Tumours of Nasal Septum: A Retrospective Study of 32 Patients
Abstract
:1. Introduction
2. Materials and Method
2.1. Study Design
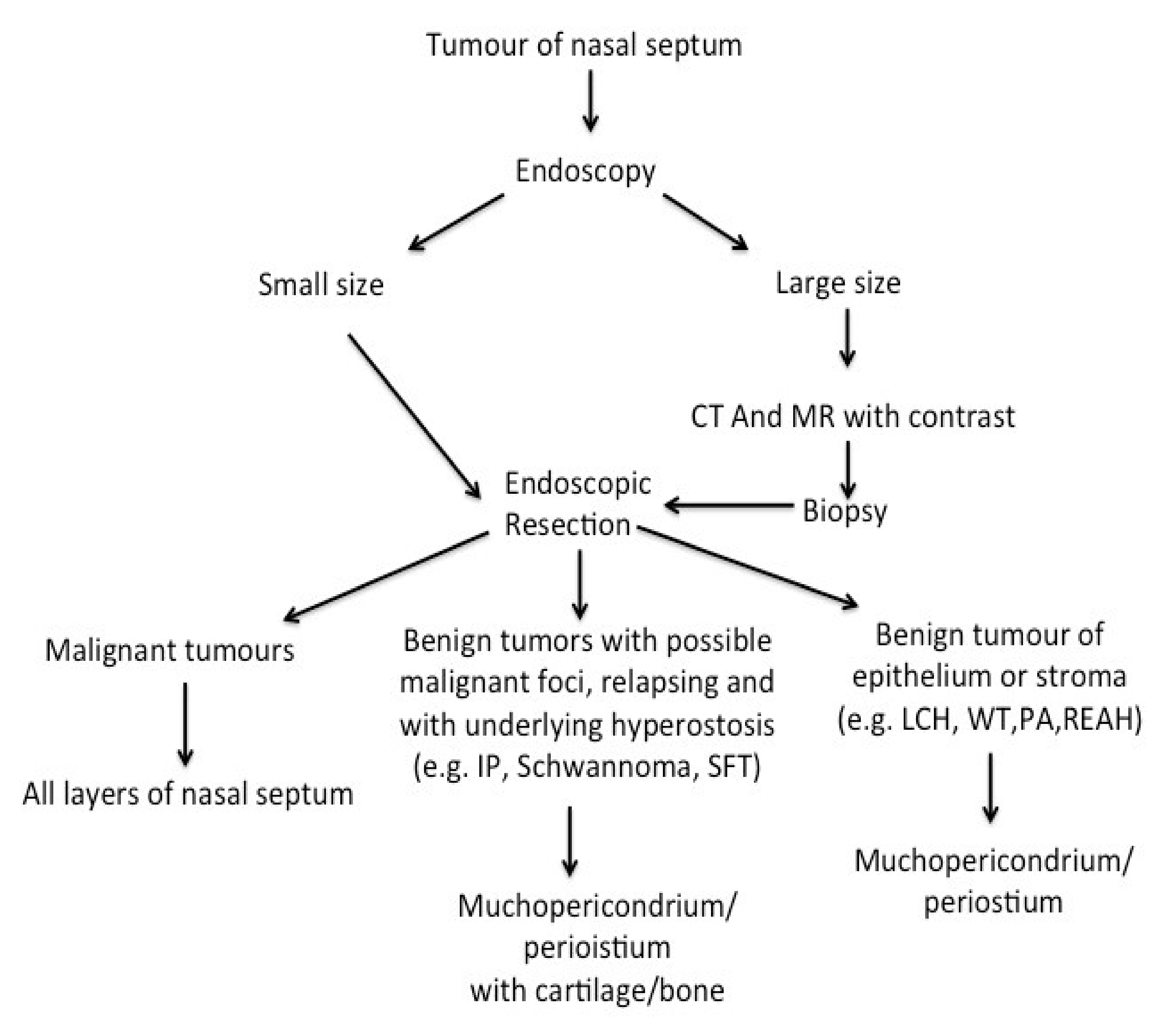
2.2. Pre-Operative Workup and Surgical Treatment
2.3. Follow-Up
3. Results
- -
- In the lobular capillary haemangioma, only the mucoperichondrium was removed.
