COVID-19: Regional Differences in Austria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dependent Variables
2.2. Independent Variables
2.3. Statistical Analysis
3. Results
3.1. Cumulative Number of Daily COVID-19 Cases
3.2. Cumulative Number of COVID-19 Deaths (Mortality) and Lethality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variable | Total Year | 1st Wave | 2nd Wave | Endemic Phase | |
|---|---|---|---|---|---|
| Correlation coefficients | |||||
| Percent no citizen | 0.024 | 0.056 | 0.031 | 0.361 ** | |
| Percent not born in Austria | −0.022 | 0.037 | −0.015 | 0.400 ** | |
| Urban vs. rural | −0.124 | −0.052 | −0.112 | 0.319 ** | |
| Sea level | 0.570 ** | 0.252 * | 0.547 ** | −0.330 ** | |
| Percent male | 0.33 ** | 0.258 * | 0.314 ** | −0.092 | |
| Percent below 15 years | 0.403 ** | 0.375 ** | 0.381 ** | 0.197 | |
| Percent aged 65 and above | −0.322 ** | −0.494 ** | −0.293 ** | −0.339 ** | |
| Percent secondary education | 0.020 | −0.065 | 0.009 | −0.400 ** | |
| Percent tertiary education | −0.287 * | 0.002 | −0.268 * | 0.298 ** | |
| Percent working out of home | −0.057 | 0.019 | −0.065 | −0.126 | |
| Proportion vote for Conservatives | 0.282 * | 0.358 ** | 0.224 * | −0.311 ** | |
| Proportion vote for Soc. Dem. | −0.288 * | −0.503 ** | −0.244 * | 0.191 | |
| Proportion vote for FP | −0.081 | −0.266* | −0.058 | −0.293 ** | |
| Proportion vote for Greens | 0.013 | 0.155 | 0.026 | 0.353 ** | |
| Proportion vote for Liberals | 0.030 | 0.204 * | 0.032 | 0.166 | |
| Proportion valid vote | −0.204 * | −0.045 | −0.206 * | 0.223 * | |
| Tourism nights per population | 0.426 ** | 0.467 ** | 0.348 ** | −0.197 | |
| Proportion working in agriculture | 0.148 | 0.063 | 0.121 | −0.416 ** | |
| Percent unemployed | −0.272 * | −0.080 | −0.308 ** | 0.155 | |
| Percent working | 0.336 ** | 0.201 | 0.340 ** | −0.046 | |
| Population density | −0.144 | −0.047 | −0.132 | 0.386 ** | |
| Proportion daily smoking | −0.379 ** | −0.182 | −0.385 ** | 0.128 | |
| Proportion never-smokers | 0.310 ** | 0.144 | 0.313 ** | −0.096 | |
| Av. persons per household | 0.370 ** | 0.265* | 0.339 ** | −0.268 * |
| Variable | Total Year | 1st Wave | 2nd Wave | Endemic Phase | |
|---|---|---|---|---|---|
| Correlation coefficients | |||||
| Percent no citizen | −0.24 * | −0.032 | −0.209 * | 0.005 | |
| Percent not born in Austria | −0.277 * | −0.044 | −0.241 * | −0.001 | |
| Urban vs. rural | 0.006 | −0.070 | 0.023 | 0.102 | |
| Sea level | 0.252 * | 0.059 | 0.223 * | −0.080 | |
| Percent male | 0.066 | 0.116 | 0.039 | 0.154 | |
| Percent below 15 years | −0.179 | 0.146 | −0.186 | 0.102 | |
| Percent aged 65 and above | 0.229 * | −0.245 * | 0.247 * | −0.208 * | |
| Percent secondary education | 0.164 | 0.066 | 0.130 | −0.058 | |
| Percent tertiary education | −0.208 * | 0.099 | −0.193 | 0.044 | |
| Percent working out of home | −0.119 | 0.051 | −0.120 | −0.104 | |
| Proportion vote for Conservatives | −0.069 | 0.176 | −0.150 | 0.045 | |
| Proportion vote for Soc. Dem. | 0.175 | −0.269 * | 0.246 * | −0.124 | |
| Proportion vote for FP | 0.422 ** | −0.073 | 0.417 ** | 0.036 | |
| Proportion vote for Greens | −0.246 * | 0.049 | −0.218 * | 0.037 | |
| Proportion vote for Liberals | −0.357 ** | 0.044 | −0.327 ** | −0.045 | |
| Proportion valid vote | −0.099 | −0.006 | −0.101 | 0.028 | |
| Tourism nights per population | 0.004 | 0.274 * | −0.072 | 0.086 | |
| Proportion working in agriculture | 0.281 * | 0.054 | 0.222 * | 0.101 | |
| Percent unemployed | −0.030 | −0.075 | −0.044 | 0.089 | |
| Percent working | 0.007 | 0.120 | −0.002 | 0.040 | |
| Population density | −0.072 | −0.038 | −0.060 | 0.164 | |
| Proportion daily smoking | −0.305 ** | −0.100 | −0.293 ** | 0.224 * | |
| Proportion never-smokers | 0.364 ** | 0.044 | 0.350 ** | −0.159 | |
| Av. persons per household | 0.063 | 0.107 | 0.027 | 0.029 |
| Variable | Total Year | 1st Wave | 2nd Wave | Endemic Phase | |
|---|---|---|---|---|---|
| Correlation coefficients | |||||
| Percent no citizen | −0.264 * | −0.115 | −0.253 * | −0.205 * | |
| Percent not born in Austria | −0.275 * | −0.117 | −0.262 * | −0.224 * | |
| Urban vs. rural | 0.047 | −0.036 | 0.053 | −0.106 | |
| Sea level | −0.025 | −0.139 | −0.021 | 0.052 | |
