Minireview: Parabens Exposure and Breast Cancer
Abstract
:1. Introduction
2. Human Parabens Exposure
3. Parabens Exposure and Breast Cancer
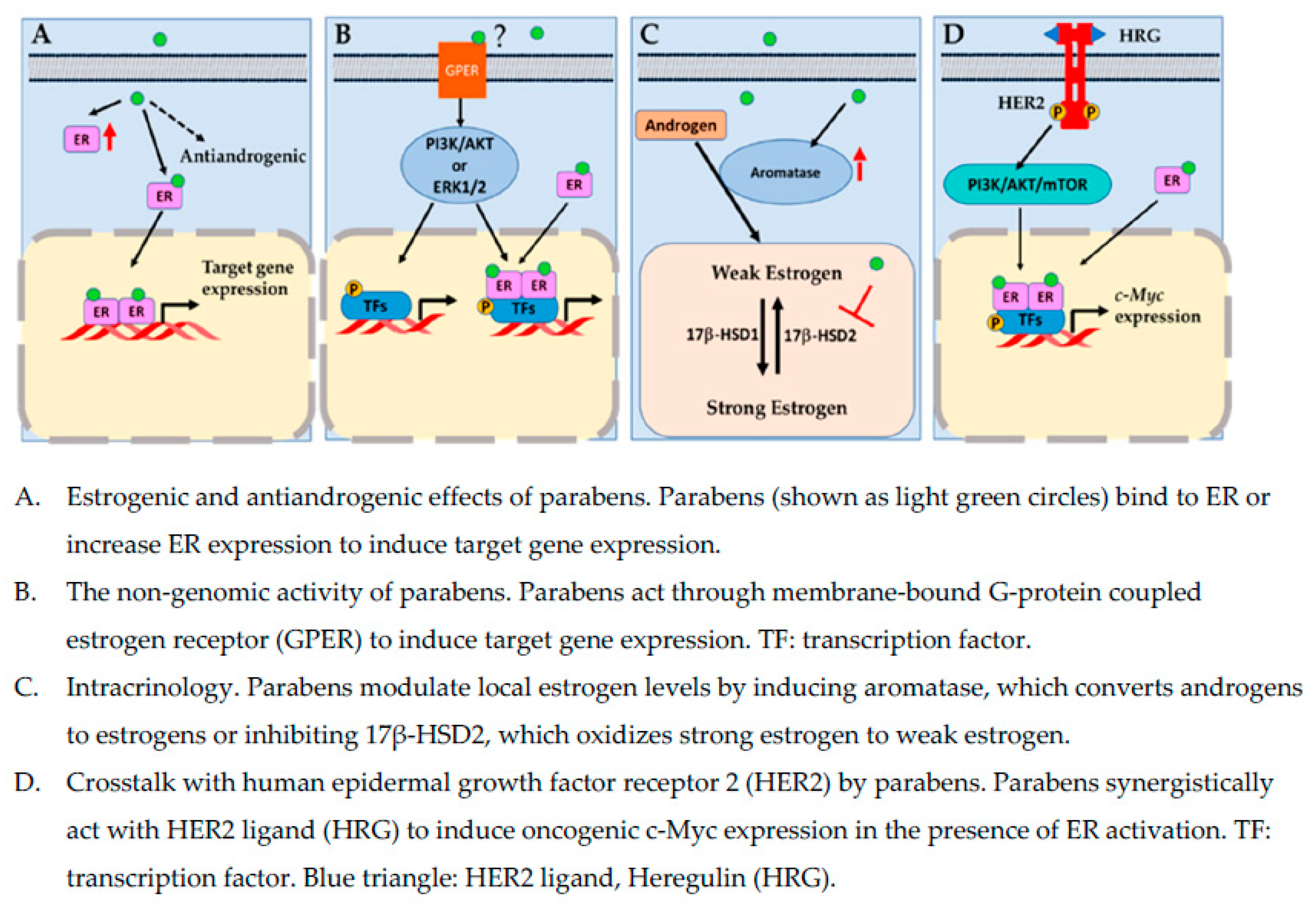
4. Estrogenic Effects of Parabens
5. The Nongenomic Activity of Parabens
6. Intracrinology: Aromatase, 17 β-Hydroxysteroid Dehydrogenases, and Parabens
7. Antiandrogenic Effects of Parabens
8. Crosstalk with Human Epidermal Growth Factor Receptor 2 Pathway by Parabens
9. Anchorage Independence, Migration, and Parabens
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 17β-HSD1 | 17β-hydroxysteroid dehydrogenases type 1 |
| 17β-HSD2 | 17β-hydroxysteroid dehydrogenases type 2 |
| AR | Androgen receptor |
| ATM | Ataxia telangiectasia mutated |
| ATR | Ataxia telangiectasia and Rad3 related |
| BP | Butylparaben |
| BMI | Body mass index |
| BzP | Benzylparaben |
| CCND1 | Cyclin D1 |
| CCND3 | Cyclin D3 |
| CCNE1 | Cyclin E1 |
| CCNE2 | Cyclin E2 |
| CCNA2 | Cyclin A2 |
| CDK2 | Cyclin Dependent Kinase 2 |
| CDK4 | Cyclin Dependent Kinase 4 |
| CDK6 | Cyclin Dependent Kinase 6 |
| CDKN1A | Cyclin Dependent Kinase Inhibitor 1A |
| CYP19A1 | cytochrome P450 19A1 (aromatase) |
| DHT | Dihydrotestosterone |
| E2F3 | Transcription factor E2F3 |
| EEF | Estradiol equivalence factor |
| E1 | Estrone |
| E2 | 17β-estradiol |
| ER | Estrogen receptor |
| EDCs | Endocrine-disruptive chemicals |
| EMT | Epithelial-to-mesenchymal transitions |
| EP | Ethylparaben |
| GPER | G-protein-coupled estrogen receptor (GPR30 or GPER1) |
| GR | Glucocorticoid receptor |
| HeP | hexylparaben |
| HepP | Heptylparaben |
| HER2 | Human epidermal growth factor receptor 2 |
| HRG | Heregulin |
| IsoBP | Isobutylparaben |
| IsoPP | Isopropylparaben |
| MMPs | Matrix metalloproteinases |
| MP | Methylparaben |
| PhP | Phenylparaben |
| PR | Progesterone receptor |
| PP | Propylparaben |
| TP53 | Tumor protein p53 |
References
- ACS. About Breast Cancer. 2021. Available online: https://www.cancer.org/cancer/breast-cancer.html (accessed on 30 December 2021).
