Electronic Nicotine Delivery Systems. Int. J. Environ. Res. Public Health 2022, 19, 2144. https://doi.org/10.3390/ijerph19042144
Hensel EC, Eddingsaas NC, Saleh QM, Jayasekera S, Sarles SE, DiFrancesco AG, Robinson RJ. Proposed Standard Test Protocols and Outcome Measures for Quantitative Comparison of Emissions from Electronic Nicotine Delivery Systems. International Journal of Environmental Research and Public Health. 2022; 19(4):2144. https://doi.org/10.3390/ijerph19042144
Chicago/Turabian StyleHensel, Edward C., Nathan C. Eddingsaas, Qutaiba M. Saleh, Shehan Jayasekera, Samantha Emma Sarles, A. Gary DiFrancesco, and Risa J. Robinson. 2022. "Proposed Standard Test Protocols and Outcome Measures for Quantitative Comparison of Emissions from Electronic Nicotine Delivery Systems" International Journal of Environmental Research and Public Health 19, no. 4: 2144. https://doi.org/10.3390/ijerph19042144
APA StyleHensel, E. C., Eddingsaas, N. C., Saleh, Q. M., Jayasekera, S., Sarles, S. E., DiFrancesco, A. G., & Robinson, R. J. (2022). Proposed Standard Test Protocols and Outcome Measures for Quantitative Comparison of Emissions from Electronic Nicotine Delivery Systems. International Journal of Environmental Research and Public Health, 19(4), 2144. https://doi.org/10.3390/ijerph19042144









