Silicone Wristbands in Exposure Assessment: Analytical Considerations and Comparison with Other Approaches
Abstract
1. Introduction
2. PDMS as a Sampler Material
3. Emergence of Silicone Wristbands in Exposure Assessment
4. Search Engine and Exclusion Criteria
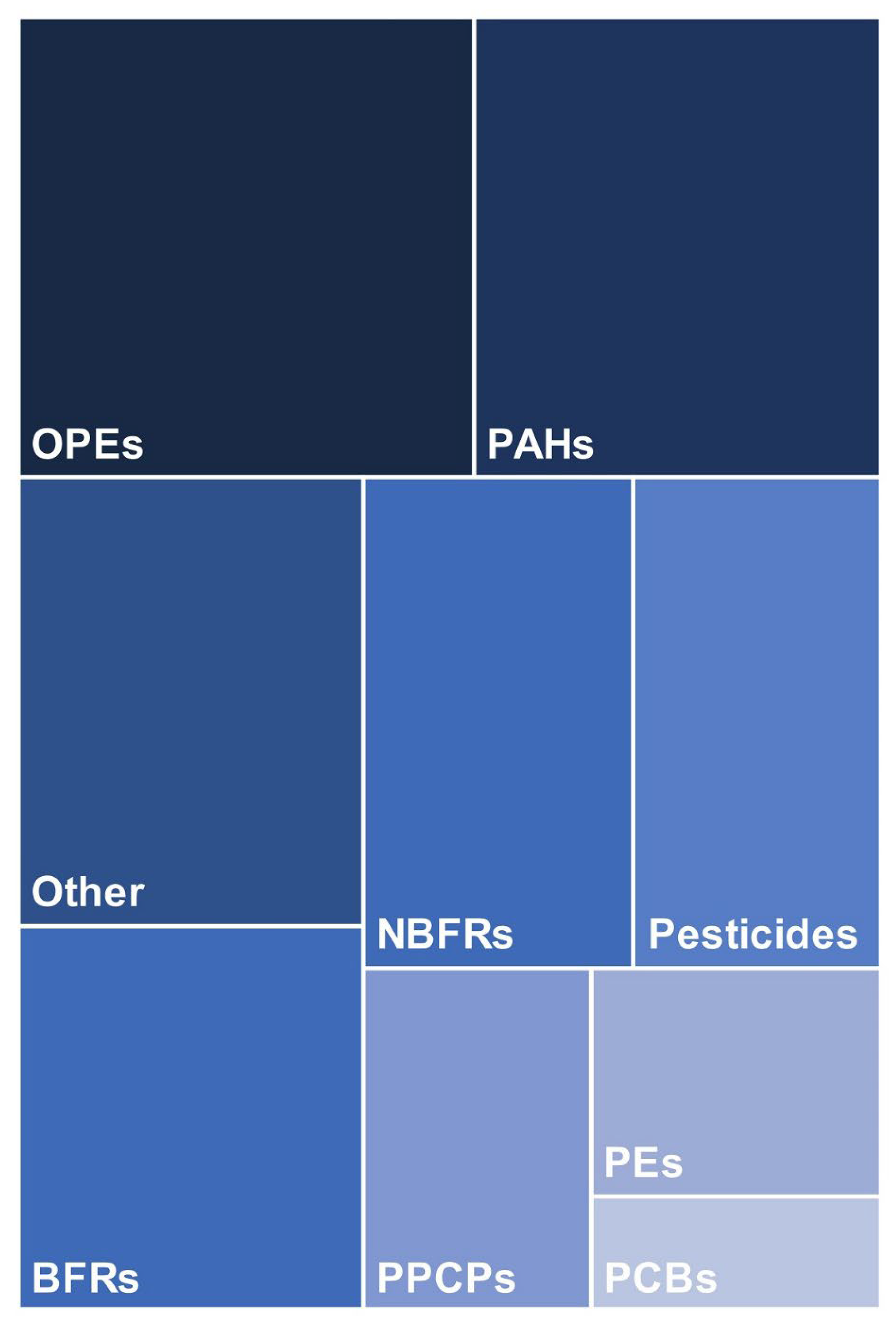
5. Chemical Analysis of Silicone Wristbands
5.1. Pre-Deployment Cleanup
5.2. Post-Deployment Cleanup
5.3. Extraction
5.4. Post-Extraction Cleanup
5.5. Other Methods
6. Qualitative and Quantitative Analysis
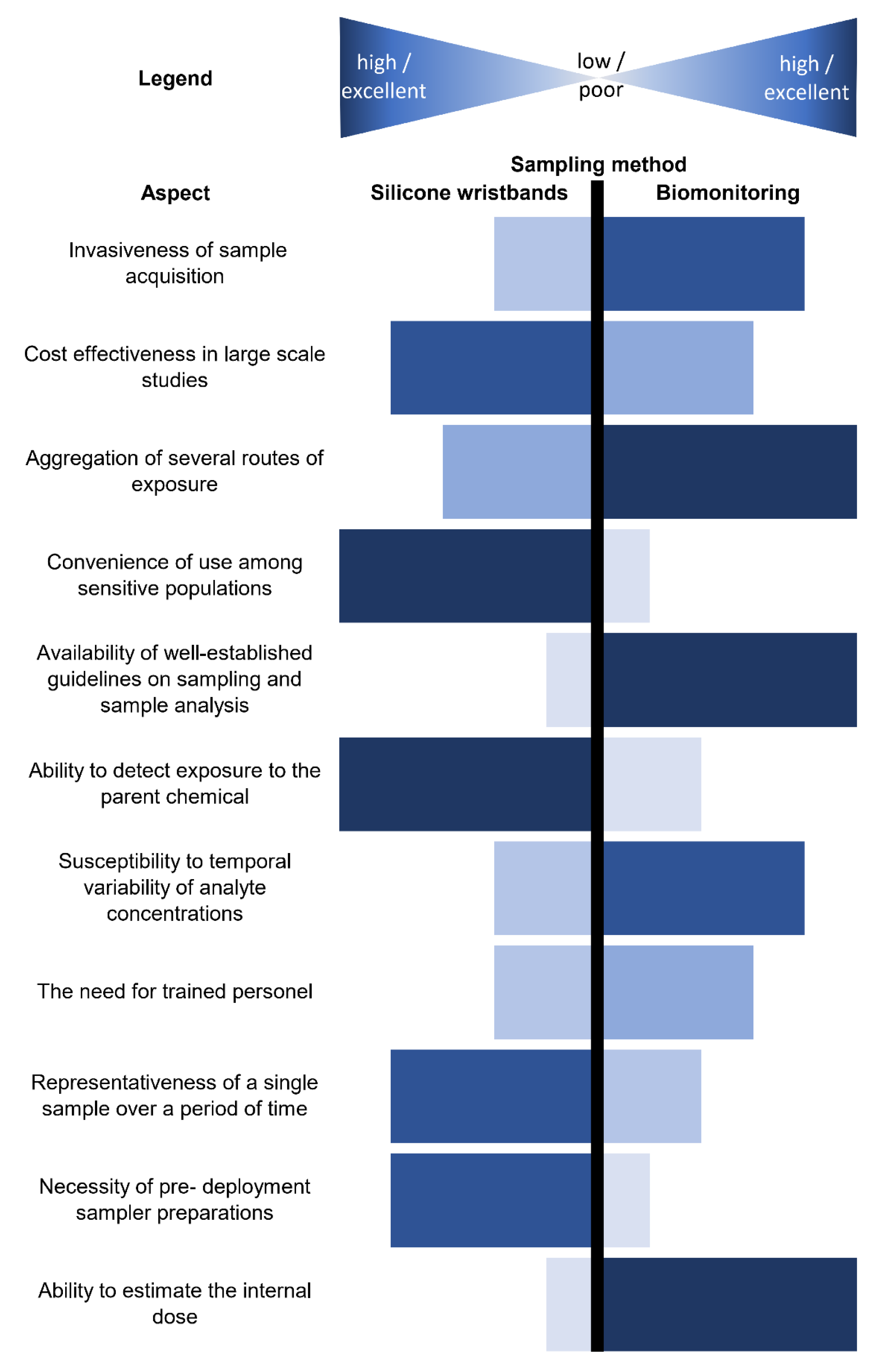
7. Comparison of Wristbands with Other Matrices
7.1. Biological Matrices
7.1.1. Urine
7.1.2. Blood
7.2. Environmental Matrices
7.2.1. Hand Wipes
7.2.2. Active Air Sampling (AAS)
7.2.3. Settled Dust
7.2.4. Other
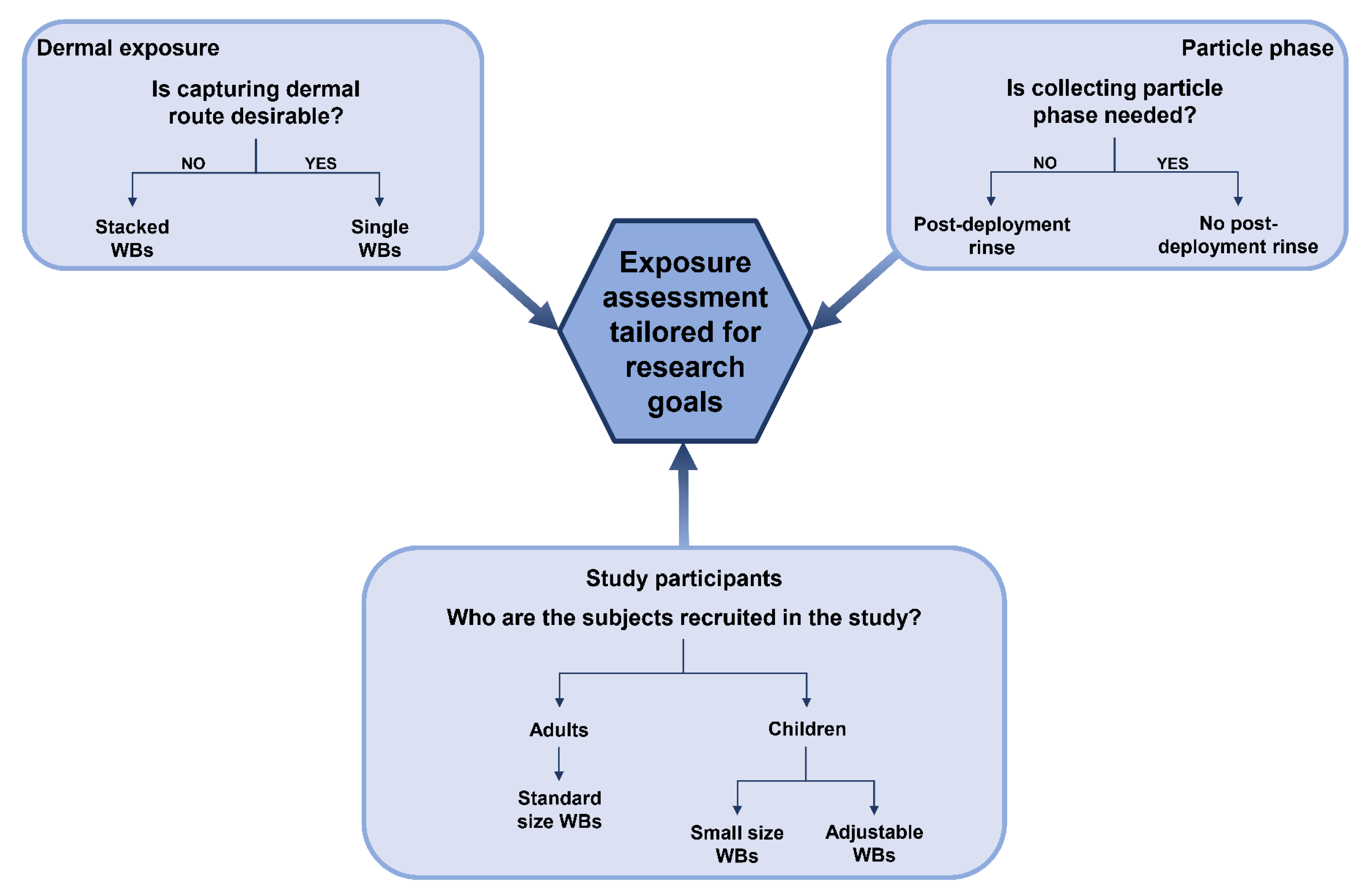
8. Future Prospects
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- UNEP. Global Chemicals Outlook—Towards Sound Management of Chemicals; UNEP: Geneva, Switzerland, 2013. [Google Scholar]
- Prüss-Ustün, A.; Vickers, C.; Haefliger, P.; Bertollini, R. Knowns and unknowns on burden of disease due to chemicals: A systematic review. Environ. Health 2011, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- EEA. Chemicals for a Sustainable Future: Report of the EEA Scientific Committee Seminar; EEA: Luxembourg, 2018. [Google Scholar]
- Lowry, L.K. Role of biomarkers of exposure in the assessment of human risk. Toxicol. Lett. 1995, 77, 31–38. [Google Scholar] [CrossRef]
- Ganzleben, C.; Antignac, J.P.; Barouki, R.; Castaño, A.; Fiddicke, U.; Klánová, J.; Lebret, E.; Olea, N.; Sarigiannis, D.; Schoeters, G.R.; et al. Human biomonitoring as a tool to support chemicals regulation in the European Union. Int. J. Hyg. Environ. Health 2017, 220, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Aylward, L.L.; Hays, S.M.; Smolders, R.; Koch, H.M.; Cocker, J.; Jones, K.; Warren, N.; Levy, L.; Bevan, R. Sources of Variability in Biomarker Concentrations. J. Toxicol. Environ. Health Part B Crit. Rev. 2014, 17, 45–61. [Google Scholar] [CrossRef]
- WHO. Human Biomonitoring: Facts and Figures; WHO: Copenhagen, Denmark, 2015. [Google Scholar]
- Haines, D.A.; Saravanabhavan, G.; Werry, K.; Khoury, C. An overview of human biomonitoring of environmental chemicals in the Canadian Health Measures Survey: 2007–2019. Int. J. Hyg. Environ. Health 2017, 220, 13–28. [Google Scholar] [CrossRef]
- Joas, A.; Knudsen, L.E.; Kolossa-Gehring, M.; Sepai, O.; Casteleyn, L.; Schoeters, G.; Angerer, J.; Castaño, A.; Aerts, D.; Biot, P.; et al. Policy recommendations and cost implications for a more sustainable framework for European human biomonitoring surveys. Environ. Res. 2015, 141, 42–57. [Google Scholar] [CrossRef]
- Lioy, P.J. Measurement Methods for Human Exposure Analysis. Environ. Health Perspect. 1995, 103, 35–43. [Google Scholar] [CrossRef][Green Version]
- Barr, J.R.; Driskell, W.J.; Hill, R.H.; Ashley, D.L.; Needham, L.L.; Head, S.L.; Sampson, E.J.; Barr, D.B. Strategies for biological monitoring of exposure for contemporary-use pesticides. Toxicol. Ind. Health 1999, 15, 169–180. [Google Scholar] [CrossRef]
- LaKind, J.S.; Idri, F.; Naiman, D.Q.; Verner, M.A. Biomonitoring and Nonpersistent Chemicals—Understanding and Addressing Variability and Exposure Misclassification. Curr. Environ. Health Rep. 2019, 6, 16–21. [Google Scholar] [CrossRef]
- Porta, M.; Puigdomènech, E.; Ballester, F.; Selva, J.; Ribas-Fitó, N.; Llop, S.; López, T. Monitoring concentrations of persistent organic pollutants in the general population: The international experience. Environ. Int. 2008, 34, 546–561. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Glass, K.; Morris, S.; Zhang, H.; McRae, I.; Anderson, N.; Alfieri, A.; Egendorf, S.P.; Holberton, S.; Owrang, S.; et al. Sediment exchange to mitigate pollutant exposure in urban soil. J. Environ. Manag. 2018, 214, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Jimenez, J.; Zanca, N.; Lan, H.; Jussila, M.; Hartonen, K.; Riekkola, M.L. Aerial drone as a carrier for miniaturized air sampling systems. J. Chromatogr. A 2019, 1597, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Mercier, F.; Glorennec, P.; Thomas, O.; Bot, B. Le Organic contamination of settled house dust, a review for exposure assessment purposes. Environ. Sci. Technol. 2011, 45, 6716–6727. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.L.R.; Bakali, U.; Killawala, C.; Santiago, K.M.; Dikici, E.; Kobetz, E.N.; Solle, N.S.; Deo, S.; Bachas, L.; Daunert, S. Evaluation of silicone-based wristbands as passive sampling systems using PAHs as an exposure proxy for carcinogen monitoring in firefighters: Evidence from the firefighter cancer initiative. Ecotoxicol. Environ. Saf. 2020, 205, 111100. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, A.J.; North, P.E.; Vasquez, L.; Bello, H.; Del Carmen Gastañaga Ruiz, M.; Anderson, K.A. Multi-class chemical exposure in rural Peru using silicone wristbands. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 560–568. [Google Scholar] [CrossRef]
- O’Connell, S.G.; Kincl, L.D.; Anderson, K.A. Silicone wristbands as personal passive samplers. Environ. Sci. Technol. 2014, 48, 3327–3335. [Google Scholar] [CrossRef]
- Wang, S.; Romanak, K.A.; Stubbings, W.A.; Arrandale, V.H.; Hendryx, M.; Diamond, M.L.; Salamova, A.; Venier, M. Silicone wristbands integrate dermal and inhalation exposures to semi-volatile organic compounds (SVOCs). Environ. Int. 2019, 132, 105104. [Google Scholar] [CrossRef]
- Craig, J.A.; Ceballos, D.M.; Fruh, V.; Petropoulos, Z.E.; Allen, J.G.; Calafat, A.M.; Ospina, M.; Stapleton, H.M.; Hammel, S.C.; Gray, R.; et al. Exposure of Nail Salon Workers to Phthalates, Di(2-ethylhexyl) Terephthalate, and Organophosphate Esters: A Pilot Study. Environ. Sci. Technol. 2019, 53, 14630–14637. [Google Scholar] [CrossRef] [PubMed]