| Percent male | −0.140 | 0.044 | −0.167 | 0.244 * | |
| Percent below 15 years | −0.454 ** | −0.116 | −0.471 ** | 0.059 | |
| Percent aged 65 and above | 0.474 ** | 0.092 | 0.506 ** | −0.065 | |
| Percent secondary education | 0.189 | 0.124 | 0.179 | 0.146 | |
| Percent tertiary education | −0.074 | 0.113 | −0.090 | −0.157 | |
| Percent working out of home | −0.069 | 0.054 | −0.073 | 0.042 | |
| Proportion vote for Conservatives | −0.225 * | −0.109 | −0.259 * | 0.237 * | |
| Proportion vote for Soc. Dem. | 0.340 ** | 0.072 | 0.395 ** | −0.243 * | |
| Proportion vote for FP | 0.512 ** | 0.245 * | 0.499 ** | 0.285 * | |
| Proportion vote for Greens | −0.298 ** | −0.105 | −0.298 ** | −0.207 * | |
| Proportion vote for Liberals | −0.384 ** | −0.143 | −0.378 ** | −0.183 | |
| Proportion valid vote | −0.023 | 0.097 | −0.036 | 0.060 | |
| Tourism nights per population | −0.227 * | −0.128 | −0.247 * | 0.124 | |
| Proportion working in agriculture | 0.214 * | 0.039 | 0.184 | 0.352 ** | |
| Percent unemployed | 0.093 | −0.087 | 0.107 | −0.097 | |
| Percent working | −0.185 | 0.009 | −0.207 * | 0.178 | |
| Population density | −0.023 | −0.002 | −0.03 | −0.087 | |
| Proportion daily smoking | −0.063 | 0.054 | −0.072 | 0.086 | |
| Proportion never-smokers | 0.16 | −0.041 | 0.171 | −0.056 | |
| Av. persons per household | −0.158 | −0.063 | −0.184 | 0.252 * |
References
- Teixeira da Silva, J.A.; Tsigaris, P.; Erfanmanesh, M. Publishing volumes in major databases related to Covid-19. Scientometrics 2021, 126, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Fry, C.V.; Wagner, C.S. International collaboration during the COVID-19 crisis: Autumn 2020 developments. Scientometrics 2021, 126, 1–10. [Google Scholar] [CrossRef]
- Uddin, M.; Mustafa, F.; Rizvi, T.A.; Loney, T.; Suwaidi, H.A.; Al-Marchzouqi, A.H.H. SARS-CoV-2/COVID-19: Viral Genomics, Epidemiology, Vaccines, and Therapeutic Interventions. Viruses 2020, 12, 526. [Google Scholar] [CrossRef] [PubMed]
- Nagel, A.; Łaszewska, A.; Haidinger, G.; Simon, J. The first 8 weeks of the Austrian SARS-CoV-2 epidemic. Wien. Klin. Wochenschr 2021, 133, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Chiu, N.-C.; Chi, H.; Tai, Y.-L.; Peng, C.-C.; Tseng, C.-Y.; Chen, C.-C. Impact of wearing masks, hand hygiene, and social distancing on influenza, enterovirus, and all-cause pneumonia during the coronavirus pandemic: Retrospective national epidemiological surveillance study. J. Med. Internet Res. 2020, 22, e21257. [Google Scholar] [CrossRef]
- Karmakar, M.; Lantz, P.M.; Tipirneni, R. Association of social and demographic factors with COVID-19 incidence and death rates in the US. JAMA Netw. Open 2021, 4, e2036462. [Google Scholar] [CrossRef]
- Hills, S.; Eraso, Y. Factors associated with non-adherence to social distancing rules during the COVID-19 pandemic: A logistic regression analysis. BMC Public Health 2021, 21, 352. [Google Scholar] [CrossRef]
- Heger, F.; Moshammer, H. COVID-19: The Austrian experience. Asian Pac. J. Env. Cancer 2020, 3, 3–4. [Google Scholar] [CrossRef]
- Moshammer, H.; Poteser, M.; Lemmerer, K.; Wallner, P.; Hutter, H.-P. Time course of COVID-19 cases in Austria. Int. J. Env. Res. Public Health 2020, 17, 3270. [Google Scholar] [CrossRef]
- Röder, M. 12 October 2020. Expertenkommission-Die Fehler von Ischgl. Available online: https://www.aerztezeitung.de/Politik/Die-Fehler-von-Ischgl-413658.html (accessed on 13 April 2021).
- Kletečka-Pulker, M.; Völkl-Kernstock, S.; Fassl, A.; Klager, E.; Willschke, H.; Klomfar, S. Telehealth in Times of COVID-19: Spotlight on Austria. Healthcare 2021, 9, 280. [Google Scholar] [CrossRef] [PubMed]
- Austrian Health and Food Safety Agency. AGES Dashboard COVID19. Available online: https://covid19-dashboard.ages.at/ (accessed on 8 March 2021).
- DW.com 2020. Corona-Fälle in Schlachthöfen in Österreich. Available online: https://www.dw.com/de/corona-f%C3%A4lle-in-schlachth%C3%B6fen-in-%C3%B6sterreich/a-54059040 (accessed on 13 April 2021).
- Kurier 17 May 2020. Corona-Hotspot Verteilerzentren der Post: Leiharbeitsfirmen im Visier. Available online: https://kurier.at/politik/inland/coronavirus-wien-nimmt-leiharbeitsfirmen-ins-visier/400844468 (accessed on 13 April 2021).
