Physical Activity, Mental Health and Wellbeing of Adults within and during the Easing of COVID-19 Restrictions, in the United Kingdom and New Zealand
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Participants
3.2. Change in PA, Mental Health and Wellbeing
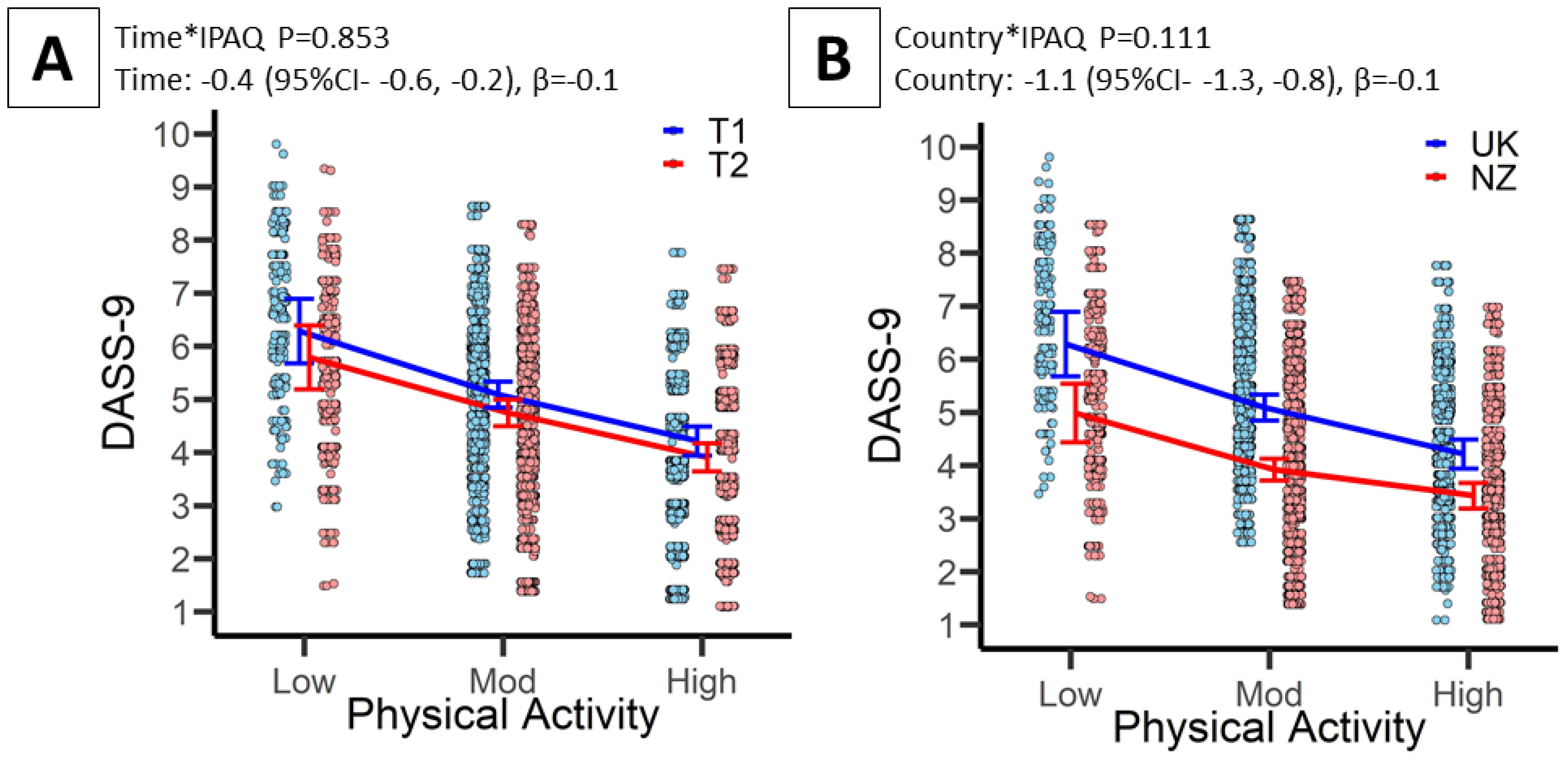
3.3. Associations between PA and Mental Health
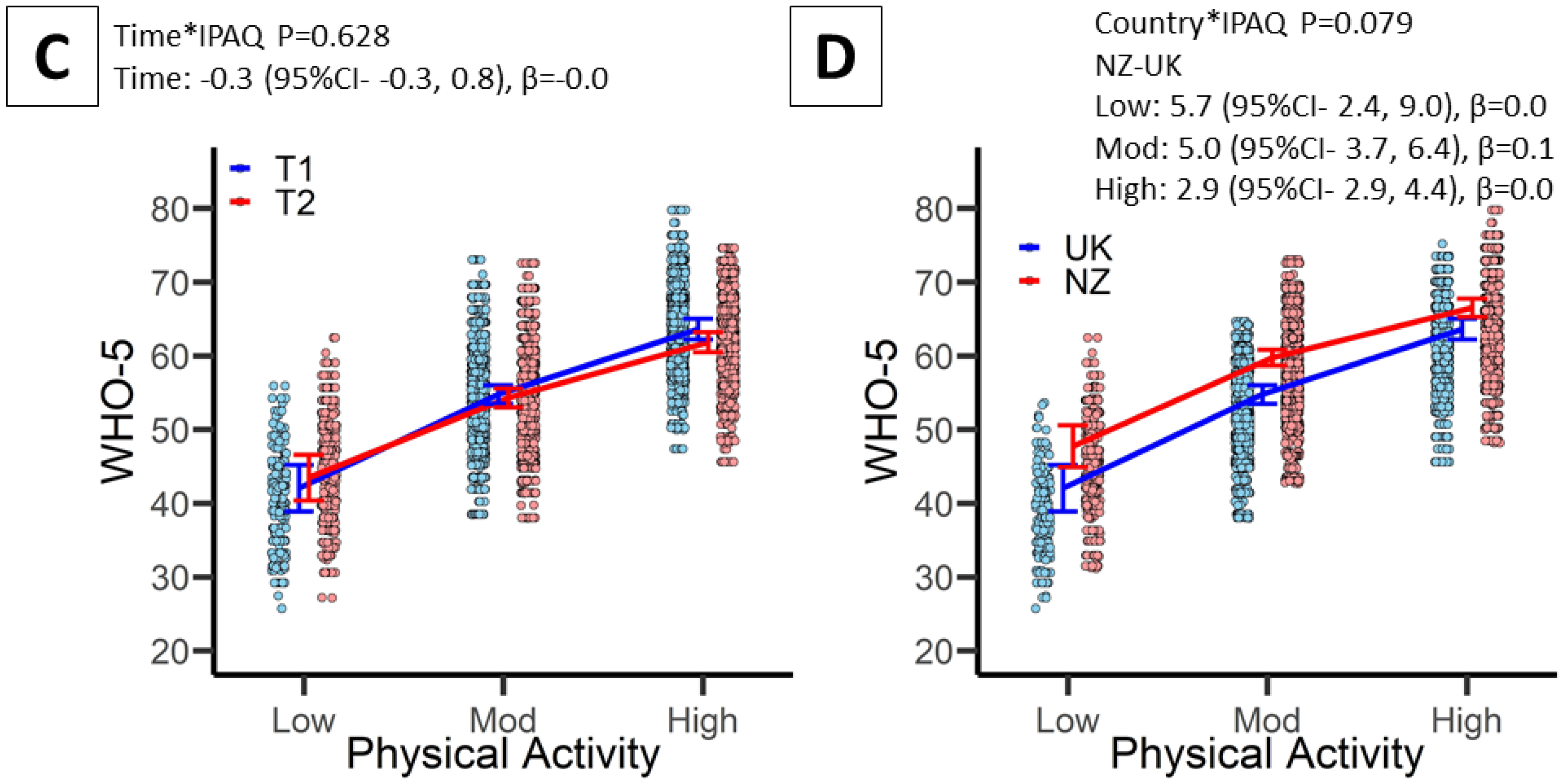
3.4. Associations between PA and Wellbeing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sallis, J.F.; Adlakha, D.; Oyeyemi, A.; Salvo, D. An international physical activity and public health research agenda to inform coronavirus disease-2019 policies and practices. J. Sport Health Sci. 2020, 9, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.; O’Brien, W.J.; McGrane, B.; Wadsworth, D.; Batten, J.; Askew, C.D.; Badenhorst, C.; Byrd, E.; Coulter, M.; Draper, N.; et al. Physical activity, mental health and well-being of adults during initial COVID-19 containment strategies: A multi-country cross-sectional analysis. J. Sci. Med. Sport 2021, 24, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Bone, J.K.; Mitchell, J.J.; Steptoe, A.; Fancourt, D. Longitudinal changes in physical activity during and after the first national lockdown due to the COVID-19 pandemic in England. Sci. Rep. 2021, 11, 17723. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; McDowell, C.; Lansing, J.; Brower, C.; Smith, L.; Tully, M.; Herring, M. Changes in physical activity and sedentary behaviour due to the COVID-19 outbreak and associations with mental health in 3052 US adults. Int. J. Environ. Res. Pub Health 2020, 17, 6469. [Google Scholar] [CrossRef] [PubMed]
- Fitbit. The Impact of Coronavirus on Global Activity. 2020. Available online: https://blogfitbitcom/covid-19-global-activity/ (accessed on 1 September 2020).