- Okeme, J.O.; Yang, C.; Abdollahi, A.; Dhal, S.; Harris, S.A.; Jantunen, L.M.; Tsirlin, D.; Diamond, M.L. Passive air sampling of flame retardants and plasticizers in Canadian homes using PDMS, XAD-coated PDMS and PUF samplers. Environ. Pollut. 2018, 239, 109–117. [Google Scholar] [CrossRef]
- Aerts, R.; Joly, L.; Szternfeld, P.; Tsilikas, K.; De Cremer, K.; Castelain, P.; Aerts, J.M.; Van Orshoven, J.; Somers, B.; Hendrickx, M.; et al. Silicone Wristband Passive Samplers Yield Highly Individualized Pesticide Residue Exposure Profiles. Environ. Sci. Technol. 2018, 52, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.J. Silicone Surface Fundamentals. Macromol. Rapid Commun. 2021, 42, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Seethapathy, S.; Górecki, T. Applications of polydimethylsiloxane in analytical chemistry: A review. Anal. Chim. Acta 2012, 750, 48–62. [Google Scholar] [CrossRef]
- Vidi, P.A.; Anderson, K.A.; Chen, H.; Anderson, R.; Salvador-Moreno, N.; Mora, D.C.; Poutasse, C.; Laurienti, P.J.; Daniel, S.S.; Arcury, T.A. Personal samplers of bioavailable pesticides integrated with a hair follicle assay of DNA damage to assess environmental exposures and their associated risks in children. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2017, 822, 27–33. [Google Scholar] [CrossRef]
- Wang, S.; Romanak, K.A.; Tarallo, S.; Francavilla, A.; Viviani, M.; Vineis, P.; Rothwell, J.A.; Mancini, F.R.; Cordero, F.; Naccarati, A.; et al. The use of silicone wristbands to evaluate personal exposure to semi-volatile organic chemicals (SVOCs) in France and Italy. Environ. Pollut. 2020, 267, 115490. [Google Scholar] [CrossRef]
- Rusina, T.P.; Smedes, F.; Klanova, J.; Booij, K.; Holoubek, I. Polymer selection for passive sampling: A comparison of critical properties. Chemosphere 2007, 68, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Carraher, C.E., Jr. Introduction to Polymer Chemistry, 4th ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 309–314. [Google Scholar]
- Mazurek, M. Silicone Copolymer Networsk and Interpretating Polymer Networks. In Silicon-Containing Polymers; Jones, R.G., Ando, W., Chojnowski, J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 113–137. [Google Scholar]
- Suwandi, M.S.; Stern, S.A. Transport of Heavy Organic Vapors Through Silicone Rubber. J. Polym. Sci. Part A-2 Polym. Phys. 1973, 11, 663–681. [Google Scholar] [CrossRef]
- Brockmeyer, B.; Kraus, U.R.; Theobald, N. Accelerated solvent extraction (ASE) for purification and extraction of silicone passive samplers used for the monitoring of organic pollutants. Environ. Sci. Pollut. Res. 2015, 22, 19887–19895. [Google Scholar] [CrossRef]
- Shahpoury, P.; Hageman, K.J. Pressurized liquid extraction of polycyclic aromatic hydrocarbons from silicone rubber passive samplers. J. Chromatogr. A 2013, 1314, 1–6. [Google Scholar] [CrossRef]
- Smedes, F.; Booij, K. Guidelines for passive sampling of hydrophobic contaminants in water using silicone rubber samplers. ICES Tech. Mar. Environ. Sci. 2012, 52, 20. [Google Scholar]
- Anderson, K.A.; Points, G.L.; Donald, C.E.; Dixon, H.M.; Scott, R.P.; Wilson, G.; Tidwell, L.G.; Hoffman, P.D.; Herbstman, J.B.; O’Connell, S.G. Preparation and performance features of wristband samplers and considerations for chemical exposure assessment. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 551–559. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, S.G.; Anderson, K.A.; Epstein, M.I. Determining chemical air equivalency using silicone personal monitors. J. Expo. Sci. Environ. Epidemiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Tromp, P.C.; Beeltje, H.; Okeme, J.O.; Vermeulen, R.; Pronk, A.; Diamond, M.L. Calibration of polydimethylsiloxane and polyurethane foam passive air samplers for measuring semi volatile organic compounds using a novel exposure chamber design. Chemosphere 2019, 227, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Okeme, J.O.; Saini, A.; Yang, C.; Zhu, J.; Smedes, F.; Klánová, J.; Diamond, M.L. Calibration of polydimethylsiloxane and XAD-Pocket passive air samplers (PAS) for measuring gas- and particle-phase SVOCs. Atmos. Environ. 2016, 143, 202–208. [Google Scholar] [CrossRef]
- Sedlačková, L.; Melymuk, L.; Vrana, B. Calibration of silicone for passive sampling of semivolatile organic contaminants in indoor air. Chemosphere 2021, 279, 130536. [Google Scholar] [CrossRef]
- Vorkamp, K.; Odsbjerg, L.; Langeland, M.; Mayer, P. Utilizing the partitioning properties of silicone for the passive sampling of polychlorinated biphenyls (PCBs) in indoor air. Chemosphere 2016, 160, 280–286. [Google Scholar] [CrossRef]
- Allan, I.J.; Harman, C.; Ranneklev, S.B.; Thomas, K.V.; Grung, M. Passive sampling for target and nontarget analyses of moderately polar and nonpolar substances in water. Environ. Toxicol. Chem. 2013, 32, 1718–1726. [Google Scholar] [CrossRef]
- The Observer: How a Yellow Wristband Became a Fashion Must. Available online: https://www.theguardian.com/society/2004/aug/08/cancercare.uknews (accessed on 11 April 2021).
- Nicole, W. Wristbands for research: Using wearable sensors to collect exposure data after Hurricane Harvey. Environ. Health Perspect. 2018, 126, 1–9. [Google Scholar] [CrossRef]
- Manzano, C.A.; Dodder, N.G.; Hoh, E.; Morales, R. Patterns of Personal Exposure to Urban Pollutants Using Personal Passive Samplers and GC × GC/ToF-MS. Environ. Sci. Technol. 2019, 53, 614–624. [Google Scholar] [CrossRef]
