Cranial Electrotherapy Stimulation to Improve the Physiology and Psychology Response, Response-Ability, and Sleep Efficiency in Athletes with Poor Sleep Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
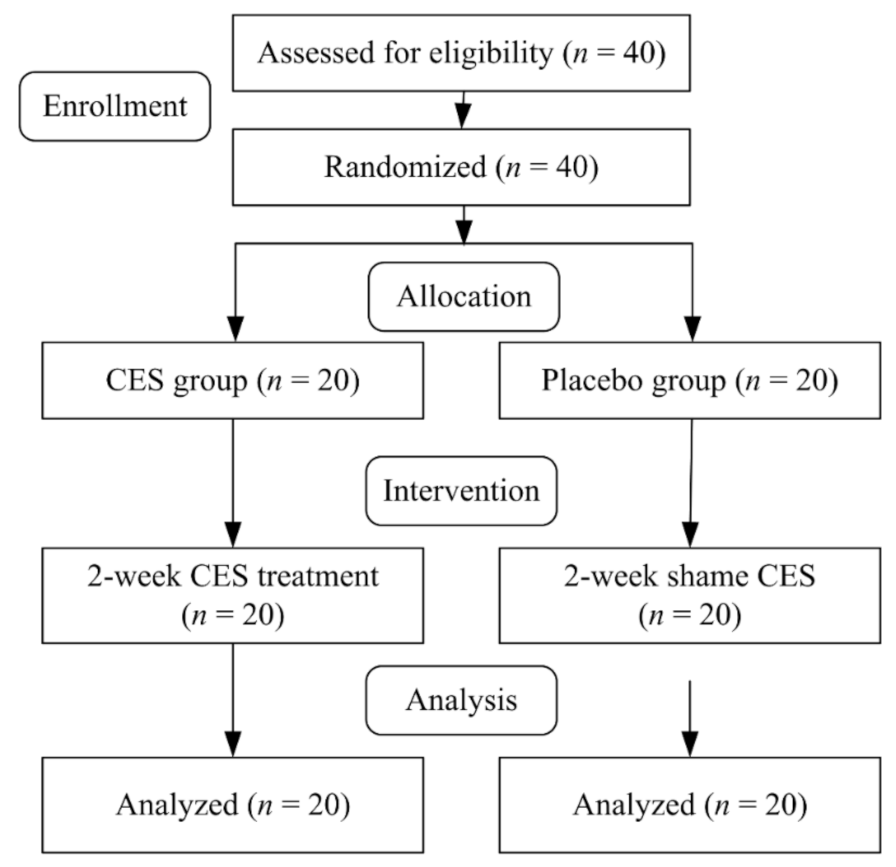
2.2. Study Procedure
2.3. Interventions
2.4. Assessments
2.4.1. Biochemistry Analysis
2.4.2. Simple and Choice Reaction Time
2.4.3. Profile of Mood State
2.4.4. Heart Rate Variability
2.4.5. Actigraph Activity Measurement
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkes, J.R.; Walter, A.E.; Chang, A.M.; Miller, S.J.; Sebastianelli, W.J.; Seidenberg, P.H.; Slobounov, S. Effects of sleep disturbance on functional and physiological outcomes in collegiate athletes: A scoping review. Sleep Med. 2021, 81, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Juliff, L.E.; Halson, S.L.; Peiffer, J.J. Understanding sleep disturbance in athletes prior to important competitions. J. Sci. Med. Sport 2015, 18, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A.; Figueiredo, P.; Nakamura, F.Y.; Rebelo, A.; Brito, J. Monitoring individual sleep and nocturnal heart rate variability indices: The impact of training and match schedule and load in high-level female soccer players. Front. Physiol. 2021, 12, 678462. [Google Scholar] [CrossRef]
- Costa, J.; Figueiredo, P.; Nakamura, F.; Rago, V.; Rebelo, A.; Brito, J. Intra-individual variability of sleep and nocturnal cardiac autonomic activity in elite female soccer players during an international tournament. PLoS ONE 2019, 14, 0218635. [Google Scholar] [CrossRef]
- Figueiredo, P.; Costa, J.; Lastella, M.; Morais, J.; Brito, J. Sleep indices and cardiac autonomic activity responses during an international tournament in a youth national soccer team. Int. J. Environ. Res. Public Health 2021, 18, 2076. [Google Scholar] [CrossRef]
- Leeder, J.; Glaister, M.; Pizzoferro, K.; Dawson, J.; Pedlar, C. Sleep duration and quality in elite athletes measured using wristwatch actigraphy. J. Sports Sci. 2012, 30, 541–545. [Google Scholar] [CrossRef]
- Lastella, M.; Lovell, G.P.; Sargent, C. Athletes’ precompetitive sleep behaviour and its relationship with subsequent precompetitive mood and performance. Eur. J. Sport Sci. 2014, 14, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Mamiya, A.; Morii, I.; Goto, K. Effects of partial sleep deprivation after prolonged exercise on metabolic responses and exercise performance on the following day. Phys. Act. Nutr. 2021, 25, 1–6. [Google Scholar] [CrossRef]
- Meeusen, R.; Duclos, M.; Foster, C.; Fry, A.; Gleeson, M.; Nieman, D.; Raglin, J.; Rietjens, G.; Steinacker, J.; Urhausen, A. Prevention, diagnosis, and treatment of the overtraining syndrome: Joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med. Sci. Sports Exerc. 2013, 45, 186–205. [Google Scholar] [CrossRef]
- Dinges, D.F.; Pack, F.; Williams, K.; Gillen, K.A.; Powell, J.W.; Ott, G.E.; Aptowicz, C.; Pack, A.I. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep 1997, 20, 267–277. [Google Scholar]