- Vienna Online 2021. Corona-Zahlen in Altenheimen: Experten warnen vor verfrühtem Optimismus. Available online: https://www.vienna.at/corona-zahlen-in-altenheimen-experten-warnen-vor-verfruehtem-optimismus/6914497 (accessed on 13 April 2021).
- Łaszewska, A.; Helter, T.; Simon, J. Perceptions of Covid-19 lockdowns and related public health measures in Austria: A longitudinal online survey. BMC Public Health 2021, 21, 1502. [Google Scholar] [CrossRef] [PubMed]
- Schaffler, Y.; Gächter, A.; Dale, R.; Jesser, A.; Probst, T.; Pieh, C. Concerns and Support after One Year of COVID-19 in Austria: A Qualitative Study Using Content Analysis with 1505 Participants. Int. J. Env. Res. Public Health 2021, 18, 8218. [Google Scholar] [CrossRef] [PubMed]
- Knabl, L.; Mitra, T.; Kimpel, J.; Rössler, A.; Volland, A.; Walser, A. High SARS-CoV-2 seroprevalence in children and adults in the Austrian ski resort Ischgl. medRxiv 2020. [Google Scholar] [CrossRef]
- Gehrke, L. 25 June 2020. Over 42 percent in Austria’s Ischgl have coronavirus antibodies, study finds. Available online: https://www.politico.eu/article/over-42-percent-in-austrias-ischgl-had-coronavirus-antibody-tests-find/ (accessed on 13 April 2021).
- Pancevski, B. 10 April 2020. Coronavirus study finds twice as many infections in Austria as earlier thought. Available online: https://www.wsj.com/articles/coronavirus-study-finds-twice-as-many-infections-in-austria-than-earlier-thought-11586523316 (accessed on 10 October 2021).
- Ogris, G.; Oberhuber, F. Spread of SARS-CoV-2 in Austria. PCR tests in a representative sample. Vienna, 30 April 2020. Available online: https://data.aussda.at/dataset.xhtml?persistentId=doi:10.11587/X2MIHW (accessed on 10 October 2021).
- gesundheit.gv.at 26 November 2020. Studie: Hälfte der Corona-Infektionen nicht bekannt und meist symptoMarchm. Available online: https://www.gesundheit.gv.at/aktuelles/studie-statistik-austria-coronavirus (accessed on 13 April 2021).
- Vindobona 09 Marchch 2021. Covid-19 in Austria: British mutation dominant. Available online: https://www.vindobona.org/article/covid-19-in-austria-british-mutation-dominant (accessed on 13 April 2021).
- World Health Organization. 31 December 2020. SARS-CoV-2 variants. Disease outbreak news. Available online: https://www.who.int/csr/don/31-december-2020-sars-cov2-variants/en/ (accessed on 13 April 2021).
- Schernhammer, E.; Weitzer, J.; Laubichler, M.D.; Birmann, B.M.; Bertau, M.; Zenk, L. Correlates of COVID-19 vaccine hesitancy in Austria: Trust and the government. J. Public Health 2021, fdab122. [Google Scholar] [CrossRef]
- Gollwitzer, A.; Marchtel, C.; Brady, W.J.; Pärnamets, P.; Freedman, I.G.; Knowles, E.D.; van Bavel, J.J. Partisan differences in physical distancing are linked to health outcomes during the COVID-19 pandemic. Nat. Hum. Behav. 2020, 4, 1186–1197. [Google Scholar] [CrossRef]
- Pummerer, L.; Böhm, R.; Lilleholt, L.; Winter, K.I.; Sassenberg, K. Conspiracy theories and their societal effects during the COVID-19 pandemic. Soc. Psychol Pers. Sci 2021, 13, 49–59. [Google Scholar] [CrossRef]
- Uddin, S.; Imam, T.; Khushi, M.; Khan, A.; Ali, M. How did socio-demographic status and personal attributes influence compliance to COVID-19 preventive behaviours during the early outbreak in Japan? Lessons for pandemic management. Pers. Individ. Dif. 2021, 175, 110692. [Google Scholar] [CrossRef]
- Vardavas, C.I.; Nikitara, K. COVID-19 and smoking: A systematic review of the evidence. Tob. Induc. Dis. 2020, 18, 20. [Google Scholar] [CrossRef]
- Reddy, R.K.; Charles, W.N.; Sklavounos, A.; Dutt, A.; Seed, P.T.; Khajuria, A. The effect of smoking on COVID-19 severity: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 1045–1056. [Google Scholar] [CrossRef]
- Usman, M.S.; Siddiqi, T.J.; Khan, M.S.; Patel, U.K.; Shahid, I.; Ahmed, J. Is there a smoker’s paradox in COVID-19? BMJ Evid Based Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Haidinger, G.; Waldhoer, T.; Vutuc, C. The prevalence of smoking in Austria. Prev. Med. 1998, 27, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Opazo Breton, M.; Gillespie, D.; Pryce, R.; Bogdanovica, I.; Angus, C.; Hernandez Alava, M. Understanding long-term trends in smoking in England, 1972–2019: An age-period-cohort approach. Addiction 2021. [Google Scholar] [CrossRef] [PubMed]
- Statistik Austria 9 December 2020. Gesundheitsbefragung 2006/07, 2014 und 2019, Mikrozensus Sonderprogramm Rauch-gewohnheiten der österreichischen Bevölkerung 1972, 1979, 1986 und 1997. Available online: https://www.statistik.at/web_de/statistiken/menschen_und_gesellschaft/gesundheit/gesundheitsdeterminanten/rauchen/index.html (accessed on 10 November 2021).