- Doherty, B.T.; Pearce, J.L.; Anderson, K.A.; Karagas, M.R.; Romano, M.E. Assessment of Multipollutant Exposures During Pregnancy Using Silicone Wristbands. Front. Public Health 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Hendryx, M.; Wang, S.; Romanak, K.A.; Salamova, A.; Venier, M. Personal exposure to polycyclic aromatic hydrocarbons in Appalachian mining communities. Environ. Pollut. 2020, 257, 113501. [Google Scholar] [CrossRef] [PubMed]
- Dixon, H.M.; Scott, R.P.; Holmes, D.; Calero, L.; Kincl, L.D.; Waters, K.M.; Camann, D.E.; Calafat, A.M.; Herbstman, J.B.; Anderson, K.A. Silicone wristbands compared with traditional polycyclic aromatic hydrocarbon exposure assessment methods. Anal. Bioanal. Chem. 2018, 410, 3059–3071. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.V.; Gravel, S.; Labrèche, F.; Bakhiyi, B.; Verner, M.A.; Zayed, J.; Jantunen, L.M.; Arrandale, V.H.; Diamond, M.L. Can Silicone Passive Samplers be Used for Measuring Exposure of e-Waste Workers to Flame Retardants? Environ. Sci. Technol. 2020, 54, 15277–15286. [Google Scholar] [CrossRef] [PubMed]
- Quintana, P.J.E.; Lopez-Galvez, N.; Dodder, N.G.; Hoh, E.; Matt, G.E.; Zakarian, J.M.; Vyas, M.; Chu, L.; Akins, B.; Padilla, S.; et al. Nicotine, Cotinine, and Tobacco-Specific Nitrosamines Measured in Children’s Silicone Wristbands in Relation to Secondhand Smoke and E-cigarette Vapor Exposure. Nicotine Tob. Res. 2020, 23, 592–599. [Google Scholar] [CrossRef]
- Hammel, S.C.; Hoffman, K.; Webster, T.F.; Anderson, K.A.; Stapleton, H.M. Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ. Sci. Technol. 2016, 50, 4483–4491. [Google Scholar] [CrossRef]
- Kile, M.L.; Scott, R.P.; O’Connell, S.G.; Lipscomb, S.; MacDonald, M.; McClelland, M.; Anderson, K.A. Using silicone wristbands to evaluate preschool children’s exposure to flame retardants. Environ. Res. 2016, 147, 365–372. [Google Scholar] [CrossRef]
- Chen, Y.; Pawliszyn, J. Time-weighted average water sampling with a solid-phase microextraction device. Anal. Chem. 2003, 75, 2004–2010. [Google Scholar] [CrossRef]
- Rohlman, D.S.; Syron, L.; Hobbie, K.; Anderson, K.A.; Scaffidi, C.; Sudakin, D.; Peterson, E.S.; Waters, K.M.; Haynes, E.; Arkin, L.; et al. A Community-Based Approach to Developing a Mobile Device for Measuring Ambient Air Exposure, Location, and Respiratory Health. Environ. Justice 2015, 8, 126–134. [Google Scholar] [CrossRef]
- Donald, C.E.; Scott, R.P.; Blaustein, K.L.; Halbleib, M.L.; Sarr, M.; Jepson, P.C.; Anderson, K.A. Silicone wristbands detect individuals’ pesticide exposures in West Africa. R. Soc. Open Sci. 2016, 3, 160433. [Google Scholar] [CrossRef]
- Lipscomb, S.T.; McClelland, M.M.; MacDonald, M.; Cardenas, A.; Anderson, K.A.; Kile, M.L. Cross-sectional study of social behaviors in preschool children and exposure to flame retardants. Environ. Health 2017, 16, 1–10. [Google Scholar] [CrossRef]
- Paulik, L.B.; Hobbie, K.A.; Rohlman, D.; Smith, B.W.; Scott, R.P.; Kincl, L.; Haynes, E.N.; Anderson, K.A. Environmental and individual PAH exposures near rural natural gas extraction. Environ. Pollut. 2018, 241, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Hammel, S.C.; Phillips, A.L.; Hoffman, K.; Stapleton, H.M. Evaluating the Use of Silicone Wristbands to Measure Personal Exposure to Brominated Flame Retardants. Environ. Sci. Technol. 2018, 52, 11875–11885. [Google Scholar] [CrossRef] [PubMed]
- De Vecchi, R.; da Silveira Carvalho Ripper, J.; Roy, D.; Breton, L.; Germano Marciano, A.; Bernardo de Souza, P.M.; de Paula Corrêa, M. Using wearable devices for assessing the impacts of hair exposome in Brazil. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rohlman, D.S.; Donatuto, J.; Heidt, M.; Barton, M.; Campbell, L.; Anderson, K.A.; Kile, M.L. A Case Study Describing a Community-Engaged Approach for Evaluating Polycyclic Aromatic Hydrocarbon Exposure in a Native American Community. Int. J. Environ. Res. Public Health 2019, 16, 327. [Google Scholar] [CrossRef]
- Harley, K.G.; Parra, K.L.; Camacho, J.; Bradman, A.; Nolan, J.E.S.; Lessard, C.; Anderson, K.A.; Poutasse, C.M.; Scott, R.P.; Lazaro, G.; et al. Determinants of pesticide concentrations in silicone wristbands worn by Latina adolescent girls in a California farmworker community: The COSECHA youth participatory action study. Sci. Total Environ. 2019, 652, 1022–1029. [Google Scholar] [CrossRef]
- Gibson, E.A.; Stapleton, H.M.; Calero, L.; Holmes, D.; Burke, K.; Martinez, R.; Cortes, B.; Nematollahi, A.; Evans, D.; Anderson, K.A.; et al. Differential exposure to organophosphate flame retardants in mother-child pairs. Chemosphere 2019, 219, 567–573. [Google Scholar] [CrossRef]
- Romanak, K.A.; Wang, S.; Stubbings, W.A.; Hendryx, M.; Venier, M.; Salamova, A. Analysis of brominated and chlorinated flame retardants, organophosphate esters, and polycyclic aromatic hydrocarbons in silicone wristbands used as personal passive samplers. J. Chromatogr. A 2019, 1588, 41–47. [Google Scholar] [CrossRef]
- Quintana, P.J.E.; Hoh, E.; Dodder, N.G.; Matt, G.E.; Zakarian, J.M.; Anderson, K.A.; Akins, B.; Chu, L.; Hovell, M.F. Nicotine levels in silicone wristband samplers worn by children exposed to secondhand smoke and electronic cigarette vapor are highly correlated with child’s urinary cotinine. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 733–741. [Google Scholar] [CrossRef]
- Zuy, Y.; Sweck, S.O.; Dockery, C.R.; Potts, G.E. HPLC detection of organic gunshot residues collected with silicone wristbands. Anal. Methods 2020, 12, 85–90. [Google Scholar] [CrossRef]
