The Distribution of Dengue Virus Serotype in Quang Nam Province (Vietnam) during the Outbreak in 2018
Abstract
1. Introduction
2. Materials and Methods
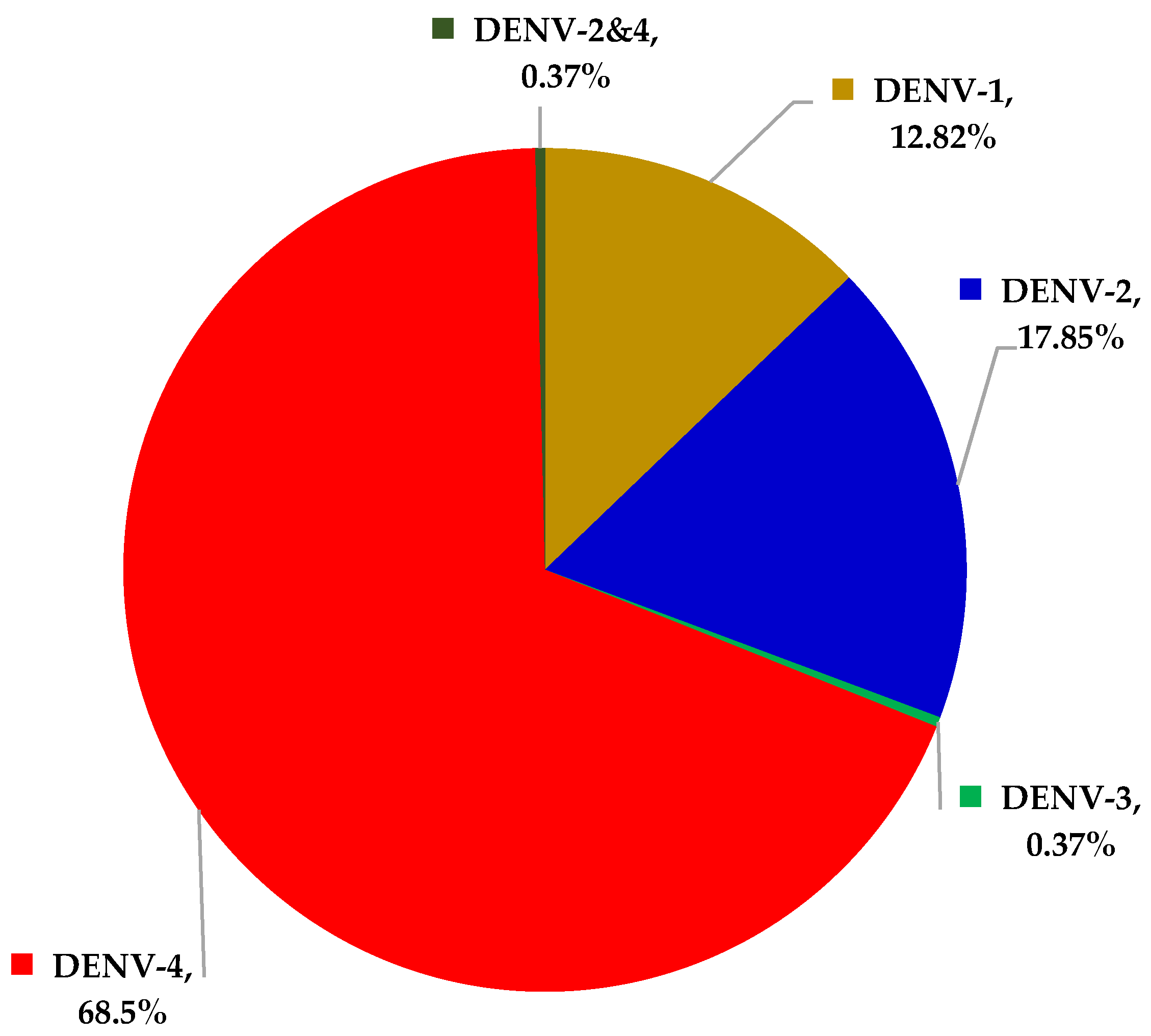
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- Katzelnick, L.C.; Coloma, J.; Harris, E. DHFD: Knowledge gaps, unmet needs, and research priorities. Lancet Infect. Dis. 2017, 17, e88–e100. [Google Scholar] [CrossRef]
- Hermann, L.L.; Gupta, S.B.; Manoff, S.B.; Kalayanarooj, S.; Gibbons, R.V.; Coller, B.A. Advances in the understanding, management, and prevention of DHFD. J. Clin. Virol. 2015, 64, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Nujum, Z.T.; Thomas, A.; Vijayakumar, K.; Nair, R.R.; Pillai, M.R.; Indu, P.S.; Sundar, S.; Gopakumar, S.; Mohan, D.; Sudheeshkumar, T.K. Comparative performance of the probable case definitions of DHFD by WHO (2009) and the WHO-SEAR expert group (2011). Pathog. Glob. Health 2014, 108, 103–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buonora, S.N.; Passos, S.R.L.; Daumas, R.P.; Machado, M.G.L.; Berardinelli, G.M.; de Oliveira, D.N.R.; de Oliveira, R.V.C. Pitfalls in acute febrile illness diagnosis: Interobserver agreement of signs and symptoms during a DHFD epidemics. J. Clin. Nurs. 2020, 29, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Aubry, M.; Mapotoeke, M.; Teissier, A.; Paoaafaite, T.; Dumas-Chastang, E.; Giard, M.; Cao-Lormeau, V.M. DHFD virus serotype 2 (DENV-2) epidemics, French Polynesia 2019. Euro Surveill. 2019, 24, 1900407. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, S.; de Silva, N.L.; Weeratunga, P.; Rodrigo, C.; Fernando, S.D. Prophylactic and therapeutic interventions for bleeding in DHFD: A systematic review. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 433–439. [Google Scholar] [CrossRef]
- López-Díaz, T.; Lugo, F.; Rodríguez, J.M.; Sabao, E.; Sierra, K.; Valdés, Y.; Vera, J. Compliance with management guidelines in patients with suspected DHFD. Bol. Asoc. Med. Puerto Rico 2016, 108, 53–56. [Google Scholar]
- Laodong. Available online: https:laodong.vn/y-te/dich-sot-xuat-huyet-tang-cao-tai-quang-nam-747103.ldo (accessed on 10 March 2019).
- Guzman, M.G.; Alvarez, M.; Halstead, S.B. Secondary infection as a risk factor for DHFD hemorrhagic fever/DHFD shock syndrome: An historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol. 2013, 158, 1445–1459. [Google Scholar] [CrossRef]
- Fried, J.R.; Gibbons, R.V.; Kalayanarooj, S.; Thomas, S.J.; Srikiatkhachorn, A.; Yoon, I.K.; Jarman, R.G.; Green, S.; Rothman, A.L.; Cummings, D.A. Serotype-Specific Differences in the Risk of DHFD Hemorrhagic Fever: An Analysis of Data Collected in Bangkok, Thailand from 1994 to 2006. PLoS Negl. Trop. Dis. 2010, 4, e617. [Google Scholar] [CrossRef]