- Hawkins, R.B.; Charles, E.J.; Mehaffey, J.H. Socio-economic status and COVID-19-related cases and fatalities. Public Health 2020, 189, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, Y.; Kawachi, I. Association of socioeconomic characteristics with disparities in COVID-19 outcomes in Japan. JAMA Netw. Open 2021, 4, e2117060. [Google Scholar] [CrossRef]
- Strang, P.; Fürst, P.; Schultz, T. Excess deaths from COVID-19 correlate with age and socio-economic status. A database study in the Stockholm region. Ups. J. Med. Sci. 2020, 125, 297–304. [Google Scholar] [CrossRef]
- Wachtler, B.; Michalski, N.; Nowossadeck, E.; Diercke, M.; Wahrendorf, M.; Santos-Hövener, C. Socioeconomic inequalities and COVID-19–A review of the current international literature. J. Health Monit. 2020, 5, e1–e12. [Google Scholar] [CrossRef]
- Sannigrahi, S.; Pilla, F.; Basu, B.; Basu, A.S.; Molter, A. Examining the association between socio-demographic composition and COVID-19 fatalities in the European region using spatial regression approach. Sustain. Cities Soc. 2020, 62, 102418. [Google Scholar] [CrossRef]
- Arias-Reyes, C.; Zubieta-DeUrioste, N.; Poma-Machicao, L.; Aliaga-Raduan, F.; Carvajal-Rodriguez, F.; Dutschmann, M. Does the pathogenesis of SARS-CoV-2 virus decrease at high-altitude? Respir Physiol. Neurobiol. 2020, 277, 103443. [Google Scholar] [CrossRef]
- Accinelli, R.A.; Leon-Abarca, J.A. En la altura la COVID-19 es menos frecuente: La experiencia del Perú. Arch. Bronconeumol. 2020, 56, 760–761. [Google Scholar] [CrossRef]
- Hutter, H.-P.; Poteser, M.; Moshammer, H.; Lemmerer, K.; Mayer, M.; Weitensfelder, L. Air pollution is associated with COVID-19 incidence and mortality in Vienna, Austria. Int. J. Environ. Res. Public Health 2020, 17, 9275. [Google Scholar] [CrossRef] [PubMed]
- Katoto, P.D.M.C.; Brand, A.S.; Bakan, B.; Obadia, P.M.; Kuhangana, C.; Kayembe-Kitenge, T. Acute and chronic exposure to air pollution in relation with incidence, prevalence, severity and mortality of COVID-19: A rapid systematic review. Environ. Health 2021, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Moshammer, H.; Poteser, M.; Hutter, H.-P. COVID-19 and air pollution in Vienna—A time series approach. Wien. Klin. Wochenschr. 2021. [Google Scholar] [CrossRef]
- Garcia, E.; Marchian, B.; Chen, Z.; Li, K.; Lurmann, F.; Gilliland, F.; Eckel, S.P. Long-term air pollution and COVID-19 mortality rates in California: Findings from the spring/summer and winter surges of COVID-19. Environ. Pollut. 2021, 292, 118396. [Google Scholar] [CrossRef]
- Statistik Austria. Bevölkerung am 1 January 2020 nach Politischen Bezirken, Alter und Geschlecht. Available online: https://www.statistik.at/web_de/statistiken/menschen_und_gesellschaft/bevoelkerung/bevoelkerungsstruktur/bevoelkerung_nach_alter_geschlecht/index.html (accessed on 8 March 2021).
- Statistik Austria. Bevölkerung am 1 January 2020 nach Staatsangehörigkeit bzw. Geburtsland und Gemeinden. Available online: https://www.statistik.at/web_de/statistiken/menschen_und_gesellschaft/bevoelkerung/bevoelkerungsstruktur/bevoelkerung_nach_staatsangehoerigkeit_geburtsland/index.html (accessed on 8 March 2021).
- Statistik Austria. Ein Blick auf die Gemeinde. Available online: https://www.statistik.at/blickgem/index (accessed on 8 March 2021).
- Statistik Austria. Bevölkerung 2019 nach Altersgruppen, Geschlecht und Bezirken bzw. NUTS 3-Regionen. 2021. Available online: https://www.statistik.at/wcm/idc/idcplg?IdcService=GET_NATIVE_FILE&RevisionSelectionMethod=LatestReleased&dDocName=078576 (accessed on 26 November 2021).
- Haluza, D.; Simic, S.; Moshammer, H. Temporal and spatial melanoma trends in Austria: An ecological study. Int. J. Environ. Res. Public Health 2014, 11, 734–748. [Google Scholar] [CrossRef] [Green Version]
- Statistik Austria. Gesundheitsbefragung 2019. Available online: http://www.statistik.at/web_de/statistiken/menschen_und_gesellschaft/gesundheit/gesundheitsdeterminanten/rauchen/index.html (accessed on 8 March 2021).