- Tison, G.H.; Avram, R.; Kuhar, P.; Abreau, S.; Marcus, G.M.; Pletcher, M.J.; Olgin, J.E. Worldwide Effect of COVID-19 on Physical Activity: A Descriptive Study. Ann. Intern. Med. 2020, 173, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Meiring, R.M.; Gusso, S.; McCullough, E.; Bradnam, L. The Effect of the COVID-19 Pandemic Movement Restrictions on Self-Reported Physical Activity and Health in New Zealand: A Cross-Sectional Survey. Int. J. Environ. Res. Public Health 2021, 18, 1719. [Google Scholar] [CrossRef] [PubMed]
- Sallis, R.; Young, D.R.; Tartof, S.Y.; Sallis, J.F.; Sall, J.; Li, Q.; Smith, G.N.; A Cohen, D. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: A study in 48,440 adult patients. Br. J. Sports Med. 2021, 55, 1099–1105. [Google Scholar] [CrossRef]
- Maugeri, G.; Castrogiovanni, P.; Battaglia, G.; Pippi, R.; D’Agata, V.; Palma, A.; Di Rosa, M.; Musumeci, G. The impact of physical activity on psychological health during COVID-19 pandemic in Italy. Heliyon 2020, 6, e04315. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Pandemics Throughout History. Front. Microbiol. 2021, 11, 631736. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Biener, L.; Abrams, D.B. The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991, 10, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. Compendium of physical activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [Green Version]
- Topp, C.W.; Østergaard, S.D.; Søndergaard, S.; Bech, P. The WHO-5 Well-Being Index: A Systematic Review of the Literature. Psychother. Psychosom. 2015, 84, 167–176. [Google Scholar] [CrossRef]
- Youssof, M.; Bahri, S. Psychometric Properties of the Depression Anxiety Stress Scale in a Sample of Medical Degree Applicants. Int. Med. J. 2013, 20, 295–300. [Google Scholar]
- Tucker, P.; Gilliland, J. The effect of season and weather on physical activity: A systematic review. Public Health 2007, 121, 909–922. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Piot, P. The Potential Future of the COVID-19 Pandemic: Will SARS-CoV-2 Become a Recurrent Seasonal Infection? JAMA 2021, 325, 1249–1250. [Google Scholar] [CrossRef]
- Callow, D.D.; Arnold-Nedimala, N.A.; Jordan, L.S.; Pena, G.S.; Won, J.; Woodard, J.L.; Smith, J.C. The Mental Health Benefits of Physical Activity in Older Adults Survive the COVID-19 Pandemic. Am. J. Geriatr. Psychiatry 2020, 28, 1046–1057. [Google Scholar] [CrossRef]
- Parrish, E. The next pandemic: COVID-19 mental health pandemic. Perspect. Psychiatr. Care 2020, 56, 485. [Google Scholar] [CrossRef]
- Gasteiger, N.; Vedhara, K.; Massey, A.; Jia, R.; Ayling, K.; Chalder, T.; Coupland, C.; Broadbent, E. Depression, anxiety and stress during the COVID-19 pandemic: Results from a New Zealand cohort study on mental well-being. BMJ Open 2021, 11, e045325. [Google Scholar] [CrossRef]
- Richardson, E.; Pearce, J.; Mitchell, R.; Day, P.; Kingham, S. The association between green space and cause-specific mortality in urban New Zealand: An ecological analysis of green space utility. BMC Public Health 2010, 10, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson Coon, J.; Boddy, K.; Stein, K.; Whear, R.; Barton, J.; Depledge, M.H. Does participating in physical activity in outdoor natural environments have a greater effect on physical and mental wellbeing than physical activity indoors? A Systematic Review. Environ. Sci. Technol. 2011, 45, 1761–1772. [Google Scholar] [CrossRef]
- Jimenez, M.; DeVille, N.; Elliott, E.; Schiff, J.; Wilt, G.; Hart, J.; James, P. Associations between Nature Exposure and Health: A Review of the Evidence. Int. J. Environ. Res. Public Health 2021, 18, 4790. [Google Scholar] [CrossRef] [PubMed]
- Webb Hooper, M.; Nápoles, A.M.; Pérez-Stable, E.J. COVID-19 and racial/ethnic disparities. JAMA 2020, 323, 2466–2467. [Google Scholar] [CrossRef]
- Ministry of Health. Annual Data Explorer 2020/21: New Zealand Health Survey [Data File]. 2021. Available online: https://minhealthnz.shinyapps.io/nz-health-survey-2020-21-annual-data-explorer/ (accessed on 1 December 2021).