- Darbre, P.D. Environmental oestrogens, cosmetics and breast cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Ghosn, B.; Benisi-Kohansal, S.; Ebrahimpour-Koujan, S.; Azadbakht, L.; Esmaillzadeh, A. Association between healthy lifestyle score and breast cancer. Nutr. J. 2020, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.M.; Udesky, J.O.; Rudel, R.A.; Brody, J.G. Environmental chemicals and breast cancer: An updated review of epidemiological literature informed by biological mechanisms. Environ. Res. 2018, 160, 152–182. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D. Endocrine disrupting chemicals and breast cancer cells. Adv. Pharm. 2021, 92, 485–520. [Google Scholar]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Endocrine disrupting chemicals and breast cancer: A systematic review of epidemiological studies. Crit. Rev. Food Sci. Nutr. 2021, 5, 1–27, Online ahead of print. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef] [Green Version]
- NIEHS. Endocrine Disruptors. 2021. Available online: https://www.niehs.nih.gov/health/topics/agents/endocrine/index.cfm (accessed on 30 December 2021).
- Nowak, K.; Ratajczak–Wrona, W.; Górska, M.; Jabłońska, E. Parabens and their effects on the endocrine system. Mol. Cell Endocrinol. 2018, 474, 238–251. [Google Scholar] [CrossRef]
- Karpuzoglu, E.; Holladay, S.D.; Gogal, R.M., Jr. Parabens: Potential impact of low-affinity estrogen receptor binding chemicals on human health. J. Toxicol. Environ. Health B Crit. Rev. 2013, 16, 321–335. [Google Scholar] [CrossRef]
- Darbre, P.D.; Harvey, P.W. Parabens can enable hallmarks and characteristics of cancer in human breast epithelial cells: A review of the literature with reference to new exposure data and regulatory status. J. Appl. Toxicol. 2014, 34, 925–938. [Google Scholar] [CrossRef]
- Freire, C.; Molina-Molina, J.-M.; Iribarne-Durán, L.M.; Jiménez-Díaz, I.; Vela-Soria, F.; Mustieles, V.; Arrebola, J.P.; Fernández, M.F.; Artacho-Cordón, F.; Olea, N. Concentrations of bisphenol A and parabens in socks for infants and young children in Spain and their hormone-like activities. Environ. Int. 2019, 127, 592–600. [Google Scholar] [CrossRef]
- Lange, C.; Kuch, B.; Metzger, J.W. Estrogenic activity of constituents of underarm deodorants determined by E-Screen assay. Chemosphere 2014, 108, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Kolatorova, L.; Duskova, M.; Vitku, J.; Starka, L. Prenatal exposure to bisphenols and parabens and impacts on human physiology. Physiol. Res. 2017, 66, S305–S315. [Google Scholar] [CrossRef]
- Sandanger, T.M.; Huber, S.; Moe, M.K.; Braathen, T.; Leknes, H.; Lund, E. Plasma concentrations of parabens in postmenopausal women and self-reported use of personal care products: The NOWAC postgenome study. J. Environ. Sci. Environ. Epidemiol. 2011, 21, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liang, J.; Zheng, L.; Lv, Q.; Wang, H. Application of dispersive liquid-liquid microextraction for the preconcentration of eight parabens in real samples and their determination by high-performance liquid chromatography. J. Sep. Sci. 2017, 40, 4385–4393. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.T.; Chen, H.C.; Ding, W.H. Accurate analysis of parabens in human urine using isotope-dilution ultrahigh-performance liquid chromatography-high resolution mass spectrometry. J. Pharm. Biomed. Anal. 2018, 150, 469–473. [Google Scholar] [CrossRef]
- Darbre, P.D. Underarm cosmetics and breast cancer. Eur. J. Cancer Prev. 2004, 13, 153. [Google Scholar] [CrossRef]
- Darbre, P.D.; Aljarrah, A.; Miller, W.R.; Coldham, N.G.; Sauer, M.J.; Pope, G.S. Concentrations of parabens in human breast tumours. Concentrations of parabens in human breast tumours. J. Appl. Toxicol. 2004, 24, 5–13. [Google Scholar] [CrossRef]
- Chen, J.; Ahn, K.C.; Gee, N.A.; Gee, S.J.; Hammock, B.D.; Lasley, B.L. Antiandrogenic properties of parabens and other phenolic containing small molecules in personal care products. Toxicol. Appl. Pharm. 2007, 221, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Harvey, P.W.; Darbre, P. Endocrine disrupters and human health: Could oestrogenic chemicals in body care cosmetics adversely affect breast cancer incidence in women? J. Appl. Toxicol. 2004, 24, 167–176. [Google Scholar] [CrossRef]