- Jarraya, M.; Jarraya, S.; Chtourou, H.; Souissi, N.; Chamari, K. The effect of partial sleep deprivation on the reaction time and the attentional capacities of the handball goalkeeper. Biol. Rhythm Res. 2013, 44, 503–510. [Google Scholar] [CrossRef]
- Blumert, P.A.; Crum, A.J.; Ernsting, M.; Volek, J.S.; Hollander, D.B.; Haff, E.E.; Haff, G.G. The acute effects of twenty-four hours of sleep loss on the performance of national-caliber male collegiate weightlifters. J. Strength Cond. Res. 2007, 21, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, D.L.; Nichols, F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr. Clin. N. Am. 2013, 36, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Fricke, K.; Henschke, U.; Schlitterlau, A.; Liebetanz, D.; Lang, N.; Henning, S.; Tergau, F.; Paulus, W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J. Physiol. 2003, 553, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Klawansky, S.; Yeung, A.; Berkey, C.; Shah, N.; Phan, H.; Chalmers, T.C. Meta-analysis of randomized controlled trials of cranial electrostimulation. Efficacy in treating selected psychological and physiological conditions. J. Nerv. Ment. Dis. 1995, 183, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Holubec, J.T. Cumulative response from cranial electrotherapy stimulation (CES) for chronic pain. Pract. Pain Manag. 2009, 9, 80–83. [Google Scholar]
- Erlacher, D.; Ehrlenspiel, F.; Adegbesan, O.A.; El-Din, H.G. Sleep habits in German athletes before important competitions or games. J. Sports Sci. 2011, 29, 859–866. [Google Scholar] [CrossRef]
- Herman, D.; Macknight, J.M.; Stromwall, A.E.; Mistry, D.J. The international athlete—Advances in management of jet lag disorder and anti-doping policy. Clin. Sports Med. 2011, 30, 641–659. [Google Scholar] [CrossRef]
- Tsai, P.S.; Wang, S.Y.; Wang, M.Y.; Su, C.T.; Yang, T.T.; Huang, C.J.; Fang, S.C. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual. Life Res. 2005, 14, 1943–1952. [Google Scholar] [CrossRef]
- Omobomi, O.; Quan, S.F. A Requiem for the clinical use of the Epworth sleepiness scale. J. Clin. Sleep Med. 2018, 14, 711–712. [Google Scholar] [CrossRef]
- Peng, L.L.; Li, J.R.; Sun, J.J.; Li, W.Y.; Sun, Y.M.; Zhang, R.; Yu, L.L. Reliability and validity of the simplified Chinese version of Epworth sleepiness scale. Chin. J. Otorhinolaryngol. Head Neck Surg. 2011, 46, 44–49. [Google Scholar]
- Jafari, B.; Mohsenin, V. Polysomnography. Clin. Chest Med. 2010, 31, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Swinbourne, R.; Gill, N.; Vaile, J.; Smart, D. Prevalence of poor sleep quality, sleepiness and obstructive sleep apnoea risk factors in athletes. Eur. J. Sport Sci. 2016, 16, 850–858. [Google Scholar] [CrossRef]
- Feighner, J.P.; Brown, S.L.; Olivier, J.E. Electrosleep therapy. A controlled double blind study. J. Nerv. Ment. Dis. 1973, 157, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Erdfelder, E.; Faul, S.; Buchner, A. G-power: A general power analysis program. Behav. Res. Methods Instrum. Comp. 1996, 28, 1–11. [Google Scholar] [CrossRef]