- -
- In the others lesions, the mucoperichondrium with cartilage and/or bone was removed.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lund, V.J.; Stammberger, H.; Nicolai, P.; Castelnuovo, P.; Beal, T.; Beham, A.; Bernal-Sprekelsen, M.; Braun, H.; Cappabianca, P.; Carrau, R. European Rhinologic Society Advisory Board on Endoscopic Techniques in the Management of Nose, Paranasal Sinus and Skull Base Tumours. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol. Suppl. 2010, 22, 1–143. [Google Scholar] [PubMed]
- Jafek, B.W.; Wood, R.P.; Dion, M. Granuloma pyogenicum. Ear Nose Throat J. 1977, 56, 228–233. [Google Scholar] [PubMed]
- Compagno, J.; Wong, R.T. Intranasal mixed tumours (pleomorphic adenomas): A clinicopathologic study of 40 cases. Am. J. Clin. Pathol. 1977, 68, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.M.; Coman, W.B. Nasal septum malignancy. ANZ J. Surg. 2011, 81, 533–536. [Google Scholar] [CrossRef]
- Olsen, K.D. Nose and sinus tumours. In Rhinologic Diagnosis and Treatment; McCaffrey, T., Ed.; Thieme: New York, NY, USA, 1997; pp. 334–359. [Google Scholar]
- Sireci, F.; Dispenza, F.; Zambito, P.; Salvago, P.; Lorusso, F.; Canevari, F.R. The Minimally Invasive Sinus Surgery Technique. In Advances in Health and Disease; Duncan, L.T., Ed.; Nova Publisher: Hauppauge, NY, USA, 2021; Chapter 6; Volume 37, pp. 191–200. [Google Scholar]
- Sireci, F.; Lorusso, F.; Martines, F.; Salvago, P.; Immordino, A.; Dispenza, F.; Gallina, S.; Canevari, F.R. Guide to the Management of complications in Endoscopic Sinus Surgery (ESS). In Advances in Health and Disease; Duncan, L.T., Ed.; Nova Publisher: Hauppauge, NY, USA, 2021; Chapter 4; Volume 37, pp. 159–176. [Google Scholar]
- Sireci, F.; Speciale, R.; Sorrentino, R.; Turri-Zanoni, M.; Nicolotti, M.; Canevari, F.R. Nasal packing in sphenopalatine artery bleeding: Therapeutic or harmful? Eur. Arch. Otorhinolaryngol. 2016, 274, 1501–1505. [Google Scholar] [CrossRef]
- Krouse, J.H. Development of a staging system for inverted papilloma. Laryngoscope 2000, 110, 965–968. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Oxford, UK, 2017. [Google Scholar]
- Sireci, F.; Dispenza, F.; Immordino, A.; Gazia, F.; Maniaci, A.; Canevari, F.R. The Role of Clarithromycin in the Management of Chronic Rhinosinusitis. In Advances in Health and Disease; Duncan, L.T., Ed.; Nova Publisher: Hauppauge, NY, USA, 2021; Chapter 7; Volume 37, pp. 201–208. [Google Scholar]
- Uraih, L.C.; Maronpot, R.R. Normal histology of the nasal cavity and application of special techniques. Environ. Health Perspect. 1990, 85, 187–208. [Google Scholar] [CrossRef] [Green Version]
- Puxeddu, R.; Berlucchi, M.; Ledda, G.P.; Parodo, G.; Farina, D.; Nicolai, P. Lobular capillary hemangioma of the nasal cavity: A retrospective study on 40 patients. Am. J. Rhinol. 2006, 20, 480–484. [Google Scholar] [CrossRef]