- Pugazhendhi, D.; Pope, G.S.; Darbre, P.D. Oestrogenic activity of p-hydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines. J. Appl. Toxicol. 2005, 25, 301–309. [Google Scholar] [CrossRef]
- Wang, L.; Asimakopoulos, A.G.; Kannan, K. Accumulation of 19 environmental phenolic and xenobiotic heterocyclic aromatic compounds in human adipose tissue. Environ. Int. 2015, 78, 45–50. [Google Scholar] [CrossRef]
- Ye, X.; Bishop, A.M.; Reidy, J.A.; Needham, L.L.; Calafat, A.M. Parabens as urinary biomarkers of exposure in humans. Environ. Health Perspect. 2006, 114, 1843–1846. [Google Scholar] [CrossRef] [Green Version]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Bishop, A.M.; Needham, L.L. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ. Health Perspect. 2010, 118, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Kyung, M.S.; Ko, A.; Park, J.H.; Hwang, M.S.; Kwon, J.E.; Suh, J.H.; Lee, H.S.; Moon, G.I.; Hong, I.G.; et al. Urinary concentrations of parabens and their association with demographic factors: A population-based cross-sectional study. Environ. Res. 2016, 146, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Y.; Zhang, W.; Kannan, K. Characteristic profiles of urinary p-hydroxybenzoic acid and its esters (parabens) in children and adults from the United States and China. Environ. Sci Technol. 2013, 47, 2069–2076. [Google Scholar] [CrossRef]
- Li, C.; Cui, X.; Chen, Y.; Liao, C. Paraben concentrations in human fingernail and its association with personal care product use. Ecotoxicol. Environ. Saf. 2020, 202, 110933. [Google Scholar] [CrossRef] [PubMed]
- Karwacka, A.; Zamkowska, D.; Radwan, M.; Jurewicz, J. Exposure to modern, widespread environmental endocrine disrupting chemicals and their effect on the reproductive potential of women: An overview of current epidemiological evidence. Hum. Fertil. 2019, 22, 2–25. [Google Scholar] [CrossRef]
- Harley, K.G.; Berger, K.P.; Kogut, K.; Parra, K.; Lustig, R.H.; Greenspan, L.C.; Calafat, A.M.; Ye, X.; Eskenazi, B. Association of phthalates, parabens and phenols found in personal care products with pubertal timing in girls and boys. Hum Reprod. 2019, 34, 109–117. [Google Scholar] [CrossRef]
- Yager, J.D.; Davidson, N.E. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006, 354, 270–282. [Google Scholar] [CrossRef] [Green Version]
- Barr, L.; Metaxas, G.; Harbach, C.A.; Savoy, L.A.; Darbre, P.D. Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J. Appl. Toxicol. 2012, 32, 219–232. [Google Scholar] [CrossRef]
- Sprague, B.L.; Gangnon, R.E.; Burt, V.; Trentham-Dietz, A.; Hampton, J.M.; Wellman, R.D.; Kerlikowske, K.; Miglioretti, D.L. Prevalence of mammographically dense breasts in the United States. J. Natl. Cancer Inst. 2014, 106, 255. [Google Scholar] [CrossRef] [PubMed]
- Sprague, B.L.; Trentham-Dietz, A.; Hedman, C.J.; Wang, J.; Hemming, J.D.; Hampton, J.M.; Buist, D.S.; Bowles, E.J.; Sisney, G.S.; Burnside, E.S. Circulating serum xenoestrogens and mammographic breast density. Breast Cancer Res. 2013, 15, R45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parada, H., Jr.; Gammon, M.D.; Ettore, H.L.; Chen, J.; Calafat, A.M.; Neugut, A.I.; Santella, R.M.; Wolff, M.S.; Teitelbaum, S.L. Urinary concentrations of environmental phenols and their associations with breast cancer incidence and mortality following breast cancer. Environ. Int. 2019, 130, 104890. [Google Scholar] [CrossRef]
- Wu, A.H.; Franke, A.A.; Wilkens, L.R.; Tseng, C.; Conroy, S.M.; Li, Y.; Sangaramoorthy, M.; Polfus, L.M.; DeRouen, M.C.; Caberto, C.; et al. Risk of breast cancer and prediagnostic urinary excretion of bisphenol A, triclosan and parabens: The Multiethnic Cohort Study. Int. J. Cancer 2021, 149, 1426–1434. [Google Scholar] [CrossRef]
- Golden, R.; Gandy, J.; Vollmer, G. A review of the endocrine activity of parabens and implications for potential risks to human health. Crit. Rev. Toxicol. 2005, 35, 435–458. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar]
- Lee, H.R.; Kim, T.H.; Choi, K.C. Functions and physiological roles of two types of estrogen receptors, ERalpha and ERbeta, identified by estrogen receptor knockout mouse. Lab. Anim. Res. 2012, 28, 71–76. [Google Scholar] [CrossRef]
- Arendt, L.M.; Kuperwasser, C. Form and function: How estrogen and progesterone regulate the mammary epithelial hierarchy. J. Mammary Gland. Biol. Neoplasia 2015, 20, 9–25. [Google Scholar] [CrossRef] [Green Version]