- Kirsch, D.L.; Price, L.R.; Nichols, F.; Marksberry, J.A.; Platoni, K.T. Military service member and veteran self reports of efficacy of cranial electrotherapy stimulation for anxiety, posttraumatic stress disorder, insomnia, and depression. US Army Med. Dep. J. 2014, 46–54. [Google Scholar] [PubMed]
- Perroni, F.; Migliaccio, S.; Borrione, P.; Vetrano, M.; Amatori, S.; Sisti, D.; Rocchi, M.B.L.; Salerno, G.; Vescovo, R.D.; Cavarretta, E.; et al. Can haematological and hormonal biomarkers predict fitness parameters in youth soccer players? A pilot study. Int. J. Environ. Res. Public Health 2020, 17, 6294. [Google Scholar] [CrossRef]
- Brancher, J.A.; Morodome, F.; Madalena, I.R.; Reis, C.L.B.; Von Held, R.; Antunes, L.A.A.; Winckler, C.; Salgueirosa, F.; Neto, Z.C.O.; Storrer, C.L.M.; et al. Salivary pH and oral health of Brazilian para-athletes: Saliva and oral health of para-athletes. Spec. Care Dentist. 2021, 41, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Stoet, G. PsyToolkit: A software package for programming psychological experiments using Linux. Behav. Res. Methods 2010, 42, 1096–1104. [Google Scholar] [CrossRef]
- Kim, J.; Gabriel, U.; Gygax, P. Testing the effectiveness of the Internet-based instrument PsyToolkit: A comparison between web-based (PsyToolkit) and lab-based (E-Prime 3.0) measurements of response choice and response time in a complex psycholinguistic task. PLoS ONE 2019, 14, 0221802. [Google Scholar] [CrossRef]
- Chen, K.M.; Snyder, M.; Krichbaum, K. Translation and equivalence: The Profile of Mood States Short Form in English and Chinese. Int. J. Nurs. Stud. 2002, 39, 619–624. [Google Scholar] [CrossRef]
- McNair, D.M.; Lorr, M.; Droppleman, L.F. EdITS Manual of the Profile of Mood States; Ed-ITS/Educational and Industrial Testing Service: San Diego, CA, USA, 1992. [Google Scholar]
- Tsutsui, Y.; Mizuno, J.; Sunada, K. Does the aroma of a patient’s preferred dental topical anaesthetic affect anxiety, fear, and autonomic nervous system activity prior to dental local anaesthesia? A randomized trial. Flavour Fragr. J. 2018, 33, 405–410. [Google Scholar] [CrossRef]
- Beauchaine, T.P.; Thayer, J.F. Heart rate variability as a transdiagnostic biomarker of psychopathology. Int. J. Psychophysiol. 2015, 98, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Degroote, L.; Hamerlinck, G.; Poels, K.; Maher, C.; Crombez, G.; De Bourdeaudhuij, I.; Vandendriessche, A.; Curtis, R.G.; DeSmet, A. Low-cost consumer-based trackers to measure physical activity and sleep duration among adults in free-living conditions: Validation study. JMIR Mhealth Uhealth 2020, 8, 16674. [Google Scholar] [CrossRef]
- Sargent, C.; Lastella, M.; Halson, S.L.; Roach, G.D. The validity of activitymonitors for measuring sleep in elite athletes. J. Sci. Med. Sport 2016, 19, 848–853. [Google Scholar] [CrossRef]