- Lee, T.J.; Huang, C.C.; Chen, Y.W.; Chang, K.P.; Fu, C.H.; Chang, P.H. Medially originated inverted papilloma. Otolaryngol. Head Neck Surg. 2009, 140, 324–329. [Google Scholar] [CrossRef]
- Rha, M.S.; Jeong, S.; Cho, H.J.; Yoon, J.H.; Kim, C.H. Sinonasal pleomorphic adenoma: A single institution case series combined with a comprehensive review of literatures. Auris Nasus Larynx 2019, 46, 223–229. [Google Scholar] [CrossRef]
- Montana, M.; Salvago, P.; Dispenza, F. Pleomorphic adenoma of the nasal septum: A rare case report of a 14 year-old patient. EMBJ 2018, 13, 28–30. [Google Scholar]
- Johnson, P.E.; Karnezis, T.T.; Storper, I.S. Papillary cystadenoma of the nasal cavity. Otolaryngol. Head Neck Surg. 2007, 137, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, F.; Verro, B.; Dispenza, F.; Gallina, S. Respiratory epithelial adenomatoid hamartoma: An unusual case report. Otorinolaringologia 2020, 70, 86–90. [Google Scholar] [CrossRef]
- Bignami, M.; Volpi, L.; Karligkiotis, A.; de Bernardi, F.; Pistochini, A.; AlQahtani, A.; Meloni, F.; Verillaud, B.; Herman, P.; Castelnuovo, P. Endoscopic endonasal resection of respiratory epithelial adenomatoid hamartomas of the sinonasal tract. Int. Forum Allergy Rhinol. 2014, 4, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Canevari, F.R.; Sireci, F.; Sollini, G. Huge septal schwannoma: Endoscopic transnasal resection using disassembling technique. Rhinologist 2017, 4, 4–11. [Google Scholar]
- Sireci, F.; Immordino, V.; Galletti, F.; Galletti, B.; Cimino, G.; Sbacchi, C. Cerebrospinal Fluid Leak Following Nasal Packing for Epistaxis. J. Craniofac. Surg. 2019, 30, 2536–2538. [Google Scholar] [CrossRef]
- Min, H.J.; Hong, S.C.; Kim, K.S. Nasal Septal Schwannoma: Advances in Diagnosis and Treatment. J. Craniofac. Surg. 2017, 28, e97–e101. [Google Scholar] [CrossRef]
- Thompson, L.D.R.; Lau, S.K. Sinonasal Tract Solitary Fibrous Tumor: A Clinicopathologic Study of Six Cases with a Comprehensive Review of the Literature. Head Neck Pathol. 2018, 12, 471–480. [Google Scholar] [CrossRef]
- Lim, L.M.; Tan, K.B.; Petersson, F.; Thong, M. Sinonasal blue naevus: Case report and clinicopathological review. J. Laryngol. Otol. 2013, 127, 939–941. [Google Scholar] [CrossRef]
- Dauer, E.H.; Lewis, J.E.; Rohlinger, A.L.; Weaver, A.L.; Olsen, K.D. Sinonasal melanoma: A clinicopathologic review of 61 cases. Otolaryngol.—Head Neck Surg. 2008, 138, 347–352. [Google Scholar] [CrossRef]
- Canevari, F.R.; Giourgos, G.; Pistochini, A. The endoscopic transnasal paraseptal approach to a sphenoid sinus osteoma: Case report and literature review. Ear Nose Throat J. 2013, 92, E7–E10. [Google Scholar] [PubMed]
- Giger, R.; Kurt, A.M.; Lacroix, J.S. Endoscopic removal of a nasal septum chondrosarcoma. Rhinology 2002, 40, 96–99. [Google Scholar] [PubMed]