- Van de Kassteele, J.; van Eijkeren, J.; Wallinga, J. Efficient estimation of age-specific social contact rates between men and women. Ann. Appl. Stat. 2017, 11. [Google Scholar] [CrossRef]
- Wallinga, J.; Teunis, P.; KretzschMarch, M. Using data on social contacts to estimate age-specific transmission parameters for respiratory-spread infectious agents. Am. J. Epidemiol. 2006, 164, 936–944. [Google Scholar] [CrossRef]
- Gallo Marchin, B.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S. Predictors of COVID-19 severity: A literature review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef]
- Pun, M.; Turner, R.; Strapazzon, G.; Brugger, H.; Swenson, E.R. Lower incidence of COVID-19 at high altitude: Facts and confounders. High. Alt. Med. Biol. 2020, 21, 217–222. [Google Scholar] [CrossRef]
- Hopkinson, N.S.; Rossi, N.; El-Sayed Moustafa, J.; Laverty, A.A.; Quint, J.K.; Freidin, M. Current smoking and COVID-19 risk: Results from a population symptom app in over 2.4 million people. Thorax 2021, 76, 714–722. [Google Scholar] [CrossRef]
- Simons, D.; Shahab, L.; Brown, J.; Perski, O. The association of smoking status with SARS-CoV-2 infection, hospitalization and mortality from COVID-19: A living rapid evidence review with Bayesian meta-analyses (version 7). Addiction 2021, 116, 1319–1368. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.; Bagos, P.G.; Giannouchos, T.; Niaura, R.; Barbouni, A.; Poulas, K. Smoking prevalence among hospitalized COVID-19 patients and its association with disease severity and mortality: An expanded re-analysis of a recent publication. Harm. Reduct. J. 2021, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Korzeniowska, A.; Ręka, G.; Bilska, M.; Piecewicz-Szczęsna, H. The smoker’s paradox during the COVID-19 pandemic? The influence of smoking and vaping on the incidence and course of SARS-CoV-2 virus infection as well as possibility of using nicotine in the treatment of COVID-19-Review of the literature. Przegl. Epidemiol. 2021, 75, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Karić, T.; Međedović, J. Covid-19 conspiracy beliefs and containment-related behaviour: The role of political trust. Pers. Individ. Dif. 2021, 175, 110697. [Google Scholar] [CrossRef] [PubMed]