- Rohlman, D.S.; Dixon, H.M.; Kincl, L.; Larkin, A.; Evoy, R.; Barton, M.; Phillips, A.; Peterson, E.; Scaffidi, C.; Herbstman, J.B.; et al. Development of an environmental health tool linking chemical exposures, physical location and lung function. BMC Public Health 2019, 19, 854. [Google Scholar] [CrossRef]
- Ulrich, E.M.; Sobus, J.R.; Grulke, C.M.; Richard, A.M.; Newton, S.R.; Strynar, M.J.; Mansouri, K.; Williams, A.J. EPA’s non-targeted analysis collaborative trial (ENTACT): Genesis, design, and initial findings. Anal. Bioanal. Chem. 2019, 411, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Travis, S.C.; Aga, D.S.; Queirolo, E.I.; Olson, J.R.; Daleiro, M.; Kordas, K. Catching flame retardants and pesticides in silicone wristbands: Evidence of exposure to current and legacy pollutants in Uruguayan children. Sci. Total Environ. 2020, 740, 140136. [Google Scholar] [CrossRef] [PubMed]
- Reche, C.; Viana, M.; van Drooge, B.L.; Fernández, F.J.; Escribano, M.; Castaño-Vinyals, G.; Nieuwenhuijsen, M.; Adami, P.E.; Bermon, S. Athletes’ exposure to air pollution during World Athletics Relays: A pilot study. Sci. Total Environ. 2020, 717, 137161. [Google Scholar] [CrossRef] [PubMed]
- Reddam, A.; Tait, G.; Herkert, N.; Hammel, S.C.; Stapleton, H.M.; Volz, D.C. Longer commutes are associated with increased human exposure to tris(1,3-dichloro-2-propyl) phosphate. Environ. Int. 2020, 136, 105499. [Google Scholar] [CrossRef]
- Caban-Martinez, A.J.; Louzado-Feliciano, P.; Santiago, K.M.; Baum, J.; Schaefer Solle, N.; Rivera, G.; Miric, M.; Perez-Then, E.; Kobetz-Kerman, E.N.; Daunert, S. Objective Measurement of Carcinogens Among Dominican Republic Firefighters Using Silicone-Based Wristbands. J. Occup. Environ. Med. 2020, 62, e611–e615. [Google Scholar] [CrossRef]
- Hammel, S.C.; Hoffman, K.; Phillips, A.L.; Levasseur, J.L.; Lorenzo, A.M.; Webster, T.F.; Stapleton, H.M. Comparing the Use of Silicone Wristbands, Hand Wipes, and Dust to Evaluate Children’s Exposure to Flame Retardants and Plasticizers. Environ. Sci. Technol. 2020, 54, 4484–4494. [Google Scholar] [CrossRef]
- Wang, Y.; Peris, A.; Rifat, M.R.; Ahmed, S.I.; Aich, N.; Nguyen, L.V.; Urík, J.; Eljarrat, E.; Vrana, B.; Jantunen, L.M.; et al. Measuring exposure of e-waste dismantlers in Dhaka Bangladesh to organophosphate esters and halogenated flame retardants using silicone wristbands and T-shirts. Sci. Total Environ. 2020, 720, 137480. [Google Scholar] [CrossRef]
- Wise, C.F.; Wise, C.F.; Hammel, S.C.; Herkert, N.; Ma, J.; Ma, J.; Motsinger-Reif, A.; Stapleton, H.M.; Stapleton, H.M.; Breen, M.; et al. Comparative Exposure Assessment Using Silicone Passive Samplers Indicates That Domestic Dogs Are Sentinels to Support Human Health Research. Environ. Sci. Technol. 2020, 54, 7409–7419. [Google Scholar] [CrossRef]
- Santiago, K.M.; Louzado-Feliciano, P.; Baum, J.; Bakali, U.; Caban-Martinez, A.J. Self-reported and objectively measured occupational exposures, health, and safety concerns among fishermen: A cross-sectional Fishing Industry Safety and Health (FISH) pilot study. Am. J. Ind. Med. 2021, 64, 58–69. [Google Scholar] [CrossRef]
- Levasseur, J.L.; Hammel, S.C.; Hoffman, K.; Phillips, A.L.; Zhang, S.; Ye, X.; Calafat, A.M.; Webster, T.F.; Stapleton, H.M. Young children’s exposure to phenols in the home: Associations between house dust, hand wipes, silicone wristbands, and urinary biomarkers. Environ. Int. 2021, 147, 106317. [Google Scholar] [CrossRef]
- Arcury, T.A.; Chen, H.; Quandt, S.A.; Talton, J.W.; Anderson, K.A.; Scott, R.P.; Jensen, A.; Laurienti, P.J. Pesticide exposure among Latinx children: Comparison of children in rural, farmworker and urban, non-farmworker communities. Sci. Total Environ. 2021, 763, 144233. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Guan, Q.; Li, L.; Pan, X.; Ho, C. Exposure of children and mothers to organophosphate esters: Prediction by house dust and silicone wristbands. Environ. Pollut. 2021, 282, 117011. [Google Scholar] [CrossRef] [PubMed]
- Dixon, H.M.; Armstrong, G.; Barton, M.; Bergmann, A.J.; Bondy, M.; Halbleib, M.L.; Hamilton, W.; Haynes, E.; Herbstman, J.; Hoffman, P.; et al. Discovery of common chemical exposures across three continents using silicone wristbands. R. Soc. Open Sci. 2019, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mantinieks, D.; Gerostamoulos, D.; Wright, P.; Drummer, O. The effectiveness of decontamination procedures used in forensic hair analysis. Forensic Sci. Med. Pathol. 2018, 14, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Żwir-Ferenc, A.; Biziuk, M. Solid Phase Extraction Technique—Trends, Opportunities and Applications. Pol. J. Environ. Stud. 2006, 15, 677–690. [Google Scholar]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Travis, S.C.; Kordas, K.; Aga, D.S. Optimized workflow for unknown screening using gas chromatography high-resolution mass spectrometry expands identification of contaminants in silicone personal passive samplers. Rapid Commun. Mass Spectrom. 2021, 35, 1–13. [Google Scholar] [CrossRef]
- Wang, Z.; Dewitt, J.C.; Higgins, C.P.; Cousins, I.T. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol. 2017, 51, 2508–2518. [Google Scholar] [CrossRef]
- Lohmann, R.; Booij, K.; Smedes, F. Use of passive sampling devices for monitoring and compliance checking of POP concentrations in water. Environ. Sci. Pollut. Res. 2012, 1885–1895. [Google Scholar] [CrossRef]
- Villaverde-de-Sáa, E.; Racamonde, I.; Quintana, J.B.; Rodil, R.; Cela, R. Ion-pair sorptive extraction of perfluorinated compounds from water with low-cost polymeric materials: Polyethersulfone vs. polydimethylsiloxane. Anal. Chim. Acta 2012, 740, 50–57. [Google Scholar] [CrossRef]
- Vrana, B.; (Masaryk University, Brno, Czech Republic). Personal communication, 2021.