- Betz, C.S.; Janda, P.; Arbogast, S.; Leunig, A. Myxoma and myxoid chondrosarcoma of the nasal septum: Two case reports. HNO 2007, 55, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Jenny, L.; Harvinder, S.; Gurdeep, S. Endoscopic resection of primary nasoseptal chondrosarcoma. Med. J. Malays. 2008, 63, 335–336. [Google Scholar]
- Kleinsasser, O.; Schroeder, H.G. Adenocarcinomas of the inner nose after exposure to wood dust. Morphological findings and relationships between histopathology and clinical behavior in 79 cases. Arch. Oto-Rhino-Laryngol. 1988, 245, 1–15. [Google Scholar] [CrossRef]
- Barnes, L. Intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Am. J. Surg. Pathol. 1986, 10, 192–202. [Google Scholar] [CrossRef]
- Franchi, A.; Gallo, O.; Santucci, M. Clinical relevance of the histological classification of sinonasal intestinal-type adenocarcinomas. Hum. Pathol. 1999, 30, 1140–1145. [Google Scholar] [CrossRef]
- Franquemont, D.W.; Fechner, R.E.; Mills, S.E. Histologic classification of sinonasal intestinal-type adenocarcinoma. Am. J. Surg. Pathol. 1991, 15, 368–375. [Google Scholar] [CrossRef]
- Heffner, D.K.; Hyams, V.J.; Hauck, K.W.; Lingeman, C. Low-grade adenocarcinoma of the nasal cavity and paranasal sinuses. Cancer 1982, 50, 312–322. [Google Scholar] [CrossRef]
- McKay, S.P.; Shibuya, T.Y.; Armstrong, W.B.; Wong, H.S.; Panossian, A.M.; Ager, J. Cell carcinoma of the paranasal sinuses and skull base. Am. J. Otolaryngol. 2007, 28, 294–301. [Google Scholar] [CrossRef]
- Von Buchwald, C.; Bradley, P.J. Risks of malignancy in inverted papilloma of the nose and paranasal sinuses. Curr. Opin. Otolaryngol. Head Neck Surg. 2007, 15, 95–98. [Google Scholar] [CrossRef] [PubMed]
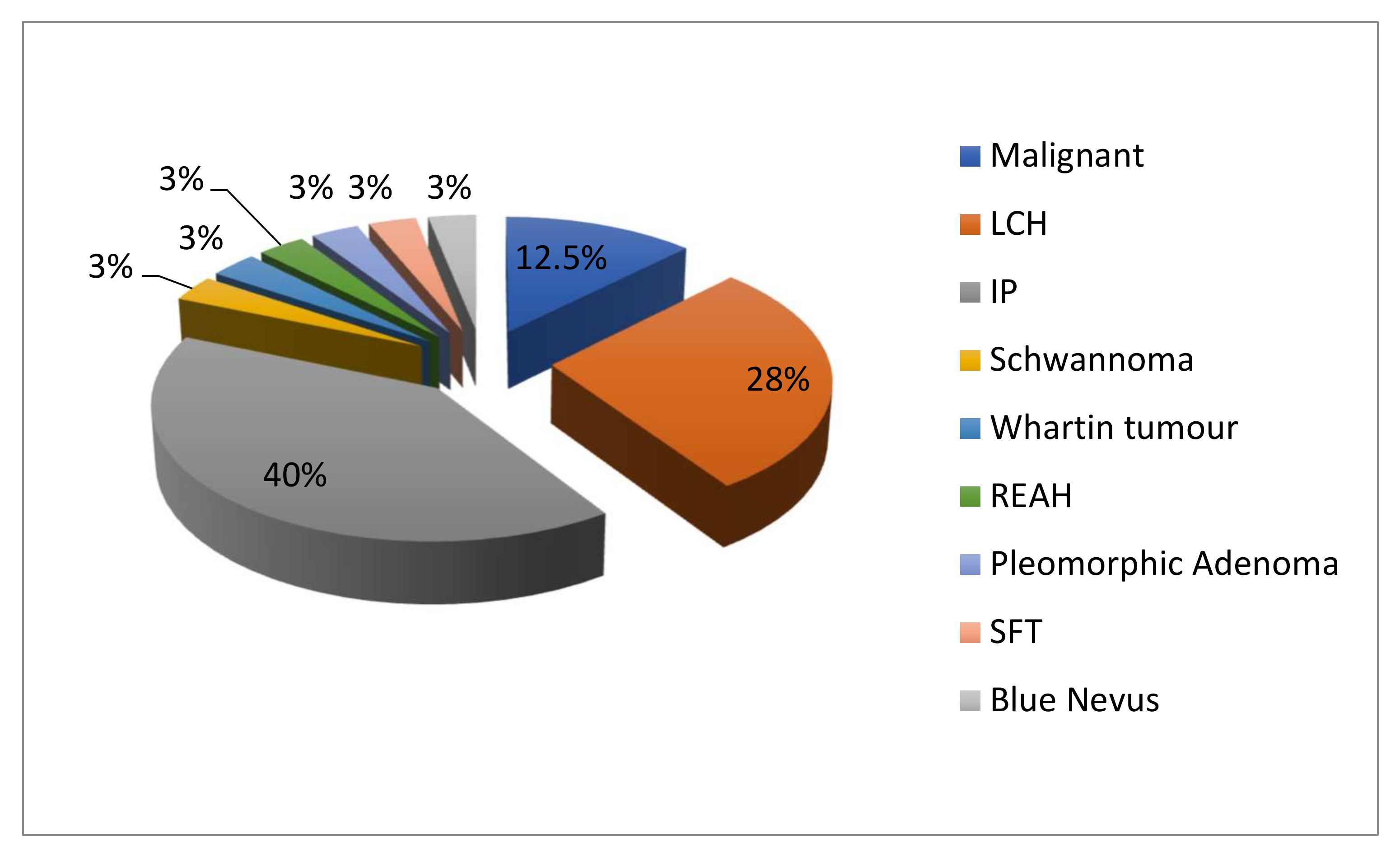

| Patients (N°) | Gender/Age | Histology | Side (Left/Right/Bilateral) | Type of Septum Resection |
|---|---|---|---|---|
| 1 | F/72 | LCH | Right | MP |
| 2 | M/44 | Inverted Papilloma | Right | MPwC |
| 3 | M/56 | n-ITAC | Left | NS |
| 4 | M/51 | Inverted Papilloma | Right | MPwC |
| 5 | F/54 | LCH | Left | MPwC |
| 6 | M/65 | Inverted Papilloma | Right | MPwC |
| 7 | M/73 | Schwannoma | Right | MPwC |
| 8 | M/58 | Inverted Papilloma | Left | MPwB |
| 9 | F/67 | Chondrosarcoma | Bilateral | NS |
| 10 | F/51 | Inverted Papilloma | Right | MPwB |
| 11 | F/54 | Warthin Tumour | Right | MP |
| 12 | M/71 | Inverted Papilloma | Right | MPwC |
| 13 | F/86 | LCH | Right | MP |
| 14 | M/69 | Inverted Papilloma | Right | MPwC |
| 15 | F/75 | Inverted Papilloma | Right | MPwB |
| 16 | F/54 | Blue Nevus | Left | NS |
| 17 | M/46 | Inverted Papilloma | Left | MPwC |
| 18 | M/57 | SCC | Right | NS |
| 19 | M/67 | Mucosal Melanoma | Right | NS |
| 20 | M/68 | Inverted Papilloma | Left | MPwC |
| 21 | M/32 | Inverted Papilloma | Left | MPwC |
| 22 | M/41 | Inverted Papilloma | Left | MPwC |
| 23 | M/62 | Inverted Papilloma | Left | MPwC |