- Lilleholt, L.; Zettler, I.; Betsch, C.; Böhm, R. Pandemic fatigue: Measurement, correlates, and consequences. PsyArXiv 2020. [Google Scholar] [CrossRef]
- Blagov, P.S. Adaptive and dark personality in the COVID-19 pandemic: Predicting health-behavior endorsement and the appeal of public-health messages. Soc. Psychol. Pers. Sci. 2020, 12, 697–707. [Google Scholar] [CrossRef]
- Rammstedt, B.; Lechner, C.M.; Weiß, B. Does personality predict responses to the COVID-19 crisis? Evidence from a prospective large-scale study. Eur. J. Pers. 2021, 36, 47–60. [Google Scholar] [CrossRef]
- Kittel, B. Wer geht testen? Zur Häufigkeit der Nutzung des Testangebots. Available online: https://viecer.univie.ac.at/corona-blog/corona-blog-beitraege/blog108/ (accessed on 10 May 2021).
- Filippidis, F.T.; Girvalaki, C.; Mechili, E.-A.; Vardavas, C.I. Are political views related to smoking and support for tobacco control policies? A survey across 28 European countries. Tob. Induc. Dis. 2017, 15, 45. [Google Scholar] [CrossRef] [Green Version]
- Hersch, J.; Rossi, A.F.D.; Viscusi, W.K. Voter preferences and state regulation of smoking. Econ. Inq. 2007, 42, 455–468. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Pandemic fatigue-reinvigorating the public to prevent COVID-19: Policy framework for supporting pandemic prevention and management: Revised version November 2020. 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/337574/WHO-EURO-2020–1573–41324–56242-eng.pdf (accessed on 25 November 2021).
- Mattos Dos Santos, R. Isolation, social stress, low socioeconomic status and its relationship to immune response in Covid-19 pandemic context. Brain Behav. Immun. Health 2020, 7, 100103. [Google Scholar] [CrossRef]

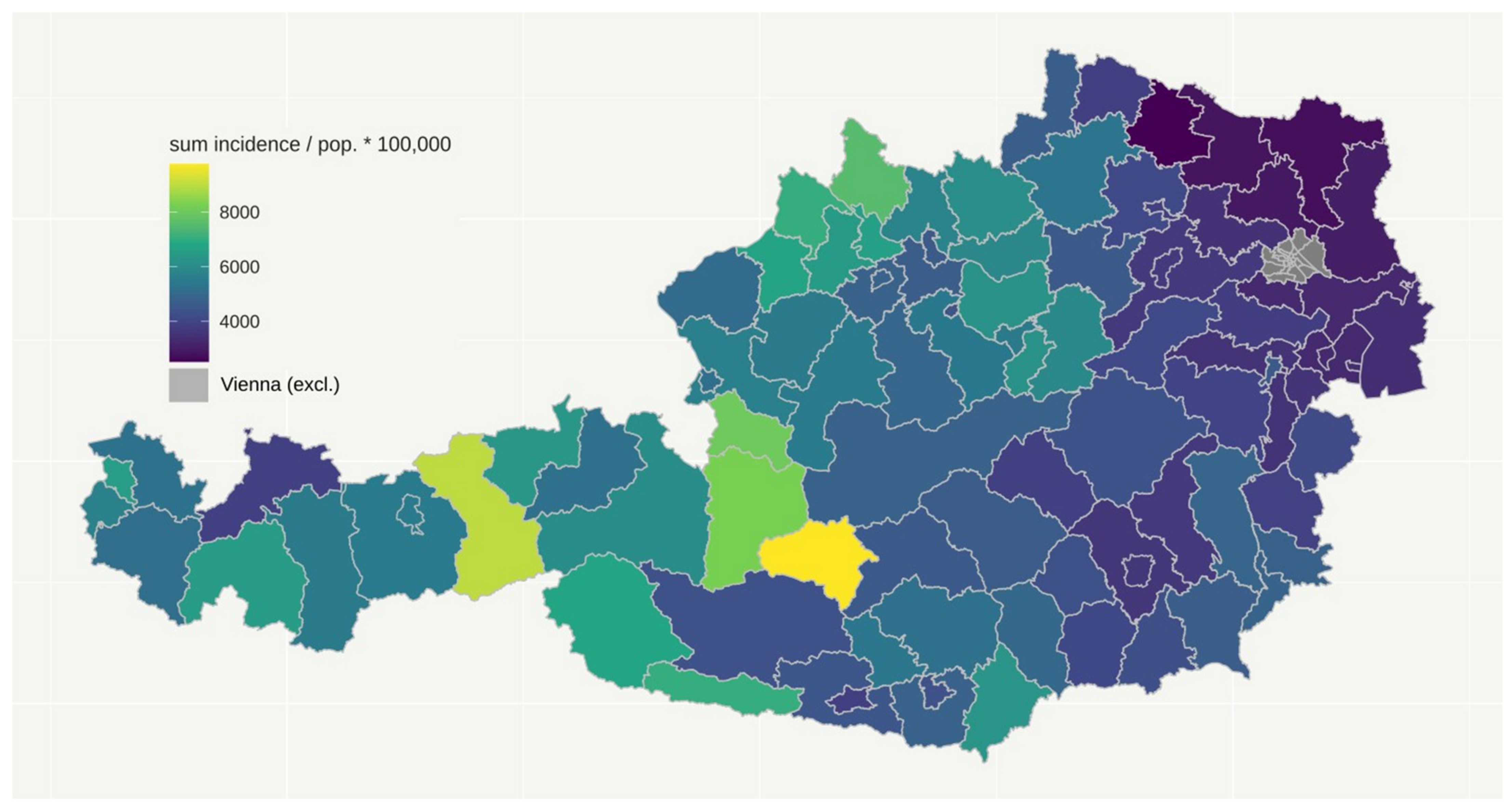
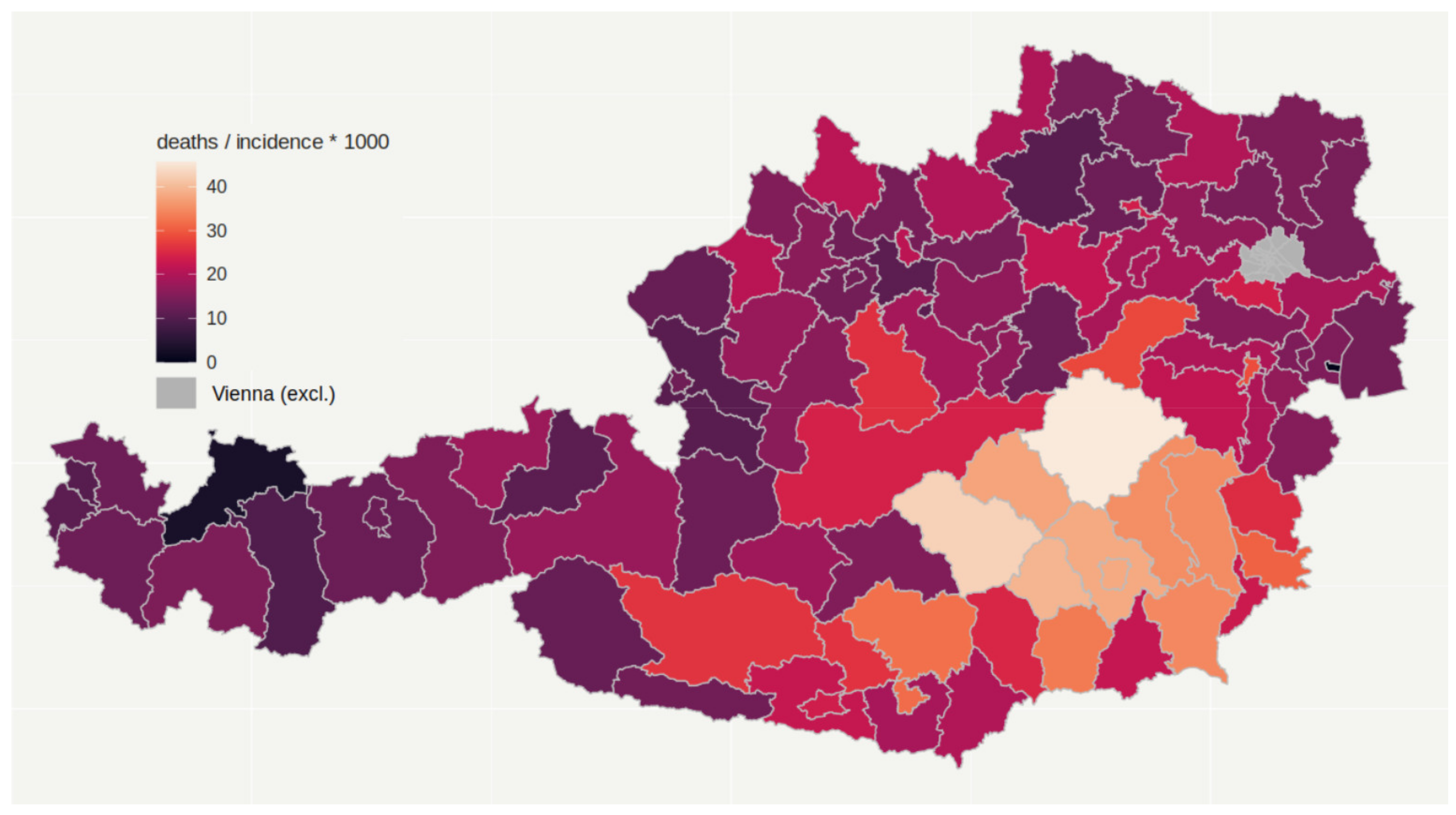
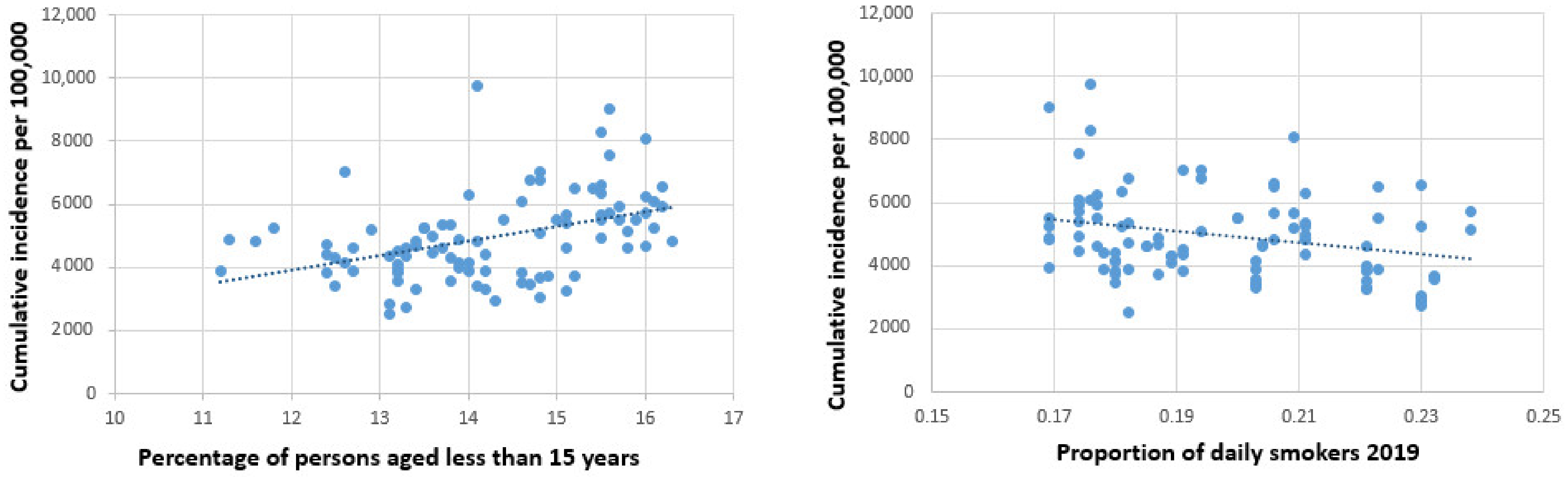
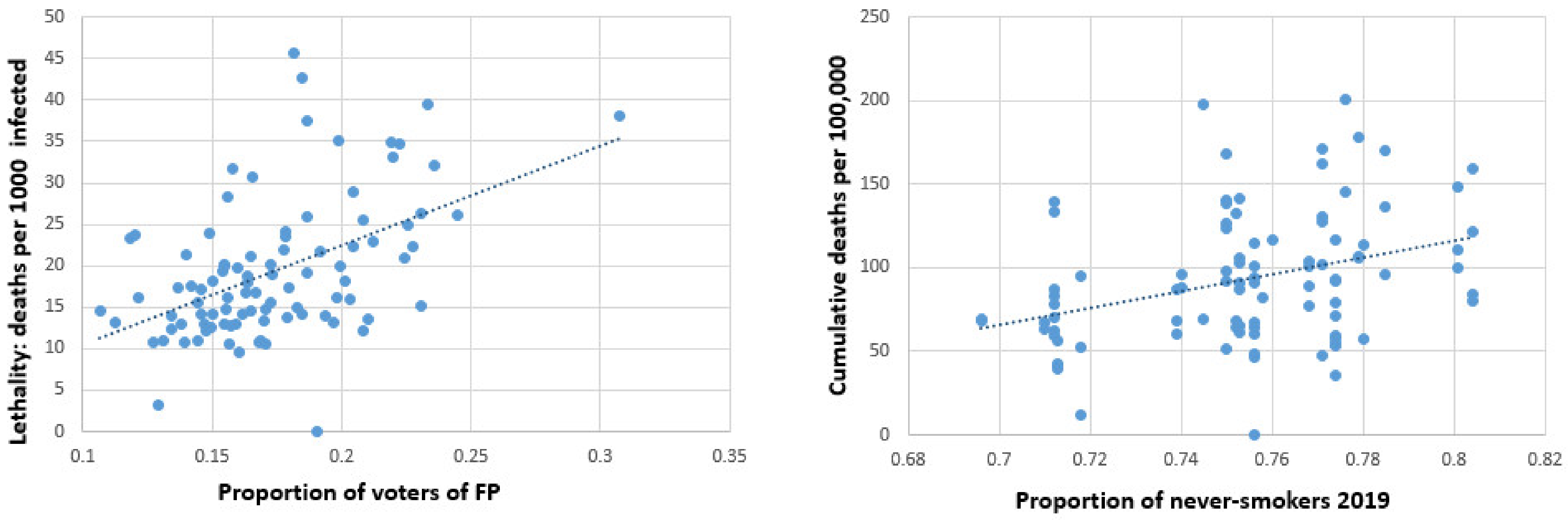
| Federal Country | Number of Districts | Number of Inhabitants | Peak per 100,000 | Total Cases per 100,000 | Deaths per 100,000 |
|---|---|---|---|---|---|
| Burgenland | 9 | 294,436 | 105.63 | 3846.00 | 74.04 |