- Sadeh, A.; Sharkey, K.M.; Carskadon, M.A. Activity-based sleep-wake identification: An empirical test of methodological issues. Sleep 1994, 17, 201–207. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef]
- Rose, K.M.; Taylor, A.G.; Bourguignon, C.; Utz, S.W.; Goehler, L.E. Cranial electrical stimulation: Potential use in reducing sleep and mood disturbances in persons with dementia and their family caregivers. Fam. Community Health 2008, 31, 240–246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anderson, T.; Lane, A.R.; Hackney, A.C. Cortisol and testosterone dynamics following exhaustive endurance exercise. Eur. J. Appl. Physiol. 2016, 116, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.G.; Pata, R.W.; D’Addario, J.; Yuknis, L.; Kingston, R.; Feinn, R. Impact of age on haematological markers pre- and post-marathon running. J. Sports Sci. 2015, 33, 1988–1997. [Google Scholar] [CrossRef]
- Nunes, J.A.; Moreira, A.; Crewther, B.T.; Nosaka, K.; Viveiros, L.; Aoki, M.S. Monitoring training load, recovery-stress state, immune-endocrine responses, and physical performance in elite female basketball players during a periodized training program. J. Strength Cond. Res. 2014, 28, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Halson, S.L. Monitoring training load to understand fatigue in athletes. Sports Med. 2014, 44, 139–147. [Google Scholar] [CrossRef]
- Barclay, T.H.; Barclay, R.D. A clinical trial of cranial electrotherapy stimulation for anxiety and comorbid depression. J. Affect. Disord. 2014, 164, 171–177. [Google Scholar] [CrossRef]
- Southworth, S. A study of the effects of cranial electrical stimulation on attention and concentration. Integr. Physiol. Behav. Sci. 1999, 34, 43–53. [Google Scholar] [CrossRef]
- Zaghi, S.; Acar, M.; Hultgren, B.; Boggio, P.S.; Fregni, F. Non-invasive brain stimulation with low-intensity electrical currents: Putative mechanisms of action for direct and alternating current stimulation. Neuroscientist 2010, 16, 285–307. [Google Scholar] [CrossRef] [PubMed]
- Gilula, M.F.; Kirsch, D.L. Cranial electrotherapy stimulation review: A safer alternative to psychopharmaceuticals in the treatment of depression. J. Neurother. 2005, 9, 7–26. [Google Scholar] [CrossRef]
- Shekelle, P.G.; Cook, I.A.; Miake-Lye, I.M.; Booth, M.S.; Beroes, J.M.; Mak, S. Benefits and harms of cranial electrical stimulation for chronic painful conditions, depression, anxiety, and insomnia: A systematic review. Ann. Intern. Med. 2018, 168, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Tai, H.L.; Yang, C.C.; Chen, Y.S. Acute effects of self-selected music intervention on golf performance and anxiety level in collegiate golfers: A crossover study. Int. J. Environ. Res. Public Health 2020, 17, 7478. [Google Scholar] [CrossRef]
- Fortes, L.S.; da Costa, B.D.; Paes, P.P.; do Nascimento Júnior, J.R.; Fiorese, L.; Ferreira, M.E. Influence of competitiveanxiety on heart rate variability in swimmers. J. Sports Sci. Med. 2017, 16, 498–504. [Google Scholar]