- Alvarez, M.; Rodriguez-Roche, R.; Bernardo, L.; Vázquez, S.; Morier, L.; Gonzalez, D.; Castro, O.; Kouri, G.; Halstead, S.B.; Guzman, M.G. DHFD Hemorrhagic Fever Caused by Sequential DHFD 1–3 Virus Infections Over a Long Time Interval: Havana Epidemic, 2001–2002. Am. J. Trop. Med. Hyg. 2006, 75, 1113–1117. [Google Scholar] [CrossRef]
- Aguas, R.; Dorigatti, I.; Coudeville, L.; Luxemburger, C.; Ferguson, N.M. Cross-serotype interactions and disease outcome prediction of DHFD infections in Vietnam. Sci. Rep. 2019, 9, 9395. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B.; Lan, N.T.; Myint, T.T.; Shwe, T.N.; Nisalak, A.; Kalyanarooj, S.; Nimmannitya, S.; Soegijanto, S.; Vaughn, D.W.; Endy, T.P. DHFD hemorrhagic fever in infants: Research opportunities ignored. Emerg. Infect. Dis. 2002, 8, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Santiago, G.A.; Vergne, E.; Quiles, Y.; Cosme, J.; Vazquez, J.; Medina, J.F.; Medina, F.; Colón, C.; Margolis, H.; Muñoz-Jordán, J.L. Analytical and clinical performance of the CDC real time RT-PCR assay for detection and typing of DHFD virus. PLoS Negl. Trop. Dis. 2013, 7, e2311. [Google Scholar] [CrossRef]
- Santiago, G.A.; Vázquez, J.; Courtney, S.; Matías, K.Y.; Andersen, L.E.; Colón, C.; Butler, A.E.; Roulo, R.; Bowzard, J.; Villanueva, J.M.; et al. Performance of the Trioplexreal-time RT-PCR assay for detection of Zika, DHFD, and chikungunya virus. Nat. Commun. 2018, 9, 1391. [Google Scholar] [CrossRef]
- Pham, V.H. The solution for the low-income countries to establish the automatic extraction of the nucleic acid from the clinical samples. In Proceedings of the Asean Congress on Medical Biotechnology and Molecular Biosciences, Bangkok, Thailand, 8–9 October 2015. [Google Scholar]
- Pham, V.H.; Thanh, B.T.; Quoc, N.V. Production and Evaluation of the Kit Using Magnetic Silica Coated Nano-Ironbeads to Extract the Nucleic Acid from Different Samples; ISAAR, BEXCO: Busan, Korea, 2017. [Google Scholar]
- Gubler, D.J. Epidemic DHFD/DHFD hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends. Microbiol. 2002, 10, 100–103. [Google Scholar] [CrossRef]
- Stephan, C.; Allwinn, R.; Brodt, H.R.; Knupp, B.; Preiser, W.; Just-Nübling, G. Travel-acquired DHFD infection: Clinical spectrum and diagnostic aspects. Infections 2002, 30, 225–228. [Google Scholar] [CrossRef]
- Patumanond, J.; Tawichasri, C.; Nopparat, S. DHFD hemorrhagic fever, Uttaradit, Thailand. Emerg. Infect. Dis. 2003, 9, 1348–1350. [Google Scholar] [CrossRef]