- Verner, M.A.; Salame, H.; Housand, C.; Birnbaum, L.S.; Bouchard, M.F.; Chevrier, J.; Aylward, L.L.; Naiman, D.Q.; Lakind, J.S. How many urine samples are needed to accurately assess exposure to non-persistent chemicals? The biomarker reliability assessment tool (BRAT) for scientists, research sponsors, and risk managers. Int. J. Environ. Res. Public Health 2020, 17, 9102. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Longnecker, M.P.; Koch, H.M.; Swan, S.H.; Hauser, R.; Goldman, L.R.; Lanphear, B.P.; Rudel, R.A.; Engel, S.M.; Teitelbaum, S.L.; et al. Optimal Exposure Biomarkers for Nonpersistent Chemicals in Environmental Epidemiology. Environ. Health Perspect. 2015, 123, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Carignan, C.C.; McClean, M.D.; Cooper, E.M.; Watkins, D.J.; Fraser, A.J.; Heiger-Bernays, W.; Stapleton, H.M.; Webster, T.F. Predictors of tris(1,3-dichloro-2-propyl) phosphate metabolite in the urine of office workers. Environ. Int. 2013, 55, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Van den Eede, N.; Heffernan, A.L.; Aylward, L.L.; Hobson, P.; Neels, H.; Mueller, J.F.; Covaci, A. Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ. Int. 2015, 74, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bastiaensen, M.; Gys, C.; Malarvannan, G.; Fotache, M.; Bombeke, J.; Ait, Y.; Araki, A.; Covaci, A. Short-term temporal variability of urinary biomarkers of organophosphate flame retardants and plasticizers. Environ. Int. 2021, 146, 106147. [Google Scholar] [CrossRef]
- Ding, J.; Deng, T.; Xu, M.; Wang, S.; Yang, F. Residuals of organophosphate esters in foodstuffs and implication for human exposure. Environ. Pollut. 2018, 233, 986–991. [Google Scholar] [CrossRef]
- Poma, G.; Sales, C.; Bruyland, B.; Christia, C.; Goscinny, S.; Van Loco, J.; Covaci, A. Occurrence of Organophosphorus Flame Retardants and Plasticizers (PFRs) in Belgian Foodstuffs and Estimation of the Dietary Exposure of the Adult Population. Environ. Sci. Technol. 2018, 52, 2331–2338. [Google Scholar] [CrossRef]
- Koch, H.M.; Lorber, M.; Christensen, K.L.Y.; Pälmke, C.; Koslitz, S.; Brüning, T. Identifying sources of phthalate exposure with human biomonitoring: Results of a 48h fasting study with urine collection and personal activity patterns. Int. J. Hyg. Environ. Health 2013, 216, 672–681. [Google Scholar] [CrossRef]
- Li, Z.; Romanoff, L.; Bartell, S.; Pittman, E.N.; Trinidad, D.A.; McClean, M.; Webster, T.F.; Sjödin, A. Excretion Profiles and half-lives of ten urinary polycyclic aromatic hydrocarbon metabolites after dietary exposure. Chem. Res. Toxicol. 2012, 25, 1452–1461. [Google Scholar] [CrossRef]
- Percy, Z.; Vuong, A.M.; Ospina, M.; Calafat, A.M.; La Guardia, M.J.; Xu, Y.; Hale, R.C.; Dietrich, K.N.; Xie, C.; Lanphear, B.P.; et al. Organophosphate esters in a cohort of pregnant women: Variability and predictors of exposure. Environ. Res. 2020, 184, 109255. [Google Scholar] [CrossRef]
- Vernet, C.; Philippat, C.; Calafat, A.M.; Ye, X.; Lyon-caen, S.; Siroux, V.; Schisterman, E.F.; Slama, R. Concentrations of Phenol Biomarkers in Pregnant Women. Environ. Health Perspect. 2017, 126, 1–12. [Google Scholar] [CrossRef]
- Wielgomas, B. Variability of urinary excretion of pyrethroid metabolites in seven persons over seven consecutive days-Implications for observational studies. Toxicol. Lett. 2013, 221, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Knudsen, L.E.; Mizrak, S.; Joas, A. Identification of exposure to environmental chemicals in children and older adults using human biomonitoring data sorted by age: Results from a literature review. Int. J. Hyg. Environ. Health 2017, 220, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Conrad, A.; Becker, K.; Kolossa-Gehring, M.; Seiwert, M.; Seifert, B. Twenty years of the German Environmental Survey (GerES): Human biomonitoring - Temporal and spatial (West Germany/East Germany) differences in population exposure. Int. J. Hyg. Environ. Health 2007, 210, 271–297. [Google Scholar] [CrossRef] [PubMed]
- Faÿs, F.; Palazzi, P.; Hardy, E.M.; Schaeffer, C.; Phillipat, C.; Zeimet, E.; Vaillant, M.; Beausoleil, C.; Rousselle, C.; Slama, R.; et al. Is there an optimal sampling time and number of samples for assessing exposure to fast elimination endocrine disruptors with urinary biomarkers? Sci. Total Environ. 2020, 747, 141185. [Google Scholar] [CrossRef]
- Franklin, C.A.; Muir, N.I.; Moody, R.P. The use of biological monitoring in the estimation of exposure during the application of pesticides. Toxicol. Lett. 1986, 33, 127–136. [Google Scholar] [CrossRef]
- Klimowska, A.; Amenda, K.; Rodzaj, W.; Wileńska, M.; Jurewicz, J.; Wielgomas, B. Evaluation of 1-year urinary excretion of eight metabolites of synthetic pyrethroids, chlorpyrifos, and neonicotinoids. Environ. Int. 2020, 145, 106119. [Google Scholar] [CrossRef]
- Meeker, J.D.; Barr, D.B.; Ryan, L.; Herrick, R.F.; Bennett, D.H.; Bravo, R.; Hauser, R. Temporal variability of urinary levels of nonpersistent insecticides in adult men. J. Expo. Anal. Environ. Epidemiol. 2005, 15, 271–281. [Google Scholar] [CrossRef]
- Needham, L.L.; Calafat, A.M.; Barr, D.B. Uses and issues of biomonitoring. Int. J. Hyg. Environ. Health 2007, 210, 229–238. [Google Scholar] [CrossRef]
- Thuresson, K.; Höglund, P.; Hagmar, L.; Sjödin, A.; Bergman, Å.; Jakobsson, K. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ. Health Perspect. 2006, 114, 176–181. [Google Scholar] [CrossRef]
- Alves, A.; Kucharska, A.; Erratico, C.; Xu, F.; Hond, E.D.; Koppen, G.; Vanermen, G.; Covaci, A.; Voorspoels, S. Human biomonitoring of emerging pollutants through non-invasive matrices: State of the art and future potential. Anal. Bioanal. Chem. 2014, 406, 4063–4088. [Google Scholar] [CrossRef]
- Appel, K.E.; Gundert-Remy, U.; Fischer, H.; Faulde, M.; Mross, K.G.; Letzel, S.; Rossbach, B. Risk assessment of Bundeswehr (German Federal Armed Forces) permethrin-impregnated battle dress uniforms (BDU). Int. J. Hyg. Environ. Health 2008, 211, 88–104. [Google Scholar] [CrossRef] [PubMed]
- Lees, P.S.J.; Corn, M.; Breysse, P.N. Evidence for Dermal Absorption as the Major Route of Body Entry During Exposure of Transformer Maintenance and Repairmen to PCBs. Am. Ind. Hyg. Assoc. J. 1987, 48, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, J. Further investigations on the evaluation of exposure to nitrobenzene. Br. J. Ind. Med. 1967, 24, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Weschler, C.J.; Bekö, G.; Koch, H.M.; Salthammer, T.; Schripp, T.; Toftum, J.; Clausen, G. Transdermal uptake of diethyl phthalate and di(n-butyl) phthalate directly from air: Experimental verification. Environ. Health Perspect. 2015, 123, 928–934. [Google Scholar] [CrossRef]
- McArthur, B. Dermal Measurement and Wipe Sampling Methods: A Review. Appl. Occup. Environ. Hyg. 1992, 7, 599–606. [Google Scholar] [CrossRef]