- Pinna, G.D.; Maestri, R.; Torunski, A.; Danilowicz-Szymanowicz, L.; Szwoch, M.; La Rovere, M.T.; Raczak, G. Heart rate variability measures: A fresh look at reliability. Clin. Sci. 2007, 113, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Wagenseil, B.; Garcia, C.; Suvorov, A.V.; Fietze, I.; Penzel, T. The effect of cranial electrotherapy stimulation on sleep in healthy women. Physiol. Meas. 2018, 39, 114007. [Google Scholar] [CrossRef] [PubMed]
- Kennerly, R.C. QEEG analysis of cranial electrotherapy: A pilot study. J. Neurother. 2004, 8, 112–113. [Google Scholar]
- Yennurajalingam, S.; Kang, D.H.; Hwu, W.J.; Padhye, N.S.; Masino, C.; Dibaj, S.S.; Liu, D.D.; Williams, J.L.; Lu, Z.; Bruera, E. Cranial electrotherapy stimulation for the management of depression, anxiety, sleep disturbance, and pain in patients with advanced cancer: A preliminary study. J. Pain Symptom Manag. 2018, 55, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Pozos, R.S.; Richardson, A.W.; Kaplan, H.M. Mode of production and locus of action of electroanesthesia in dogs. Anesth. Analg. 1969, 48, 342–345. [Google Scholar] [CrossRef]


| CES Group (n = 20) | Placebo Group (n = 20) | p Value | |
|---|---|---|---|
| Age (years) | 21.55 ± 2.26 | 21.05 ± 1.46 | 0.41 |
| Height (cm) | 171.84 ± 9.66 | 171.17 ± 8.42 | 0.91 |
| Weight (kg) | 69.70 ± 12.51 | 70.33 ± 13.79 | 0.87 |
| BMI(kg/m2) | 23.56 ± 2.79 | 23.93 ± 3.97 | 0.73 |
| Body fat(%) | 21.29 ± 8.44 | 21.81 ± 7.78 | 0.83 |
| Frequency of insomnia (time/week) | 2.48 ± 0.94 | 2.71 ± 1.81 | 0.56 |
| Total PSQI | 9.05 ± 2.46 | 9.14 ± 2.39 | 0.89 |
| Sleep architecture | |||
| REM stage (%) | 20.90 ± 6.23 | 20.52 ± 6.75 | 0.85 |
| N1 stage (%) | 9.28 ± 5.37 | 9.77 ± 5.41 | 0.76 |
| N2 stage (%) | 58.77 ± 8.73 | 56.80 ± 10.94 | 0.52 |
| N3 stage (%) | 11.14 ± 9.21 | 12.90 ± 11.91 | 0.59 |
| N4 stage (%) | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.98 |
| Periodic limb movement | 2.01 ± 7.93 | 1.26 ± 2.44 | 0.68 |
| Lowest SpO2 (%) | 91.14 ± 2.68 | 92.09 ± 2.11 | 0.21 |
| AHI (times/h) | 2.88 ± 5.75 | 1.01 ± 1.16 | 0.15 |
| Sleep onset latency (min) | 15.36 ± 20.24 | 17.81 ± 16.77 | 0.67 |
| Sleep efficiency (%) | 88.01 ± 11.56 | 87.20 ± 7.49 | 0.79 |
| ESS | 9.38 ± 3.81 | 9.71 ± 4.02 | 0.78 |
| CES Group (n = 20) | Placebo Group (n = 20) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre- | 95% CI | Post- | 95% CI | Pre- | 95% CI | Post- | 95% CI | |
| Blood Urea Nitrogen (mg/dL) | 16.01 ± 3.91 | 14.29–17.72 | 15.01 ± 3.90 | 13.30–16.71 | 15.10 ± 3.49 | 13.57–16.63 | 15.24 ± 3.25 | 13.81–16.66 |
| Creatine Phosphate (U/L) | 240.19 ± 198.58 | 153.16–327.22 | 206.95 ± 193.08 | 122.33–291.56 | 239.67 ± 154.62 | 171.90–307.43 | 239.71 ± 112.38 | 190.458–288.96 |
| Testosterone (ng/dL) | 409.60 ± 334.39 | 263.050–556.15 | 396.39 ± 325.19 | 253.87–538.90 | 441.34 ± 340.38 | 292.16–590.51 | 452.89 ± 331.38 | 307.65–598.12 |
| Cortisol (ug/dL) | 12.09 ± 3.45 | 10.57–13.60 | 12.19 ± 3.79 | 10.52–13.85 | 13.59 ± 4.58 | 11.58–15.59 | 13.49 ± 4.89 | 11.347–15.63 |