- NHS Digital (Great Britain). Statistics on Obesity, Physical Activity and Diet, England, 2019: Main Report. Dandy Booksellers Limited. Available online: https://digital.N.H.S.uk/data-and-information/publications/statistical/statistics-on-obesity-physical-activity-and-diet/statistics-on-obesityphysical-activity-and-diet-england-2019 (accessed on 1 December 2021).
| T1 | T2 | |||||
|---|---|---|---|---|---|---|
| UK | NZ | Total | UK | NZ | Total | |
| Sample [n] | 3121 | 4007 | 7128 | 1333 | 2030 | 3363 |
| Sex [n (%)] | ||||||
| Male | 1024 (32.8%) | 1087 (27.1%) | 2111 (29.7%) | 426 (32.0%) | 507 (25.0%) | 933 (27.7%) |
| Female | 2094 (67.1%) | 2886 (72.0%) | 4980 (70.0%) | 905 (67.9%) | 1510 (74.4%) | 2415 (71.8%) |
| Prefer not to say | 3 (0.1%) | 24 (0.9%) | 27 (0.4%) | 2 (0.2%) | 13 (0.6%) | 15 (0.5%) |
| Mean age (years) [x(sd)] | 43.9 (14.9) | 46.5 (14.7) | 45.4 (14.8) | 48.6 (15.0) | 49.3 (14.7) | 49.0 (14.8) |
| Age groups [n (%)] | ||||||
| 20–29 years | 627 (20.2%) | 619 (15.5%) | 1246 (17.5%) | 171 (12.9%) | 229 (11.3%) | 400 (11.9%) |
| 30–39 years | 722 (23.2%) | 775 (19.3%) | 1497 (21.0%) | 249 (18.7%) | 339 (16.7%) | 588 (17.5%) |
| 40–49 years | 650 (20.9%) | 910 (22.7%) | 1560 (21.9%) | 260 (19.6%) | 447 (22.0%) | 707 (21.0%) |
| 50–59 years | 559 (18.0%) | 853 (21.3%) | 1412 (19.9%) | 293 (22.0%) | 460 (22.7%) | 753 (22.4%) |
| 60–69 years | 392 (12.6%) | 578 (14.4%) | 970 (13.6%) | 253 (19.0%) | 386 (18.1%) | 621 (18.5%) |
| 70–79 years | 141 (4.5%) | 250 (6.2%) | 391 (5.5%) | 97 (7.3%) | 170 (8.4%) | 267 (8.0%) |
| 80+ years | 15 (0.5%) | 22 (0.5%) | 37 (0.5%) | 7 (0.7%) | 17 (0.8%) | 24 (0.7%) |
| Ethnicity | ||||||
| White | 3010 (96.4%) | 3630 (90.6%) | 6640 (93.2%) | 1295 (97.2%) | 1886 (92.9%) | 3181 (94.6%) |
| Polynesian | 0 (0.0%) | 156 (3.9%) | 156 (2.2%) | 0 (0.0%) | 62 (3.1%) | 62 (1.8%) |
| Indian | 37 (1.2%) | 48 (1.2%) | 85 (1.2%) | 14 (1.1%) | 23 (1.1%) | 37 (1.1%) |
| Asian | 0 (0.0%) | 95 (2.4%) | 95 (1.3%) | 0 (0.0%) | 29 (1.4%) | 29 (0.9%) |
| Black | 14 (0.5%) | 8 (0.2%) | 22 (0.3%) | 6 (0.5%) | 2 (0.1%) | 8 (0.2%) |
| Mixed groups | 37 (1.2%) | 18 (0.5%) | 55 (0.8%) | 13 (1.0%) | 8 (0.4%) | 21 (0.6%) |
| Other | 23 (0.7%) | 52 (1.3%) | 75 (1.1%) | 5 (0.4%) | 20 (1.0%) | 25 (0.7%) |
| Living situation [n (%)] | ||||||
| Live alone | - | 459 (11.5%) | - | 179 (13.5%) | 261 (12.9%) | 440 (13.1%) |
| Couple | - | 1287 (32.1%) | - | 525 (39.5%) | 720 (35.5%) | 1245 (37.1%) |
| Two parent family | - | 1164 (29.1%) | - | 381 (28.7%) | 553 (27.2%) | 934 (27.8%) |
| Single parent family | - | 114 (2.9%) | - | 32 (2.4%) | 59 (2.9%) | 91 (2.7%) |