- Simmons, M.; Burgess, T.; Lynch, J.; Putnak, R. Protection against DHFD virus by non-replicating and live attenuated vaccines used together in a prime boost vaccination strategy. Virology 2010, 396, 280–288. [Google Scholar] [CrossRef]
- Tsai, T.T.; Chuang, Y.J.; Lin, Y.S.; Wan, S.W.; Chen, C.L.; Lin, C.F. An emerging role for the anti-inflammatory cytokine interleukin-10 in DHFD virus infection. J. Biomed. Sci. 2013, 20, 40. [Google Scholar] [CrossRef]
- Cummings, D.A.; Irizarry, R.A.; Huang, N.E.; Endy, T.P.; Nisalak, A.; Ungchusak, K.; Burke, D.S. Travelling waves in the occurrence of DHFD haemorrhagic fever in Thailand. Nature 2004, 427, 344–347. [Google Scholar] [CrossRef]
- Adams, B.; Holmes, E.C.; Zhang, C.; Mammen, M.P., Jr.; Nimmannitya, S.; Kalayanarooj, S.; Boots, M. Cross-protective immunity.can account for the alternating epidemic pattern of DHFD virus.serotypes circulating in Bangkok. Proc. Natl. Acad. Sci. USA 2006, 103, 14234–14239. [Google Scholar] [CrossRef] [PubMed]
- Phuong, C.X.; Nhan, N.T.; Kneen, R.; Thuy, P.T.; van Thien, C.; Nga, N.T.; Thuy, T.T.; Solomon, T.; Stepniewska, K.; Wills, B.; et al. Clinical diagnosis and assessment of severity of confirmed DHFD infection in Vietnamese childrens: Is the WHO classification system helpful? Am. J. Trop. Med. Hyg. 2004, 70, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Quoc, C.H.; Henrik, S.; Isabel, R.B.; In-Kyu, Y.; Chau, N.V.; Hung, N.T.; Tuan, H.M.; Lan, P.T.; Willis, B.; Nisalak, A.; et al. Synchrony of DHFD Incidence in Ho Chi Minh City and Bangkok. PLoS Negl. Trop. Dis. 2016, 10, e0005188. [Google Scholar] [CrossRef]
- Lien, P.T.K.; Briant, L.; Tang, T.B.; Trang, B.M.; Gavotte, L.; Cornillot, E.; Duoc, V.T.; Duong, T.N.; Frutos, R.; Nga, P.T. Surveillance of DHFD and chikungunya infection in Dong Thap, Vietnam: A 13-month study. Asian Pac. J. Trop. Med. 2016, 9, 39–43. [Google Scholar] [CrossRef]
- Blacksell, S.D. Commercial DHFD Rapid Diagnostic Tests for Point-of-Care Application: Recent Evaluations and Future Needs? J. Biomed. Biotechnol. 2012, 2012, 151967. [Google Scholar] [CrossRef] [PubMed]
| One-Step RT-rPCR | Total | |||
|---|---|---|---|---|
| [+] | [−] | |||
| NS1 | [+] | 241 | 63 | 304 |
| [−] | 32 | 152 | 184 | |
| Total | 273 | 215 | 488 | |
| Day of Infection | DHF Case | NS1(+) Case | RT-rPCR(+) Case | Sensitivity of NS1 | Sensitivity of RT-rPCR |
|---|---|---|---|---|---|
| D9 | 1 | 1 | 0 | 100.00% (1/1) | 0.00% (0/1) |
| D8 | 3 | 3 | 1 | 100.00% (3/3) | 33.33% (1/3) |