- Stapleton, M.H.; Kelly, S.M.; Allen, J.G.; Mcclean, M.D.; Webster, T.F. Measurement of polybrominated diphenyl ethers on hand wipes: Estimating exposure from hand-to-mouth contact. Environ. Sci. Technol. 2008, 42, 3329–3334. [Google Scholar] [CrossRef]
- Brouwer, D.H.; Boeniger, M.F.; Van Hemmen, J. Hand wash and manual skin wipes. Ann. Occup. Hyg. 2000, 44, 501–510. [Google Scholar] [CrossRef]
- Durham, W.F.; Wolfe, H.R. Measurement of the exposure of workers to pesticides. Bull. World Health Organ. 1962, 26, 75–91. [Google Scholar]
- Stapleton, H.M.; Eagle, S.; Sjödin, A.; Webster, T.F. Serum PBDEs in a North Carolina toddler cohort: Associations with handwipes, house dust, and socioeconomic variables. Environ. Health Perspect. 2012, 120, 1049–1054. [Google Scholar] [CrossRef]
- Behroozy, A. On dermal exposure assessment. Int. J. Occup. Environ. Med. 2013, 4, 113–127. [Google Scholar] [PubMed]
- Curwin, B.D.; Hein, M.J.; Sanderson, W.T.; Nishioka, M.G.; Buhler, W. Nicotine exposure and decontamination on tobacco harvesters’ hands. Ann. Occup. Hyg. 2005, 49, 407–413. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gong, M.; Zhang, Y.; Weschler, C.J. Measurement of phthalates in skin wipes: Estimating exposure from dermal absorption. Environ. Sci. Technol. 2014, 48, 7428–7435. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, G.; Cao, Z.; Wang, B.; Huang, J.; Deng, S.; Wang, Y. Occurrence of organophosphorus flame retardants on skin wipes: Insight into human exposure from dermal absorption. Environ. Int. 2017, 98, 113–119. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Padilla-Sanchez, J.A.; Collins, C.D.; Cousins, I.T.; Covaci, A.; de Wit, C.A.; Leonards, P.E.G.; Voorspoels, S.; Thomsen, C.; Harrad, S.; et al. Sampling strategy for estimating human exposure pathways to consumer chemicals. Emerg. Contam. 2016, 2, 26–36. [Google Scholar] [CrossRef]
- Watkins, D.J.; McClean, M.D.; Fraser, A.J.; Weinberg, J.; Stapleton, H.M.; Sjödin, A.; Webster, T.F. Impact of dust from multiple microenvironments and diet on PentaBDE body burden. Environ. Sci. Technol. 2012, 46, 1192–1200. [Google Scholar] [CrossRef][Green Version]
- Bohlin, P.; Jones, K.C.; Strandberg, B. Occupational and indoor air exposure to persistent organic pollutants: A review of passive sampling techniques and needs. J. Environ. Monit. 2007, 9, 501–509. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, Y.; Lan, Z.; Yao, Y.; Wang, L.; Sun, H. Sampling methods of emerging organic contaminants in indoor air. Trends Environ. Anal. Chem. 2016, 12, 13–22. [Google Scholar] [CrossRef]
- National Research Council. Exposure Science in the 21st Century: A Vision and a Strategy; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Nieuwenhuijsen, M. Personal Exposure Monitoring and Modeling. In Exposure Assessment in Environmental Epidemiology; Nieuwenhuijsen, M., Ed.; Oxford University Press: Oxford, UK, 2016; pp. 1–32. [Google Scholar]
- Chen, Y.; Du, W.; Shen, G.; Zhuo, S.; Zhu, X.; Shen, H.; Huang, Y.; Su, S.; Lin, N.; Pei, L.; et al. Household air pollution and personal exposure to nitrated and oxygenated polycyclic aromatics (PAHs) in rural households: Influence of household cooking energies. Indoor Air 2017, 27, 169–178. [Google Scholar] [CrossRef]
- Xu, F.; Giovanoulis, G.; Van Waes, S.; Padilla-Sanchez, J.A.; Papadopoulou, E.; Magnér, J.; Haug, L.S.; Neels, H.; Covaci, A. Comprehensive study of human external exposure to organophosphate flame retardants via air, dust, and hand wipes: The importance of sampling and assessment strategy. Environ. Sci. Technol. 2016, 50, 7752–7760. [Google Scholar] [CrossRef]
- Król, S.; Zabiegała, B.; Namieśnik, J. Monitoring and analytics of semivolatile organic compounds (SVOCs) in indoor air. Anal. Bioanal. Chem. 2011, 400, 1751–1769. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, R.J.; Greenhalgh, D.M. A Personal air sampler. Ann. Occup. Hyg. 1960, 2, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Cherrie, J.W. The beginning of the science underpinning occupational hygiene. Ann. Occup. Hyg. 2003, 47, 179–185. [Google Scholar] [CrossRef]
- Rappaport, S.M. Implications of the exposome for exposure science. J. Expo. Sci. Environ. Epidemiol. 2011, 5–9. [Google Scholar] [CrossRef] [PubMed]
- NIOSH Manual of Analytical Methods (NMAM). Available online: https://www.cdc.gov/niosh/nmam/ (accessed on 28 July 2021).
- ASTM D4861-17, Standard Practice for Sampling and Selection of Analytical Techniques for Pesticides and Polychlorinated Biphenyls in Air. Available online: https://www.astm.org/d4861-17.html (accessed on 28 July 2021).
- Weisskopf, M.G.; Webster, T.F. Trade-offs of Personal Versus More Proxy Exposure Measures in Environmental Epidemiology. Epidemiology 2017, 28, 635–643. [Google Scholar] [CrossRef]
- Cao, Z.; Yu, G.; Chen, Y.; Cao, Q.; Fiedler, H.; Deng, S. Particle size: A missing factor in risk assessment of human exposure to toxic chemicals in settled indoor dust. Environ. Int. 2012, 49, 24–30. [Google Scholar] [CrossRef]
- Bergh, C.; Luongo, G.; Wise, S.; Östman, C. Organophosphate and phthalate esters in standard reference material 2585 organic contaminants in house dust. Anal. Bioanal. Chem. 2012, 402, 51–59. [Google Scholar] [CrossRef]
- ASTM D5438-17, Standard Practice for Collection of Floor Dust for Chemical Analysis. Available online: https://www.astm.org/d5438-17.html (accessed on 28 July 2021).
- Whitehead, T.; Metayer, C.; Buffler, P.; Rappaport, S.M. Estimating exposures to indoor contaminants using residential dust. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 549–564. [Google Scholar] [CrossRef]
- Lioy, P.J.; Freeman, N.C.G.; Millette, J.R. Dust: A metric for use in residential and building exposure assessment and source characterization. Environ. Health Perspect. 2002, 110, 969–983. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Variations in the composition of house dust by particle size. J. Environ. Sci. Health Part A 2017, 52, 770–777. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Romanak, K.A.; Hendryx, M.; Salamova, A.; Venier, M. Association between Thyroid Function and Exposures to Brominated and Organophosphate Flame Retardants in Rural Central Appalachia. Environ. Sci. Technol. 2020, 54, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Appenzeller, B.M.R. Hair Analysis for the Biomonitoring of Human Exposure to Organic Pollutants; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Appenzeller, B.M.R.; Tsatsakis, A.M. Hair analysis for biomonitoring of environmental and occupational exposure to organic pollutants: State of the art, critical review and future needs. Toxicol. Lett. 2012, 210, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Fäys, F.; Hardy, E.M.; Palazzi, P.; Haan, S.; Beausoleil, C.; Appenzeller, B.M.R. Biomonitoring of fast-elimination endocrine disruptors—Results from a 6-month follow up on human volunteers with repeated urine and hair collection. Sci. Total Environ. 2021, 778, 146330. [Google Scholar] [CrossRef] [PubMed]