- Russo, J.; Russo, I.H. Development of the human breast. Maturitas 2004, 49, 2–15. [Google Scholar] [CrossRef]
- Hinck, L.; Silberstein, G.B. Key stages in mammary gland development: The mammary end bud as a motile organ. Breast Cancer Res. 2005, 7, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Silberstein, G.B. Tumour-stromal interactions. Role of the stroma in mammary development. Breast Cancer Res. 2001, 3, 218–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javed, A.; Lteif, A. Development of the human breast. Semin. Plast. Surg. 2013, 27, 5–12. [Google Scholar]
- Darbre, P.D.; Byford, J.R.; Shaw, L.E.; Hall, S.; Coldham, N.G.; Pope, G.S.; Sauer, M.J. Oestrogenic activity of benzylparaben. J. Appl. Toxicol. 2003, 23, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D.; Byford, J.R.; Shaw, L.E.; Horton, R.A.; Pope, G.S.; Sauer, M.J. Oestrogenic activity of isobutylparaben in vitro and in vivo. J. Appl Toxicol. 2002, 22, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D.; Harvey, P.W. Paraben esters: Review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J. Appl. Toxicol. 2008, 28, 561–578. [Google Scholar] [CrossRef]
- Lemini, C.; Jaimez, R.; Avila, M.E.; Franco, Y.; Larrea, F.; Lemus, A.E. In vivo and in vitro estrogen bioactivities of alkyl parabens. Toxicol. Ind. Health 2003, 19, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yu, T.; Guo, J.; Zhang, Z.; Hu, Y.; Xiao, X.; Sun, Y.; Xiao, H.; Li, J.; Zhu, D.; et al. The estrogenicity of methylparaben and ethylparaben at doses close to the acceptable daily intake in immature Sprague-Dawley rats. Sci. Rep. 2016, 6, 25173. [Google Scholar] [CrossRef]
- Vo, T.T.; Yoo, Y.M.; Choi, K.C.; Jeung, E.B. Potential estrogenic effect(s) of parabens at the prepubertal stage of a postnatal female rat model. Reprod. Toxicol. 2010, 29, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, A.; Gregoraszczuk, E.L. Effects of single and repeated in vitro exposure of three forms of parabens, methyl-, butyl- and propylparabens on the proliferation and estradiol secretion in MCF-7 and MCF-10A cells. Pharm. Rep. 2013, 65, 484–493. [Google Scholar] [CrossRef]
- Wrobel, A.M.; Gregoraszczuk, E.L. Actions of methyl-, propyl- and butylparaben on estrogen receptor-alpha and -beta and the progesterone receptor in MCF-7 cancer cells and non-cancerous MCF-10A cells. Toxicol. Lett. 2014, 230, 375–381. [Google Scholar] [CrossRef]
- Dall, G.V.; Hawthorne, S.; Seyed-Razavi, Y.; Vieusseux, J.; Wu, W.; Gustafsson, J.A.; Byrne, D.; Murphy, L.; Risbridger, G.P.; Britt, K.L. Estrogen receptor subtypes dictate the proliferative nature of the mammary gland. J. Endocrinol. 2018, 237, 323–336. [Google Scholar] [CrossRef]
- Treeck, O.; Lattrich, C.; Springwald, A.; Ortmann, O. Estrogen receptor beta exerts growth-inhibitory effects on human mammary epithelial cells. Breast Cancer Res. Treat. 2010, 120, 557–565. [Google Scholar] [CrossRef]
- Kunc, M.; Biernat, W.; Senkus-Konefka, E. Estrogen receptor-negative progesterone receptor-positive breast cancer–”Nobody’s land” or just an artifact? Cancer Treat. Rev. 2018, 67, 78–87. [Google Scholar] [CrossRef]
- Lanari, C.; Molinolo, A.A. Progesterone receptors–Animal models and cell signalling in breast cancer. Diverse activation pathways for the progesterone receptor: Possible implications for breast biology and cancer. Breast Cancer Res. 2002, 4, 240–243. [Google Scholar] [CrossRef]
- Wrobel, A.M.; Gregoraszczuk, E.L. Differential effect of methyl-, butyl- and propylparaben and 17beta-estradiol on selected cell cycle and apoptosis gene and protein expression in MCF-7 breast cancer cells and MCF-10A non-malignant cells. J. Appl. Toxicol. 2014, 34, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Terasaka, S.; Inoue, A.; Tanji, M.; Kiyama, R. Expression profiling of estrogen-responsive genes in breast cancer cells treated with alkylphenols, chlorinated phenols, parabens, or bis- and benzoylphenols for evaluation of estrogenic activity. Toxicol. Lett. 2006, 163, 130–141. [Google Scholar] [CrossRef]