| Saliva pH | 7.25 ± 0.36 | 7.09–7.40 | 7.09 ± 0.51 | 6.86–7.31 | 7.29 ± 0.40 | 7.11–7.46 | 7.28 ± 0.42 | 7.09–7.46 |
| CES Group (n = 20) | Placebo Group (n = 20) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre- | 95% CI | Post- | 95% CI | Pre- | 95% CI | Post- | 95% CI | |
| POMS | ||||||||
| Confusion | 0.70 ± 0.83 | 0.33–1.06 | 0.38 ± 0.45 | 0.18–0.57 | 0.98 ± 1.18 | 0.46–1.49 | 0.74 ± 0.96 | 0.31–1.16 |
| Fatigue | 0.96 ± 0.84 | 0.59–1.32 | 0.73 ± 0.65 | 0.44–1.01 | 1.23 ± 0.96 | 0.80–1.65 | 1.12 ± 1.10 | 0.638–1.60 |
| Anger | 0.36 ± 0.45 | 0.16–0.55 | 0.11 ± 0.20 * | 0.02–0.19 | 0.24 ± 0.45 | 0.04–0.43 | 0.26 ± 0.47 | 0.05–0.46 |
| Tension | 1.62 ± 0.97 | 1.19–2.04 | 1.12 ± 0.74 * | 0.79–1.44 | 1.65 ± 1.19 | 1.12–2.17 | 1.27 ± 1.05 | 0.81–1.73 |
| Depression | 1.67 ± 1.06 | 1.20–2.13 | 0.81 ± 0.75 * | 0.48–1.13 | 1.38 ± 1.40 | 0.76–1.99 | 1.14 ± 1.28 | 0.57–1.70 |
| Vigor | 2.20 ± 0.82 | 1.84–2.55 | 2.29 ± 0.92 | 1.88–2.69 | 1.77 ± 0.91 | 1.37–2.16 | 1.83 ± 0.97 | 1.40–2.25 |
| Esteem | 1.15 ± 0.48 | 0.94–1.36 | 1.14 ± 0.41 | 0.96–1.32 | 0.97 ± 0.41 | 0.79–1.15 | 1.02 ± 0.48 | 0.81–1.23 |
| Total mood disturbance | 92.05 ± 17.37 | 84.43–99.66 | 84.01 ± 16.99 | 76.56–91.45 | 99.14 ± 22.12 | 89.44–108.83 | 94.52 ± 21.42 | 85.13–103.91 |
| HRV analysis | ||||||||
| Heart rate (bpm) | 62.31 ± 9.58 | 58.11–66.50 | 62.08 ± 9.44 | 57.94–66.21 | 62.50 ± 9.41 | 58.37–66.62 | 61.18 ± 10.76 | 56.46–65.89 |
| SDNN (ms) | 65.68 ± 30.38 | 52.36–78.99 | 68.03 ± 27.08 | 56.16–79.89 | 73.44 ± 49.85 | 51.59–95.28 | 76.62 ± 29.66 | 63.62–89.61 |
| LF (%) | 57.11 ± 16.12 | 50.04–64.17 | 49.37 ± 18.34 | 41.33–57.40 | 54.24 ± 16.70 | 46.92–61.55 | 59.85 ± 14.41 | 53.53–66.16 |
| HF (%) | 42.87 ± 16.10 | 35.81–49.92 | 50.61 ± 18.35 | 42.56–58.65 | 45.57 ± 16.76 | 38.22–52.91 | 40.14 ± 14.42 | 33.82–46.46 |
| LF/HF | 1.80 ± 1.39 | 1.19–2.40 | 1.21 ± 0.73 | 0.89–1.53 | 1.76 ± 1.87 | 0.94–2.58 | 1.85 ± 1.15 # | 1.34–2.35 |
| Reaction time test | ||||||||
| Simple reaction time (ms) | 322.25 ± 40.78 | 304.37–340.12 | 306.95 ± 29.16 | 294.17–319.73 | 307.05 ± 29.18 | 294.26–319.83 | 307.85 ± 32.83 | 293.46–322.23 |
| Choice reaction time (ms) | 420.85 ± 41.22 | 402.78–438.91 | 399.90 ± 36.71 *# | 383.81–415.98 | 419.35 ± 46.12 | 399.13–439.56 | 428.15 ± 48.73 | 406.79–449.50 |
| CES Group (n = 20) | Placebo Group (n = 20) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre- | 95% CI | Post- | 95% CI | Pre- | 95% CI | Post- | 95% CI | |
| Sleep latency | 2.19 ± 1.53 | 1.51–2.86 | 3.52 ± 2.94 | 2.23–4.80 | 2.57 ± 2.13 | 1.63–3.50 | 2.62 ± 2.32 | 1.60–3.63 |
| Sleep efficiency (%) | 87.94 ± 6.76 | 84.97–90.90 | 81.75 ± 9.62 * | 77.53–85.96 | 89.60 ± 9.19 | 85.57–93.62 | 81.36 ± 9.64 * | 77.13–85.58 |
| Total minutes in bed (min) | 370.01 ± 52.94 | 346.80–393.21 | 372.86 ± 75.06 | 339.96– 405.75 | 392.19 ± 38.29 | 375.40–408.97 | 320.62 ± 74.08 *# | 288.15–353.08 |
| Total sleep time (min) | 327.33 ± 63.35 | 299.56–355.09 | 306.71 ± 85.02 | 269.44–343.97 | 352.29 ± 56.69 | 327.44–377.13 | 262.71 ± 77.35 * | 228.81–296.61 |