| Publication Year | Sampling Year | Country * | Population | Population Age Range (<18 y.o) | n | Exposure Setting | Wearing Period [Days] | References |
|---|---|---|---|---|---|---|---|---|
| 2014 | NA | USA | NA | NA | <30 | ambient | 30 | [20] † |
| 2014 | NA | USA | NA | NA | 8 | occupational | 0.3, 1.3–1.6 | [20] † |
| 2015 | 2013 | USA | adults | NA | 50 | ambient | 7 | [54] |
| 2016 | 2015 | USA | adults | NA | 40 | ambient | 5 | [51] |
| 2016 | 2012/2013 | USA | children | 3–5 | 92 | ambient | 7 | [52] |
| 2016 | 2014 | SEN | adults, children | NR | 35 | occupational | 5 | [55] |
| 2017 | 2014 | PER | adults, children | ≥6 | 68 | ambient | 30–34 | [19] |
| 2017 | NR | USA | adults | NA | 22 | ambient | 2 | [36] |
| 2017 | NR | USA | children | 7–9 | 10 | ambient | 7 | [27] |
| 2017 | 2012–2013 | USA | children | 3–5 | 77 | ambient | 7 | [56] |
| 2018 | nd | USA | adults | NA | 19 | ambient | 21 | [57] |
| 2018 | NR | USA | adults | NA | 22 | ambient | 2 | [48] |
| 2018 | 2016 | BEL | adults | NA | 30 | ambient | 5 | [24] |
| 2018 | 2016 | USA | adults | NA | 30 | ambient | 7 | [58] |
| 2019 | 2017–2018 | USA | adults | NA | 101 | ambient | 7 | [21] |
| 2019 | 2016/2017 | USA | adults | NA | 10 | occupational | 0.83–2.08 | [22] |
| 2019 | 2016 | BRA | adults | NA | 2 | ambient | 3 | [59] |
| 2019 | 2016 | USA | adults | NA | 10 | ambient | 7 | [60] † |
| 2019 | 2017 | USA | adults | NA | 22 | ambient | 7 | [60] † |
| 2019 | 2016 | USA | adolescents | 14–16 | 97 | ambient | 7 | [61] |
| 2019 | 2008–? | USA | child-mother pairs | 3–5 | 32 | ambient | 7 | [62] |
| 2019 | NR | USA | adults | NA | 10 | ambient | 7 | [63] |
| 2019 | 2017 | USA | children | 4–14 | 31 | ambient | 7,2 | [64] |
| 2019 | NA | CAN, NED | NA | NA | NA | exposure chamber | 1, 4, 10, 30, 50, 71, 91, 161 | [38] |
| 2019 | NA | USA | NA | NA | NA | NA | 7 | [65] |
| 2019 | NR | NR | NR | NA | 10 | NR | 7 | [66] |
| 2019 | 2016–2017 | CHL | NR | NA | 27 | ambient | 5 | [45] |
| 2019 | NR | NR | NR | NA | 16 | ambient | 18 | [67] |
| 2020 | 2018 | URY | children | 6–7.8 | 24 | ambient | 7 | [68] |
| 2020 | 2019 | JPN | adults | NA | 5 | ambient | 5 | [69] |
| 2020 | 2017 | USA | adults | NA | 72 | occupational | 1 | [18] |
| 2020 | 2019 | USA | adults | NA | 88 | ambient | 5 | [70] |
| 2020 | 2017–2018 | USA | adults | NA | 101 | ambient | 7 | [47] |
| 2020 | 2019 | DOM | adults | NA | 15 | occupational | 1 | [71] |
| 2020 | 2017–2018 | USA | adults | NA | 255 | ambient | 7 | [46] † |
| 2020 | 2017–2018 | USA | adults | NA | 20 | ambient | 7 | [46] † |
| 2020 | 2015/2016 | USA | children | 3–6 | 77 | ambient | 7 | [72] |
| 2020 | 2017–2018 | USA | children | 3–14 | 53 | ambient | 7, 2 | [50] |
| 2020 | 2018–2019 | FRA | adults | NA | 40 | ambient | 5 | [28] † |
| 2020 | 2018–2019 | ITA | adults | NA | 31 | ambient | 5 | [28] † |
| 2020 | 2018 | BGD | adolescents/adults | ≥14 | 15 | occupational | 1 | [73] |
| 2020 | 2018 | USA | adults | NA | 30 | ambient | 5 | [74] |
| 2020 | 2018 | USA | adults | NA | 17 | occupational | 1 | [75] |
| 2020 | 2017 | CAN | adults | NA | 45 | occupational | 0.3 | [49] |
| 2021 | 2014–2016 | USA | children | 3–6 | 27 | ambient | 7 | [76] |
| 2021 | 2018–2019 | USA | children | 10–17 | 163 | ambient | 7 | [77] |
| 2021 | 2018–2019 | CHN | Child-mother pairs | ≤7 | 47 | ambient | 14 | [78] |
| Pre-Deployment | Post-Deployment | Extraction | Post-Extraction Sample Cleanup | Analyzed Substances | Instrumental Analysis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Publication Year | Mechanism | Protocol | Mechanism | Protocol | Mechanism | Protocol | Instrumentation | Protocol | NBRFs | OPEs | PAHs | BFRs | PCBs | PEs | Pesticides | PPCPs | Other | Ref. | |
| 2014 | Agitated wash (orbital shaker) | 3 × EtAc:n-hex (2.5 h), 60 rpm 2 × EtAc:MeOH (2.5 h), 60 rpm | Rinse | 2 × DI water 1 × IPA | Agitated wash (orbital shaker) | 2 × EtAc,100 mL, (2 h), 60 rpm | NR | NR | N | Y | Y | N | Y | Y | Y | Y | Y | GC-MS | [20] |
| 2016 | Thermal conditioning | 280–300 °C (48 h) | Rinse | 1 × DI water 1 × IPA | NR | 2 × EtAc, 100 mL | NR | NR | N | N | N | N | N | N | Y | N | N | GC-ECD | [55] |
| 2016 | Soxhlet extraction | 1 × EtAc:n-hex, (12 h) 1 × EtAc:MeOH, (12 h) | NR | NR | Soxhlet extraction | 1 × n-hex:acetone, (12 h) | Syringe filter (0.2 µm PTFE) SPE cartridges (Florisil, 500 mg) | Filtration Elution: | N | Y | N | N | N | N | N | N | N | GC-MS | [51] |
| F1:n-hex (10 mL) | |||||||||||||||||||
| F2:EtAc (10 mL) | |||||||||||||||||||
| 2017 | Wash | 3 × EtAc:n-hex 2 × EtAc:MeOH | Rinse | 2 × DI water 1 × IPA | Wash | 1 × EtAc, 100 mL, (12 h) 1 × EtAc, 100 mL, (2 h) | NR | NR | Y | Y | Y | Y | Y | Y | Y | Y | Y | GC-ECD, GC-MS | [19] |
| 2017 | Conditioning (vacuum oven) | 300 °C, 180 min, 0.1 Torr | Rinse | 2 × DI water 1 × IPA | Agitated wash (orbital shaker) | 2 × EtAc, 100 mL | NR | NR | N | Y | Y | Y | Y | N | Y | Y | Y | GC-MS, GC-MS/MS, GC-µECD | [36] |
| 2017 | Soak | EtAc, n-hex, MeOH | Rinse | 2 × water 1 × IPA | NR | 2 × EtAc, 100 mL | SPE cartridges (C18, 500 mg) | Elution: ACN | Y | Y | N | Y | N | N | N | N | N | GC-MS | [52] |
| 2018 | NR | NR | Rinse | 1 × DI water 1 × IPA | Dialysis | 2 × EtAc | NR | NR | N | N | Y | N | N | N | N | N | N | GC-MS/MS | [57] |
| 2018 | Solvent exchange | 3 × EtAc:n-hex 2 × EtAc:MeOH | Rinse | 2 × DI water 1 × IPA | Agitated wash (orbital shaker) | 2 × EtAc 100 mL, 60 rpm | NR | NR | N | N | Y | N | N | N | N | N | N | GC-MS/MS | [48] |
| 2018 | Agitated wash (overhead shaker) | 1 × EtAc:n-hex, (30 min) 1 × EtAc:MeOH, (30 min): | NR | NR | Agitated wash (overhead shaker) | 2 × EtAc, 40 mL, (30 min) | NR | NR | N | N | N | N | N | N | Y | N | N | LC-MS | [24] |
| 2018 | NR | NR | Rinse | 2 × DI water 1 × IPA | Wash | 1 × EtAc 100 mL, (12 h) 1 × EtAc, 100 mL, (2 h) | NR | NR | N | N | N | N | N | N | Y | N | N | GC-µECD | [27] |
| 2018 | Soxhlet extraction | 1 × EtAc:n-hex, (12 h) 1 × EtAc:MeOH, (12 h) | NR | NR | Sonication | 3 × n-hex:acetone, 10 mL | Custom SPE: Florisil (500 mg) and silica gel (12 g; F1 only) | Elution (Florisil): | Y | N | N | Y | N | N | N | N | N | GC-MS | [58] |
| F1:n-hex | |||||||||||||||||||
| F2:EtAc | |||||||||||||||||||
| Elution (silica gel): F3:DCM:n-hex | |||||||||||||||||||
| 2019 | Rinse, conditioning | Water rinse, thermal conditioning | Rinse | 1 × DI water 1 × IPA | Agitated wash (orbital shaker) | 2 × EtAc, 100 mL, (2 h) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | GC-MS | [67] |
| 2019 | Soxhlet extraction | 1 × EtAc:n-hex, (24 h) 1 × EtAc:MeOH, (24 h) | NR | NR | Sonication | 2 × n-hex:acetone, 30 mL, (2 h) | Custom SPE (neutral alumina, neutral silica gel, sulfuric acid- silica gel, sodium sulfate) | Elution: DCM (40 mL) | Y | Y | N | Y | N | N | N | N | Y | GC-MS | [21] |
| Custom SPE (neutral alumina, neutral silica, Florisil, sodium sulfate) | Elution: | ||||||||||||||||||