| Carinthia | 10 | 561,293 | 150.72 | 4811.93 | 120.08 |
| Lower Austria | 24 | 1,684,287 | 67.09 | 3915.13 | 70.53 |
| Upper Austria | 18 | 1,490,279 | 151.31 | 5514.47 | 92.20 |
| Salzburg | 6 | 558,410 | 144.34 | 6399.24 | 84.35 |
| Styria | 13 | 1,246,395 | 87.77 | 4127.74 | 133.67 |
| Tyrol | 9 | 757,634 | 133.31 | 6019.40 | 79.33 |
| Vorarlberg | 4 | 397,139 | 202.20 | 5662.50 | 66.73 |
| Vienna | 23 | 1,911,191 | 101.61 | 4391.40 | 83.30 |
| Austria (Total) | 116 | 8,901,064 | 103.46 | 4782.11 | 90.43 |
| Variable | Total Year | 1st Wave | 2nd Wave | Endemic Phase | |
|---|---|---|---|---|---|
| Adjusted R2 | 0.435 | 0.464 | 0.199 | 0.196 | |
| Sea level per 100 m | 0.06553 ** | −0.07372 | 0.06333 ** | ||
| Percent below 15 years | 0.10034 ** | 0.10133 ** | |||
| Percent aged 65 and above | −0.12397 ** | −0.16442 ** | |||
| Proportion vote for Soc. Dem. | −2.85619 * | ||||
| Proportion valid vote | −0.90317 * | −1.03251 * | 6.68207 ** | ||
| Tourism nights per population | 0.00913 ** | ||||
| Proportion daily smoking | −4.10789 ** | −8.47946 ** | −4.51481 ** | ||
| Av. Persons per household | −2.15088 ** | ||||
| Adjusted R2 | 0.696 | 0.529 | 0.662 | 0.625 | |
| Sea level per 100 m | 0.00026 | ||||
| Percent aged 65 and above | −0.14900 ** | −0.14689 ** | |||
| Tourism nights per population | 0.00807 ** | ||||
| Proportion daily smoking | −3.15999 ** | −8.9770 ** | −4.22585 ** | ||
| Percent secondary education | 0.039093 | ||||
| Av. Persons per household | 0.36510 ** | 0.35511 ** | −2.58785 ** |
| Variable | Total Year | 1st Wave | 2nd Wave | Endemic Phase | |
|---|---|---|---|---|---|
| Adjusted R2 | 0.267 | 0.106 | 0.308 | 0.040 | |
| Sea level per 100 m | 0.07152 ** | 0.0859 ** | |||
| Percent aged 65 and above | −0.09137 * | ||||
| Tertiary education | 0.01934 * | 0.01999 * | |||
| Proportion vote for Soc. Dem. | 1.43921 * | ||||
| Proportion vote for FP | 5.83868 ** | 5.74163 ** | |||
| Tourism nights per population | 0.00683 * | ||||
| Proportion daily smoking | 7.09339 * | ||||
| Proportion never-smokers | 5.01240 ** | 4.71345 ** | |||
| Adjusted R2 | 0.440 | 0.249 | 0.443 | 0.095 | |