| Extended family | - | 588 (14.7%) | - | 149 (11.2%) | 266 (13.1%) | 415 (12.4%) |
| Shared occupancy | - | 395 (9.9%) | - | 30 (2.3%) | 171 (8.4%) | 201 (6.0%) |
| Residential care | - | 0 (0.0%) | - | 33 (2.5%) | 0 (0.0%) | 33 (1.0%) |
| Living with LTC [n (%)] | - | 873 (21.8%) | - | 346 (26.0%) | 468 (24.0%) | 814 (24.8%) |
| T1 | T2 | |||||
|---|---|---|---|---|---|---|
| UK | NZ | Total | UK | NZ | Total | |
| Met PA guidelines [n (%)] | 983 (73.9) | 1494 (73.6) | 2477 (73.7) | |||
| Stages of change [n (%)] | ||||||
| Precontemplation | 17 (1.3) | 9 (0.4) | 26 (0.8) | 13 (1.0) | 7 (0.3) | 20 (0.6) |
| Contemplation | 56 (4.2) | 57 (2.8) | 113 (3.4) | 63 (4.7) | 95 (4.7) | 158 (4.7) |
| Preparation | 216 (16.2) | 266 (13.1) | 482 (14.3) | 238 (17.9) | 349 (17.2) | 587 (17.5) |
| Action | 220 (16.5) | 340 (16.8) | 560 (16.7) | 127 (9.5) | 218 (10.7) | 345 (10.3) |
| Maintenance | 824 (61.8) | 1358 (66.9) | 2182 (64.9) | 890 (66.9) | 1361 (67.0) | 2251 (67.0) |
| IPAQ-SF [x (SD)] | ||||||
| Total PA (MET·min–1·week–1) | 3109 (2322) | 2979 (2219) | 3030 (2261) | 3302 (2545) | 2527 * (2323) | 2833 (2443) |
| Sitting time (min) | 458 (263) | 455 (171) | 456 (212) | 414 (255) | 429 (193) | 423 (220) |
| IPAQ-SF [n (%)] | ||||||
| Low | 95 (7.1) | 127 (6.3) | 222 (6.6) | 99 (7.4) | 307 (15.1) | 406 (12.1) |
| Moderate | 693 (52.0) | 1125 (55.4) | 1818 (54.1) | 623 (46.8) | 1134 (55.9) | 1757 (52.3) |
| High | 545 (40.9) | 778 (38.3) | 1323 (39.3) | 610 (45.8) | 589 (29.0) | 1199 (35.7) |
| Exercise Behaviour (T2-T1) [n (%)] | ||||||
| Positive change | 251 (18.9) | 229 (11.3) | 480 (14.3) | |||
| Negative change | 215 (16.2) | 337 (16.6) | 552 (16.4) | |||
| No change | 865 (65.0) | 1464 (72.1) | 2329 (69.3) | |||
| WHO-5 [x (sd)] | 55.6 (21.5) | 59.9 (20.7) | 58.2 (21.1) | 54.8 (22.6) | 57.8 (21.1) | 56.6 (21.7) |
| DASS-9 [x (sd)] | ||||||
| Depression | 2.24 (2.02) | 1.89 (1.76) | 2.03 (1.88) | 2.03 (2.08) | 1.61 (1.72) | 1.78 (1.88) |
| Anxiety | 0.71 (1.39) | 0.46 (1.04) | 0.56 (1.20) | 0.73 (1.43) | 0.57 (1.20) | 0.63 (1.30) |
| Stress | 2.25 (1.94) | 1.80 (1.65) | 1.98 (1.78) | 2.21 (2.08) | 1.67 (1.63) | 1.88 (1.84) |
| Total | 5.20 (4.52) | 4.15 (3.65) | 4.57 (4.05) | 4.97 (4.79) | 3.85 (3.81) | 4.29 (4.26) |
| DASS-9 | WHO-5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | LCI | UCI | β | p | B | LCI | UCI | β | p | |
| Model 1. Unadjusted | ||||||||||
| Mod−Low | −1.1 | −1.4 | −0.8 | −0.1 | <0.001 | 12.0 | 10.3 | 13.7 | 0.2 | <0.001 |
| High−Low | −1.8 | −2.2 | −1.5 | −0.1 | <0.001 | 19.6 | 17.8 | 21.4 | 0.3 | <0.001 |