- Pugazhendhi, D.; Sadler, A.J.; Darbre, P.D. Comparison of the global gene expression profiles produced by methylparaben, n-butylparaben and 17beta-oestradiol in MCF7 human breast cancer cells. J. Appl. Toxicol. 2007, 27, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, K.; Teitelbaum, S.L.; Lambertini, L.; Wetmur, J.; Manservisi, F.; Falcioni, L.; Panzacchi, S.; Belpoggi, F.; Chen, J. Changes in mammary histology and transcriptome profiles by low-dose exposure to environmental phenols at critical windows of development. Environ. Res. 2017, 152, 233–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lillo, M.A.; Nichols, C.; Perry, C.; Runke, S.; Krutilina, R.; Seagroves, T.N.; Miranda-Carboni, G.A.; Krum, S.A. Methylparaben stimulates tumor initiating cells in ER+ breast cancer models. J. Appl. Toxicol. 2017, 37, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, M.A.; Budish, R.A.; Kashyap, S.; Lindsey, S.H. GPER-novel membrane oestrogen receptor. Clin. Sci. 2016, 130, 1005–1016. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Yu, S.; Dong, D.; Lee, L.T.O. G Protein-Coupled Estrogen Receptor: A Potential Therapeutic Target in Cancer. Front. Endocrinol. 2019, 10, 725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J. Role of G Protein-Coupled Estrogen Receptor in Cancer Progression. Toxicol. Res. 2019, 35, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrobel, A.M.; Gregoraszczuk, E.L. Action of methyl-, propyl- and butylparaben on GPR30 gene and protein expression, cAMP levels and activation of ERK1/2 and PI3K/Akt signaling pathways in MCF-7 breast cancer cells and MCF-10A non-transformed breast epithelial cells. Toxicol. Lett. 2015, 238, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.Z.; Yan, R.L.; Huang, J.W.; Li, F.L.; Zhong, Y.X.; Chen, Y.; Liu, F.N.; Hu, B.; Huang, S.B.; Yin, L.H. Activation of G protein coupled estrogen receptor (GPER) promotes the migration of renal cell carcinoma via the PI3K/AKT/MMP-9 signals. Cell Adh. Migr. 2018, 12, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Liu, H.; Wen, H.; Jiang, X.; Cao, X.; Zhang, G.; Liu, G. The novel estrogen receptor GPER regulates the migration and invasion of ovarian cancer cells. Mol. Cell. Biochem. 2013, 378, 1–7. [Google Scholar] [CrossRef]
- Hsu, L.H.; Chu, N.M.; Lin, Y.F.; Kao, S.H. G-Protein Coupled Estrogen Receptor in Breast Cancer. Int. J. Mol. Sci. 2019, 20(2), 306. [Google Scholar] [CrossRef] [Green Version]
- Girgert, R.; Emons, G.; Grundker, C. Estrogen Signaling in ERalpha-Negative Breast Cancer: ERbeta and GPER. Front Endocrinol 2018, 9, 781. [Google Scholar] [CrossRef] [Green Version]
- Marchese, S.; Silva, E. Disruption of 3D MCF-12A breast cell cultures by estrogens--an in vitro model for ER-mediated changes indicative of hormonal carcinogenesis. PLoS ONE 2012, 7, e45767. [Google Scholar]
- Périan, S.; Cerutti, C.; Forcet, C.; Tribollet, V.; Vanacker, J. A Cell-Based Method to Detect Agonist and Antagonist Activities of Endocrine-Disrupting Chemicals on GPER. Front. Endocrinol. 2020, 11, 547. [Google Scholar] [CrossRef]
- Sasano, H.; Miki, Y.; Nagasaki, S.; Suzuki, T. In situ estrogen production and its regulation in human breast carcinoma: From endocrinology to intracrinology. Pathol. Int. 2009, 59, 777–789. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, L.; Shangguan, A.J.; Bulun, S.E. Aromatase expression and regulation in breast and endometrial cancer. J. Mol. Endocrinol. 2016, 57, R19–R33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Meeuwen, J.A.; Van Son, O.; Piersma, A.H.; de Jong, P.C.; Van den Berg, M. Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicol. Appl. Pharm. 2008, 230, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Stocco, C. Tissue physiology and pathology of aromatase. Steroids 2012, 77, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, N.; Utsumi, T.; Takagi, Y. Tissue-specific expression of the human aromatase cytochrome P-450 gene by alternative use of multiple exons 1 and promoters, and switching of tissue-specific exons 1 in carcinogenesis. Proc. Natl. Acad. Sci. USA 1993, 90, 11312–11316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilborn, E.; Stal, O.; Jansson, A. Estrogen and androgen-converting enzymes 17beta-hydroxysteroid dehydrogenase and their involvement in cancer: With a special focus on 17beta-hydroxysteroid dehydrogenase type 1, 2, and breast cancer. Oncotarget 2017, 8, 30552–30562. [Google Scholar] [CrossRef] [Green Version]
- Gunnarsson, C.; Hellqvist, E.; Stal, O. 17beta-Hydroxysteroid dehydrogenases involved in local oestrogen synthesis have prognostic significance in breast cancer. Br. J. Cancer 2005, 92, 547–552. [Google Scholar] [CrossRef] [Green Version]