| WASO | 40.48 ± 22.06 | 30.81–50.14 | 62.62 ± 35.70 | 46.97–78.26 | 37.33 ± 35.63 | 21.71–52.94 | 55.29 ± 28.34 | 42.87–67.71 |
| Number of awakenings | 15.03 ± 5.74 | 12.51–17.54 | 19.43 ± 10.12 * | 14.99–23.86 | 13.29 ± 7.44 | 10.02–16.55 | 21.62 ± 6.79 * | 18.64–24.59 |
| Average awakening length | 2.61 ± 1.05 | 2.15–3.07 | 3.61 ± 2.84 | 2.36–4.85 | 2.35 ± 1.36 | 1.75–2.94 | 2.54 ± 0.90 | 2.14–2.93 |
| Movement index | 13.18 ± 5.33 | 10.84–15.51 | 20.82 ± 17.64 | 13.08–28.55 | 15.59 ± 11.63 | 10.49–20.68 | 16.78 ± 10.40 | 12.22–21.33 |
| Fragmentation index | 13.03 ± 10.05 | 8.62–17.43 | 14.74 ± 8.60 | 10.97–18.50 | 11.76 ± 7.67 | 8.39–15.12 | 15.20 ± 8.37 | 11.53–18.86 |
| Sleep fragmentation index | 26.21 ± 13.74 | 20.18–32.23 | 35.56 ± 16.84 | 28.18–42.94 | 27.35 ± 14.76 | 20.88–33.81 | 31.98 ± 14.77 | 25.507–38.45 |
| Total Mood Disturbance | LF (%) | HF (%) | LF/HF | Simple Reaction Time (ms) | Choice Reaction Time (ms) | |
|---|---|---|---|---|---|---|
| CES group | ||||||
| LF (%) | −0.26 | |||||
| HF (%) | 0.26 | −1.00 * | ||||
| LF/HF | −0.13 | 0.95 * | −0.95 * | |||
| Simple reaction time (ms) | 0.16 | 0.13 | −0.13 | 0.12 | ||
| Choice reaction time (ms) | 0.28 | −0.11 | 0.11 | −0.03 | 0.35 | |
| Sleep efficiency (%) | −0.51 * | 0.01 | −0.01 | −0.01 | −0.11 | −0.41 |
| Placebo group | ||||||
| LF (%) | 0.29 | |||||
| HF (%) | −0.30 | −1.00 * | ||||
| LF/HF | 0.10 | 0.89 * | −0.89 * | |||
| Simple reaction time (ms) | −0.08 | −0.01 | 0.01 | 0.26 | ||
| Choice reaction time (ms) | 0.02 | −0.02 | 0.03 | −0.01 | 0.26 | |
| Sleep efficiency (%) | −0.21 | −0.09 | 0.10 | −0.12 | −0.05 | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.-D.; Tsou, Y.-A.; Chen, Y.-Y.; Hung, B.-L. Cranial Electrotherapy Stimulation to Improve the Physiology and Psychology Response, Response-Ability, and Sleep Efficiency in Athletes with Poor Sleep Quality. Int. J. Environ. Res. Public Health 2022, 19, 1946. https://doi.org/10.3390/ijerph19041946
Chang W-D, Tsou Y-A, Chen Y-Y, Hung B-L. Cranial Electrotherapy Stimulation to Improve the Physiology and Psychology Response, Response-Ability, and Sleep Efficiency in Athletes with Poor Sleep Quality. International Journal of Environmental Research and Public Health. 2022; 19(4):1946. https://doi.org/10.3390/ijerph19041946
Chicago/Turabian StyleChang, Wen-Dien, Yung-An Tsou, Yi-Ying Chen, and Bao-Lien Hung. 2022. "Cranial Electrotherapy Stimulation to Improve the Physiology and Psychology Response, Response-Ability, and Sleep Efficiency in Athletes with Poor Sleep Quality" International Journal of Environmental Research and Public Health 19, no. 4: 1946. https://doi.org/10.3390/ijerph19041946
APA StyleChang, W.-D., Tsou, Y.-A., Chen, Y.-Y., & Hung, B.-L. (2022). Cranial Electrotherapy Stimulation to Improve the Physiology and Psychology Response, Response-Ability, and Sleep Efficiency in Athletes with Poor Sleep Quality. International Journal of Environmental Research and Public Health, 19(4), 1946. https://doi.org/10.3390/ijerph19041946