| Model 2. Adjusted for age, sex & ethnicity | ||||||||||
| Mod−Low | −0.9 | −1.2 | −0.6 | −0.1 | <0.001 | 10.8 | 9.2 | 12.4 | 0.2 | <0.001 |
| High−Low | −1.4 | −1.7 | −1.1 | −0.1 | <0.001 | 17.5 | 15.8 | 19.2 | 0.2 | <0.001 |
| Model 3. Addition of PA by Time interaction term | ||||||||||
| Mod−Low | −1.0 | −1.3 | −0.7 | −0.1 | <0.001 | 11.2 | 9.5 | 12.8 | 0.2 | <0.001 |
| High−Low | −1.5 | −1.9 | −1.2 | −0.1 | <0.001 | 17.8 | 16.0 | 19.5 | 0.2 | <0.001 |
| Model 4. Addition of PA by Country interaction term | ||||||||||
| Mod−Low | −1.1 | −1.4 | −0.7 | −0.1 | <0.001 | 11.4 | 9.7 | 13.2 | 0.2 | <0.001 |
| High−Low | −1.7 | −2.1 | −1.4 | −0.1 | <0.001 | 18.6 | 16.7 | 20.4 | 0.2 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faulkner, J.; O’Brien, W.J.; Stuart, B.; Stoner, L.; Batten, J.; Wadsworth, D.; Askew, C.D.; Badenhorst, C.E.; Byrd, E.; Draper, N.; et al. Physical Activity, Mental Health and Wellbeing of Adults within and during the Easing of COVID-19 Restrictions, in the United Kingdom and New Zealand. Int. J. Environ. Res. Public Health 2022, 19, 1792. https://doi.org/10.3390/ijerph19031792
Faulkner J, O’Brien WJ, Stuart B, Stoner L, Batten J, Wadsworth D, Askew CD, Badenhorst CE, Byrd E, Draper N, et al. Physical Activity, Mental Health and Wellbeing of Adults within and during the Easing of COVID-19 Restrictions, in the United Kingdom and New Zealand. International Journal of Environmental Research and Public Health. 2022; 19(3):1792. https://doi.org/10.3390/ijerph19031792
Chicago/Turabian StyleFaulkner, James, Wendy J. O’Brien, Beth Stuart, Lee Stoner, John Batten, Daniel Wadsworth, Christopher D. Askew, Claire E. Badenhorst, Erin Byrd, Nick Draper, and et al. 2022. "Physical Activity, Mental Health and Wellbeing of Adults within and during the Easing of COVID-19 Restrictions, in the United Kingdom and New Zealand" International Journal of Environmental Research and Public Health 19, no. 3: 1792. https://doi.org/10.3390/ijerph19031792
APA StyleFaulkner, J., O’Brien, W. J., Stuart, B., Stoner, L., Batten, J., Wadsworth, D., Askew, C. D., Badenhorst, C. E., Byrd, E., Draper, N., Elliot, C., Fryer, S., Hamlin, M. J., Jakeman, J. R., Mackintosh, K. A., McNarry, M. A., Mitchelmore, A., Ryan-Stewart, H., Saynor, Z., ... Lambrick, D. (2022). Physical Activity, Mental Health and Wellbeing of Adults within and during the Easing of COVID-19 Restrictions, in the United Kingdom and New Zealand. International Journal of Environmental Research and Public Health, 19(3), 1792. https://doi.org/10.3390/ijerph19031792