- Gunnarsson, C.; Olsson, B.M.; Stal, O. Abnormal expression of 17beta-hydroxysteroid dehydrogenases in breast cancer predicts late recurrence. Cancer Res. 2001, 61, 8448–8451. [Google Scholar]
- Yazawa, T.; Imamichi, Y.; Uwada, J.; Sekiguchi, T.; Mikami, D.; Kitano, T.; Sato, T.; Nemoto, T.; Nagata, S.; Khan, R.I.; et al. Evaluation of 17beta-hydroxysteroid dehydrogenase activity using androgen receptor-mediated transactivation. J. Steroid Biochem. Mol. Biol. 2020, 196, 105493. [Google Scholar] [CrossRef]
- Engeli, R.T.; Rohrer, S.R.; Vuorinen, A.; Herdlinger, S.; Kaserer, T.; Leugger, S.; Schuster, D. Odermatt, AInterference of Paraben Compounds with Estrogen Metabolism by Inhibition of 17beta-Hydroxysteroid Dehydrogenases. Int. J. Mol. Sci. 2017, 18, 2007. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.; Wheals, B.B.; Beresford, N.; Sumpter, J.P. Estrogenic activity of phenolic additives determined by an in vitro yeast bioassay. Environ. Health Perspect. 2001, 109, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Berrino, F.; Pasanisi, P.; Bellati, C.; Venturelli, E.; Krogh, V.; Mastroianni, A.; Berselli, E.; Muti, P.; Secreto, G. Serum testosterone levels and breast cancer recurrence. Int. J. Cancer 2005, 113, 499–502. [Google Scholar] [CrossRef]
- Giovannelli, P.; Di Donato, M.; Auricchio, F.; Castoria, G.; Migliaccio, A. Androgens Induce Invasiveness of Triple Negative Breast Cancer Cells Through AR/Src/PI3-K Complex Assembly. Sci. Rep. 2019, 9, 4490. [Google Scholar] [CrossRef] [Green Version]
- Giovannelli, P.; Di Donato, M.; Galasso, G.; Di Zazzo, E.; Bilancio, A.; Migliaccio, A. The Androgen Receptor in Breast Cancer. Front. Endocrinol. 2018, 9, 492. [Google Scholar] [CrossRef] [Green Version]
- Venturelli, E.; Orenti, A.; Fabricio, A.S.C.; Garrone, G.; Agresti, R.; Paolini, B.; Bonini, C.; Gion, M.; Berrino, F.; Desmedt, C.; et al. Observational study on the prognostic value of testosterone and adiposity in postmenopausal estrogen receptor positive breast cancer patients. BMC Cancer 2018, 18, 651. [Google Scholar]
- Doane, A.S.; Danso, M.; Lal, P.; Donaton, M.; Zhang, L.; Hudis, C.; Gerald, W.L. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 2006, 25, 3994–4008. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Xia, X.; Liu, N.; Cai, J.; Guo, Z.; Li, Y.; Jiang, L.; Dou, Q.P.; Tang, D.; Huang, H.; et al. Growth arrest and apoptosis induction in androgen receptor-positive human breast cancer cells by inhibition of USP14-mediated androgen receptor deubiquitination. Oncogene 2018, 37, 1896–1910. [Google Scholar] [CrossRef]
- Ni, M.; Chen, Y.; Lim, E.; Wimberly, H.; Bailey, S.T.; Imai, Y.; Rimm, D.L.; Liu, X.S.; Brown, M. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell 2011, 20, 119–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh, K.; Nonaka, R.; Ohyama, K.; Nagai, F. Androgenic and Antiandrogenic Effects ofAlkylphenols and Parabens AssessedUsing the Reporter Gene Assay withStably Transfected CHO-K1 Cells(AR-EcoScreen System). J. Health Sci. 2005, 51, 12. [Google Scholar]
- Kolsek, K.; Gobec, M.; Rascan, I.M.; Dolenc, M.S. Screening of bisphenol A, triclosan and paraben analogues as modulators of the glucocorticoid and androgen receptor activities. Toxicol. Vitr. 2015, 29, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Pelch, K.E.; Li, Y.; Perera, L.; Thayer, K.A.; Korach, K.S. Characterization of Estrogenic and Androgenic Activities for Bisphenol A-like Chemicals (BPs): In Vitro Estrogen and Androgen Receptors Transcriptional Activation, Gene Regulation, and Binding Profiles. Toxicol. Sci. 2019, 172, 23–37. [Google Scholar] [CrossRef]
- Hu, P.; Chen, X.; Whitener, R.J.; Boder, E.T.; Jones, J.O.; Porollo, A.; Chen, J.; Zhao, L. Effects of parabens on adipocyte differentiation. Toxicol. Sci. 2013, 131, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bader, A.G.; Kang, S.; Zhao, L.; Vogt, P.K. Oncogenic PI3K deregulates transcription and translation. Nat. Rev. Cancer 2005, 5, 921–929. [Google Scholar] [CrossRef]
- Davoli, A.; Hocevar, B.A.; Brown, T.L. Progression and treatment of HER2-positive breast cancer. Cancer Chemother. Pharm. 2010, 65, 611–623. [Google Scholar] [CrossRef]