| F1:DCM (40 mL) | |||||||||||||||||||
| F2:EtAc (40 mL) | |||||||||||||||||||
| 2019 | NR | NR | Rinse | 2 × DI water 1 × IPA | Agitated wash (orbital shaker) | 2 × EtAc, 100 mL, 60 rpm | NR | NR | N | Y | Y | N | N | Y | Y | Y | Y | GC-MS | [59] |
| 2019 | Thermal conditioning (vacuum oven) | 300 °C, (180 min), 0.1 Torr | Rinse | 1 × DI water 1 × IPA | NR | 2 × EtAc, 100 mL | SPE (C18, silica) | Elution: ACN | Y | Y | Y | Y | Y | Y | Y | Y | Y | GC-µECD, GC-MS | [61] |
| 2019 | Soak | EtAc, n-hex, MeOH | NR | NR | NR | 2 × EtAc, 100 mL | SPE cartridges (C18, 500 mg) | Elution: ACN | N | Y | N | N | N | N | N | N | N | GC-MS | [62] |
| 2019 | Soxhlet extraction | Agitated wash | 1 × DI water | Sonication | 1 × Acetone:n-hex, 20 mL, (2 h) | Custom SPE (neutral alumina, neutral silica, Florisil, anhydrous sodium sulfate) | Elution: | Y | Y | Y | Y | N | N | N | N | Y | GC-MS | [63] | |
| F1:DCM | |||||||||||||||||||
| F2:EtAc | |||||||||||||||||||
| Rinse | 1 × IPA | Custom SPE, (neutral alumina, neutral silica, acidic silica, anhydrous sodium sulfate) | elution: F3: DCM | ||||||||||||||||
| 2019 | NR | NR | Rinse | 2 × DI water 1 × IPA | Agitated wash (orbital shaker) | 2 × EtAc, 100 mL, (2 h), 60 rpm | - | - | N | N | Y | N | N | Y | Y | N | Y | GC-GC/ToF-MS | [45] |
| 2020 | Soxhlet extraction | 1 × EtAc (3 days) | - | - | Agitated wash (Wrist Action Shaker) | 1 × ACN, 30 mL | Syringe filter (0.2 µm, Teflon) | Filtration | Y | Y | N | Y | N | N | N | N | Y | GC-MS | [49] |
| 2020 | Agitated wash (platform shaker) | 3 × EtAc:n-hex, (2.5 h) 2 × EtAc:MeOH, (2.5 h), 60 rpm | NR | NR | Agitated wash (orbital shaker) | 2 × EtAc, 25 mL, (2 h), 60 rpm | SPE cartridges (C18, 500 mg) | Elution: ACN | Y | Y | N | Y | Y | N | Y | N | Y | GC-MS | [68] |
| 2020 | NR | NR | Rinse | 1 × DI water 1 × IPA | Agitated wash (orbital shaker) | 2 × EtAc, 25 mL, (24 h) | SPE cartridges (C18, 500 mg) | Elution: n-hex: DCM (4 mL) | N | N | Y | N | N | N | N | Y | N | GC-MS | [69] |
| 2020 | Agitated wash (orbital shaker) | 1 × MeOH (10 min) 3 × n-hex:EtAc (1 h), 2 × MeOH:EtAc | Rinse | 1 × MeOH | agitated wash (orbital shaker) | 2 × 30 mL EtAc, 30 mL, (1 h) | NR | NR | N | N | Y | N | N | N | N | N | N | GC-MS | [18] |
| 2020 | Soxhlet extraction | 1 × EtAc:n-hex, (12 h) 1 × EtAc:MeOH, (12 h) | NR | NR | Sonication | 3 × n-hex: DCM, 10 mL, (15 min) | SPE (Florisil, 8 g) | Elution: | N | Y | N | N | N | N | N | N | N | GC-MS/MS | [70] |
| F1:n-hex | |||||||||||||||||||
| F2:EtAc | |||||||||||||||||||
| 2020 | Soxhlet extraction | 1 × EtAc:n-hex, (24 h) 1 × EtAc:MeOH, (24 h) | NR | NR | Sonication | 2 × n-hex:acetone, 30 mL, (2 h) | Custom SPE (neutral alumina, neutral silica, Florisil, sodium sulfate) | Elution: DCM | N | N | Y | N | N | N | N | N | N | GC-MS | [47] |
| 2020 | Agitated wash (orbital shaker) | 2 × MeOH, (10 min), 120 rpm 2 × (1 h): n-hex:EtAc, (1 h), 120 rpm 2 × MeOH:EtAc, 120 rpm | Rinse | 1 × MeOH | Agitated wash (overhead shaker) | 2 × EtAc, 30 mL | NR | NR | N | N | Y | N | N | N | N | N | N | GC-MS | [71] |
| 2020 | Conditioning (vacuum oven) | 300 °C, (12 h), 0.1 Torr | Rinse | 2 × DI water 1 × IPA | NR | 2 × EtAc, 50 mL | SPE cartridges (C18) | Eluted: ACN | N | Y | Y | Y | Y | Y | Y | Y | Y | GC-MS | [46] |
| 2020 | Soxhlet extraction | 1 × EtAc:n-hex, (12 h) 1 × EtAc:MeOH, (12 h) | NR | NR | Sonication | 3 × n-hex:DCM, 10 mL) | SPE cartridges (Florisil, 500 mg) | Elution: | N | Y | N | N | N | Y | N | N | Y | GC-MS | [72] |
| F1: n-hex | |||||||||||||||||||
| F2: EtAc | |||||||||||||||||||
| F3: MeOH | |||||||||||||||||||
| 2020 | NR | NR | Rinse | DI water | Sonication | 2 × n-hex: acetone, 30 mL, (2 h) | Chromatography column (neutral alumina, neutral silica gel, sulfuric acid-silica gel, sodium sulfate) | Elution: DCM | Y | Y | Y | Y | N | N | N | N | N | GC-MS | [28] |
| Chromatography column (neutral alumina, neutral silica gel, Florisil, sodium sulfate) | Elution:F1:DCM | ||||||||||||||||||
| F2:EtAc | |||||||||||||||||||
| 2020 | Soxhlet extraction | 1 × pentane (3 days) | - | - | Agitated wash | ACN | SPE cartridge (Florisil, 500 mg) | Elution: EtAc | Y | Y | N | Y | N | N | N | N | Y | GC-MS | [73] |
| 2020 | Agitated wash (magnetic stir plate) | 3 × EtAc:n-hex, (30 min), 60 rpm 2 × EtAc:MeOH, (30 min), 60 rpm | NR | NR | Agitated wash (magnetic stir plate) | ACN:MeOH, 20 mL, (1 h), 60 rpm | NR | NR | N | N | N | N | N | N | N | N | Y | HPLC | [65] |
| 2020 | NR | NR | NR | NR | Sonication | 3 × n-hex:DCM, 10 mL | SPE (Florisil, 8 g) | Elution: | Y | Y | N | Y | Y | Y | Y | N | Y | GC-MS, GC-MS/MS | [74] |
| F1: n-hex, | |||||||||||||||||||
| F2: EtAc, | |||||||||||||||||||
| F3: MeOH | |||||||||||||||||||
| 2020 | Agitated wash (orbital shaker) | 1 × MeOH (10 min), 120 rpm 2 × EtAc:n-hex (1 h), 120 rpm 2 × EtAc:MeOH (1 h), 120 rpm | NR | NR | Agitated wash (orbital shaker) | 2 × EtAc, 30 mL, (1 h), 120 rpm | NR | NR | N | N | Y | N | N | N | N | N | N | GC-MS | [75] |
| 2021 | Soxhlet extraction | 1 × EtAc:n-hex (12 h) 1 × EtAc:MeOH (12 h) | NR | NR | Sonication | 3 × DCM:n-hex | SPE cartridges (Florisil, 500 mg) | Elution: | N | N | N | N | N | N | N | Y | Y | LC-MS | [76] |
| F1 | |||||||||||||||||||
| F2: EtAc | |||||||||||||||||||
| F3 | |||||||||||||||||||
| 2021 | Rinse, conditioning | DI water, 300 °C (180 min) | rinse | 1 × DI water 1 × IPA | Agitated wash (orbital shaker) | 2 × EtAc | SPE (C18, silica) | Elution: ACN | N | N | N | N | N | N | Y | N | N | GC-ECD, GC-MS | [77] |
| 2021 | Sonication | 3 × DCM:n-hex, (20 min) | NR | NR | Sonication | 2 × DCM: n-hex, 15 mL, (20 min) | SPE cartridges (Florisil, 2 g) | Elution: 1 × n-hex 1 × EtAc | N | Y | N | N | N | N | N | N | N | LC-MS | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wacławik, M.; Rodzaj, W.; Wielgomas, B. Silicone Wristbands in Exposure Assessment: Analytical Considerations and Comparison with Other Approaches. Int. J. Environ. Res. Public Health 2022, 19, 1935. https://doi.org/10.3390/ijerph19041935
Wacławik M, Rodzaj W, Wielgomas B. Silicone Wristbands in Exposure Assessment: Analytical Considerations and Comparison with Other Approaches. International Journal of Environmental Research and Public Health. 2022; 19(4):1935. https://doi.org/10.3390/ijerph19041935
Chicago/Turabian StyleWacławik, Małgorzata, Wojciech Rodzaj, and Bartosz Wielgomas. 2022. "Silicone Wristbands in Exposure Assessment: Analytical Considerations and Comparison with Other Approaches" International Journal of Environmental Research and Public Health 19, no. 4: 1935. https://doi.org/10.3390/ijerph19041935
APA StyleWacławik, M., Rodzaj, W., & Wielgomas, B. (2022). Silicone Wristbands in Exposure Assessment: Analytical Considerations and Comparison with Other Approaches. International Journal of Environmental Research and Public Health, 19(4), 1935. https://doi.org/10.3390/ijerph19041935