| Sea level per 100 m | 0.00062 | ||||
| Percent aged 65 and above | −0.12746 * | −0.09126 * | |||
| Proportion vote for FP | 2.47332 * | 3.52370 ** | |||
| Tourism nights per population | 0.00637 * | ||||
| Proportion working in agriculture | −1.95950 | ||||
| Proportion never-smokers | 5.13156 ** | 6.1099 ** |
| Variable | Total Year | 1st Wave | 2nd Wave | Endemic Phase | |
|---|---|---|---|---|---|
| Adjusted R2 | 0.420 | 0.050 | 0.443 | 0.114 | |
| Percent not born in Austria | −0.40551 * | −0.57471 ** | |||
| Percent aged 65 and above | 0.78620 | ||||
| Proportion vote for Conservatives | −32.15577 * | −26.15493 * | |||
| Proportion vote for FP | 78.35056 ** | 185.3812 * | 89.23756 ** | ||
| Proportion working in agriculture | 53.66657 * | 624.5471 ** | |||
| Percent working | −0.70916 * | ||||
| Av. persons per household | −35.2299 ** | ||||
| Adjusted R2 | 0.701 | 0.233 | 0.676 | 0.226 | |
| Percent not born in Austria | −0.34281 | −0.36662 | |||
| Percent aged 65 and above | 1.13242 ** | 1.38660 ** | |||
| Percent secondary education | −0.420333 | −0.45343 | |||
| Proportion vote for FP | 58.4355 ** | 71.90519 | 57.37606 ** | 315.0977 | |
| Proportion working in agriculture | −40.34525 * | −47.14581 * | 309.686 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moshammer, H.; Poteser, M.; Weitensfelder, L. COVID-19: Regional Differences in Austria. Int. J. Environ. Res. Public Health 2022, 19, 1644. https://doi.org/10.3390/ijerph19031644
Moshammer H, Poteser M, Weitensfelder L. COVID-19: Regional Differences in Austria. International Journal of Environmental Research and Public Health. 2022; 19(3):1644. https://doi.org/10.3390/ijerph19031644
Chicago/Turabian StyleMoshammer, Hanns, Michael Poteser, and Lisbeth Weitensfelder. 2022. "COVID-19: Regional Differences in Austria" International Journal of Environmental Research and Public Health 19, no. 3: 1644. https://doi.org/10.3390/ijerph19031644
APA StyleMoshammer, H., Poteser, M., & Weitensfelder, L. (2022). COVID-19: Regional Differences in Austria. International Journal of Environmental Research and Public Health, 19(3), 1644. https://doi.org/10.3390/ijerph19031644