- Vasan, N.; Toska, E.; Scaltriti, M. Overview of the relevance of PI3K pathway in HR-positive breast cancer. Ann. Oncol. 2019, 30, x3–x11. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Yuan, C.; Tagmount, A.; Rudel, R.A.; Ackerman, J.M.; Yaswen, P.; Vulpe, C.D.; Leitman, D.C. Parabens and Human Epidermal Growth Factor Receptor Ligand Cross-Talk in Breast Cancer Cells. Environ. Health Perspect. 2016, 124, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, S.; Darbre, P.D. Parabens enable suspension growth of MCF-10A immortalized, non-transformed human breast epithelial cells. J. Appl. Toxicol. 2013, 33, 378–382. [Google Scholar] [CrossRef]
- Fedele, M.; Cerchia, L.; Chiappetta, G. The Epithelial-to-Mesenchymal Transition in Breast Cancer: Focus on Basal-Like Carcinomas. Cancers 2017, 9, 134. [Google Scholar] [CrossRef]
- Savci-Heijink, C.D.; Halfwerk, H.; Hooijer, G.K.J.; Koster, J.; Horlings, H.M.; Meijer, S.L.; van de Vijver, M. Epithelial-to-mesenchymal transition status of primary breast carcinomas and its correlation with metastatic behavior. Breast Cancer Res. Treat. 2019, 174, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Drasin, D.J.; Robin, T.P.; Ford, H.L. Breast cancer epithelial-to-mesenchymal transition: Examining the functional consequences of plasticity. Breast Cancer Res. 2011, 13, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, S.; Dash, P.R.; Darbre, P.D. Exposure to parabens at the concentration of maximal proliferative response increases migratory and invasive activity of human breast cancer cells in vitro. J. Appl. Toxicol. 2014, 34, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
| Conclusions | Strength | Limitations | |
|---|---|---|---|
| Darbre et al. 2004 [19]. | Intact parabens were found in the human breast tumor tissues. MP was present at the highest level and represented 62% of the total parabens extracted from breast tumor tissues. | Demonstrated that after exposure, a proportion of the parabens can remain intact in human body tissues. The levels of parabens measured in this study were comparable to the levels of parabens used in in vitro studies, indicating the levels of parabens detected in breast tissues could induce estrogenic effects in the human breast. | Small sample size. Analytical blank values might contain parabens introduced from other sources. |
| Barr et al. 2011 [32]. | Parabens were detected across the human breast from axilla to sternum. PP was found at significantly higher levels in the axilla than mid or medial regions of the breast. No correlations were found between paraben concentrations and tumor location or tumor estrogen receptor content. | Measured at the earliest time point possible after cancer diagnosis and prior to any cancer therapy. Investigated the distribution of parabens across a single breast. | Parabens were detected in breast tissues from human subjects who self-reported as non-underarm cosmetics users. Parabens were measured from breast tissues adjacent to breast tumor but not from tumor directly. It is also unclear the relative importance of long-term accumulation and/or acute exposure to the levels of parabens in the breast tissue. |
| Sprague et al. 2013 [34]. | Serum concentrations of BP and PP were modestly correlated, but parabens concentrations were not associated with percentage breast density (a marker of breast cancer risk). | Evaluated mammographic breast density in relation to biological measures of xenoestrogens, including parabens. Serum measurements may better reflect the biologically relevant exposure of the target organs. | Only postmenopausal women were enrolled in the study. Single blood sample was collected, which may only reflect current exposure. The study population was predominately non-Hispanic white. The results may not apply to general population. |
| Harley, et al. 2019 [30]. | Peripubertal concentrations of MP were associated with earlier breast development, pubic hair development, and menarche in girls; peripubertal concentrations of PP were associated with earlier pubic hair development in girls; peripubertal PP concentrations were associated with earlier genital development in boys. | Evaluated prenatal as well as peripubertal parabens exposure. | Urinary parabens only reflected recent exposure. The study population was limited to Latino women and children of low socioeconomic status. Potential confounding factors from other environmental contaminants could not be ruled out. Associations of peripubertal measurements with parabens may reflect reverse causality because children going through puberty earlier may be more likely to use personal care products. |