| 24 | M/58 | LCH | Right | MP |
| 25 | M/60 | SFT | Right | MPwB |
| 26 | F/55 | LCH | Right | MP |
| 27 | F/57 | LCH | Left | MP |
| 28 | F/50 | LCH | Right | MP |
| 29 | F/25 | LCH | Left | MP |
| 30 | M/69 | LCH | Right | MP |
| 31 | F/14 | Pleomorphic Adenoma | Left | MP |
| 32 | M/46 | REAH | Right | MP |
| WHO Classifications of Tumours | Histopathology | Most Frequent Origin in the Nose | Local Recurrence | Malignant Trasformation | Therapy | Septal Resection |
|---|---|---|---|---|---|---|
| Benign Vascular Tumours | LCH | Septum | / | No | Surgery | MP |
| Benign epithelial Tumours | Inverted papilloma | Lateral wall of nasal cavity | Yes (0–78%) | Yes (3.6%) | Surgery | MPwC/B |
| Plemorphic Adenoma | Septum | / | Yes (6%) | Surgery | MP | |
| Whartin’s Tumours | / | / | No | Surgery | MP | |
| REAH | Posterior Nasal Septum | / | No | Surgery | MP | |
| Benign soft tissue tumours | Schwannoma | Naso ethmoid compartment | / | Yes (10–15% in Von Recklinghausen’s disease) | Surgery | MPwC/B |
| Borderline and low malignant potential tumours of soft tissue | SFT | Nasal cavity | / | Yes (10–20%) | Surgery | MPwC/B |
| Neuro-ectodermal Tumours | Blue nevus | Nasal cavity | Low | No | Surgery | NS |
| Mucosal melanoma | Nasal cavity | High | - | Surgery ± RT | NS | |
| Malignant tumours of bone and cartilage | Low grade chondrosarcoma | Maxillary Sinus | / | - | Surgery ± RT | NS |
| Malignant epitelial tumours | n-ITAC | Ethmoid sinus | / | - | Surgery ± RT | NS |
| Squamous cell carcionma | Ethmoid sinus | Yes (20%) | - | Surgery ± RT | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sireci, F.; Dispenza, F.; Lorusso, F.; Immordino, A.; Immordino, P.; Gallina, S.; Peretti, G.; Canevari, F.R. Tumours of Nasal Septum: A Retrospective Study of 32 Patients. Int. J. Environ. Res. Public Health 2022, 19, 1713. https://doi.org/10.3390/ijerph19031713
Sireci F, Dispenza F, Lorusso F, Immordino A, Immordino P, Gallina S, Peretti G, Canevari FR. Tumours of Nasal Septum: A Retrospective Study of 32 Patients. International Journal of Environmental Research and Public Health. 2022; 19(3):1713. https://doi.org/10.3390/ijerph19031713
Chicago/Turabian StyleSireci, Federico, Francesco Dispenza, Francesco Lorusso, Angelo Immordino, Palmira Immordino, Salvatore Gallina, Giorgio Peretti, and Frank Rikki Canevari. 2022. "Tumours of Nasal Septum: A Retrospective Study of 32 Patients" International Journal of Environmental Research and Public Health 19, no. 3: 1713. https://doi.org/10.3390/ijerph19031713
APA StyleSireci, F., Dispenza, F., Lorusso, F., Immordino, A., Immordino, P., Gallina, S., Peretti, G., & Canevari, F. R. (2022). Tumours of Nasal Septum: A Retrospective Study of 32 Patients. International Journal of Environmental Research and Public Health, 19(3), 1713. https://doi.org/10.3390/ijerph19031713