| Parada et al. 2020 [35]. | The highest (vs. lowest) quintiles of urinary MP, PP, and total parabens were associated with the risk of breast cancer. MP, PP, and total parabens were inversely associated with all-cause mortality hazard ratios. | A case-control and follow up design. Large sample size. Participants included women with breast cancer and women without breast cancer. Among women with breast cancer, phenol biomarkers were quantified in urine samples. Women with breast cancer were monitored for vital status with a median follow-up of 17.6 years. Examined whether urinary phenol biomarkers were associated with mortality following breast cancer. | A single spot urine sample may not be a reliable reflection of women’s parabens exposure. In addition, urine samples from breast cancer patients were collected after not before their diagnosis. |
| Wu et al. 2021 [36]. | Breast cancer risk was weakly inversely associated with total (but not individual) parabens exposure. Risk of hormone receptor positive (HR+) cancer was lower among women in the upper two tertiles of paraben exposure. | A multiethnic population-based nested case-control study. Examined the association between breast cancer risk and prediagnostic exposures paraben. Potential differences in terms of hormone receptor status, tumor stage (invasive vs in situ) and the length of follow-up time were considered in the analysis. | All cases and controls were postmenopausal at the time of urine collection. A single measure of parabens to capture long-term exposures could lead to misclassification of exposure. |
| MCF-7 | ||||||||
| ERα | PR | |||||||
| mRNA | Protein | mRNA | Protein | |||||
| 6 h | 24 h | 48 h | 72 h | 6 h | 24 h | 48 h | 72 h | |
| MP | NC | ↑ | ↑ | ↑ | NC | NC | ↑ | ↑ |
| BP | NC | ↑ | ↑ | NC | NC | ↑ | NC | NC |
| PP | NC | ↑ | ↑ | NC | NC | ↑ | ↑ | ↑ |
| E2 | NC | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| MCF-10A | ||||||||
| ERα | PR | |||||||
| mRNA | Protein | mRNA | Protein | |||||
| 6 h | 24 h | 48 h | 72 h | 6 h | 24 h | 48 h | 72 h | |
| MP | ↑ | NC | ↑ | ↑ | NC | NC | NC | NC |
| BP | NC | NC | NC | NC | NC | ↑ | NC | NC |
| PP | ↑ | NC | NC | ↑ | NC | ↑ | NC | NC |
| E2 | NC | ↑ | ↑ | ↑ | NC | NC | NC | NC |
| MCF-7 | MCF-10A | |||||||
| ERβ | ERβ | |||||||
| mRNA | Protein | mRNA | Protein | |||||
| 6 h | 24 h | 48 h | 72 h | 6 h | 24 h | 48 h | 72 h | |
| MP | NC | ↑ | ↑ | ↑ | NC | NC | NC | ↑ |
| BP | NC | ↑ | ↑ | NC | NC | NC | NC | ↑ |
| PP | NC | ↑ | ↑ | NC | NC | NC | NC | ↑ |
| E2 | NC | ↑ | ↑ | ↑ | NC | NC | NC | NC |
| MCF-7 | ||||||||||||||
| Genes | CCND1 | CCND3 | CCNE1 | CCNE2 | CCNA2 | CDK2 | CDK4 | CDK6 | CDKN1A | ATR | ATM | E2F3 | TP53 | |
| E2 | ↑ | ↑ | NC | ↑ | ↑ | ↑ | ↑ | ↓ | ↑ | ↑ | ↓ | ↓ | NC | |
| MP | NC | NC | ↓ | NC | NC | NC | NC | ↓ | NC | ↑ | ↓ | NC | NC | |
| PP | NC | NC | ↑ | NC | NC | ↑ | NC | ↓ | NC | ↑ | ↓ | ↓ | NC | |
| BP | NC | ↑ | ↓ | ↑ | NC | NC | NC | ↓ | NC | ↑ | ↓ | ↓ | NC | |
| MCF-10A | ||||||||||||||
| Genes | CCND1 | CCND3 | CCNE1 | CCNE2 | CCNA2 | CDK2 | CDK4 | CDK6 | CDKN1A | ATR | ATM | E2F3 | TP53 | |
| E2 | ↑ | NC | ↑ | ↑ | NC | ↑ | ↑ | NC | ↓ | NC | NC | ↑ | ↑ | |
| MP | ↑ | NC | ↑ | NC | ↑ | ↑ | ↑ | ↓ | ↓ | NC | NC | ↑ | NC | |
| PP | ↑ | ↓ | ↑ | NC | ↑ | ↑ | ↑ | NC | ↓ | NC | ↑ | ↑ | ↑ | |
| BP | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | NC | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hager, E.; Chen, J.; Zhao, L. Minireview: Parabens Exposure and Breast Cancer. Int. J. Environ. Res. Public Health 2022, 19, 1873. https://doi.org/10.3390/ijerph19031873
Hager E, Chen J, Zhao L. Minireview: Parabens Exposure and Breast Cancer. International Journal of Environmental Research and Public Health. 2022; 19(3):1873. https://doi.org/10.3390/ijerph19031873
Chicago/Turabian StyleHager, Emily, Jiangang Chen, and Ling Zhao. 2022. "Minireview: Parabens Exposure and Breast Cancer" International Journal of Environmental Research and Public Health 19, no. 3: 1873. https://doi.org/10.3390/ijerph19031873
APA StyleHager, E., Chen, J., & Zhao, L. (2022). Minireview: Parabens Exposure and Breast Cancer. International Journal of Environmental Research and Public Health, 19(3), 1873. https://doi.org/10.3390/ijerph19031873