| D7 | 11 | 10 | 7 | 90.91% (10/11) | 63.64% (7/11) |
| D6 | 28 | 24 | 16 | 85.71% (24/28) | 57.14% (16/28) |
| D5 | 60 | 55 | 47 | 91.67% (55/60) | 78.33% (47/60) |
| D4 | 85 | 76 | 70 | 89.41% (76/85) | 82.35% (70/85) |
| D3 | 75 | 68 | 71 | 90.67% (68/75) | 94.67% (71/75) |
| D2 | 38 | 34 | 33 | 89.47% (34/38) | 86.84% (33/38) |
| D1 | 4 | 3 | 4 | 75.00% (3/4) | 100.00% (4/4) |
| D0 | 0 | 0 | 0 | ||
| Undetermined | 31 | 30 | 24 | 96.77% (30/31) | 77.42% (24/31) |
| Total | 336 | 304 | 273 | 90.48% (304/336) | 81.25% (273/336) |
| Children | Adults | Total | |
|---|---|---|---|
| Fever | 93.15 | 92.09 | 92.33 |
| Orbital pain | 79.71 | 90.28 | 87.97 |
| Muscular/joint pain | 68.12 | 86.59 | 82.54 |
| Positive tourniquet test | 0 | 0.82 | 0.63 |
| Cutaneous hemorrhages | 25.00 | 21.63 | 22.36 |
| Gum bleeding | 13.24 | 15.10 | 14.70 |
| Mucous membrane hemorrhages | 2.94 | 2.04 | 2.24 |
| Lethargy | 35.29 | 47.76 | 45.05 |
| Abdominal pain | 2.70 | 0.78 | 1.21 |
| Hepatomegaly | 0 | 0 | 0 |
| Hct increase > 42% | 24.66 | 28.19 | 27.41 |
| Platelet decrease < 20 K | 2.70 | 7.72 | 6.61 |
| Platelet decrease < 100 K | 25.68 | 35.91 | 33.63 |
| DHFD with warning signs | 51.35 | 57.63 | 56.25 |
| Liver enzyme increase | 33.78 | 41.31 | 39.64 |
| Abdominal fluid accumulation | 0 | 2.32 | 1.80 |
| DENV-1 | DENV-2 | DENV-4 | |
|---|---|---|---|
| Sustained fever | 63.64 | 72.34 | 69.73 |
| Fever | 83.33 | 92.31 | 78.57 |
| Orbital pain | 78.13 | 88.89 | 91.67 |
| Muscular/joint pain | 78.13 | 88.89 | 81.01 |
| Positive tourniquet test | 0 | 0 | 1.12 |
| Cutaneous hemorrhages | 31.25 | 11.11 | 17.42 |
| Gum bleeding | 15.63 | 11.11 | 14.04 |
| Subconjunctival hemorrhage | 0 | 0 | 0.56 |
| Hematuria | 3.13 | 0 | 0 |
| GI bleeding | 0 | 2.22 | 0 |
| Lethargy | 46.88 | 37.78 | 47.75 |
| Abdominal pain | 0 | 2.08 | 1.62 |
| Hepatomegaly | 0 | 0 | 0 |
| Hct ≥ 42% | 2.94 | 12.5 | 9.14 |
| PLT < 10,000 | 0 | 4.17 | 0 |
| PLT 10,000–20,000 | 0.03 | 0.06 | 0.06 |
| PLT 20,000–100,000 | 44.12 | 39.58 | 29.41 |
| DHFD with warning signs | 48.57 | 59.18 | 59.36 |
| Liver enzymes > 40–< 400 | 50 | 37.5 | 36.02 |
| Abdominal fluid (ultrasounds) | 0 | 8.33 | 1.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, D.Q.; Nguyen, L.D.N.; Pham, S.T.; Nguyen, T.; Pham, P.T.T.; Nguyen, S.T.H.; Pham, D.T.; Pham, H.T.; Tran, D.K.; Le, S.H.; et al. The Distribution of Dengue Virus Serotype in Quang Nam Province (Vietnam) during the Outbreak in 2018. Int. J. Environ. Res. Public Health 2022, 19, 1285. https://doi.org/10.3390/ijerph19031285
Phan DQ, Nguyen LDN, Pham ST, Nguyen T, Pham PTT, Nguyen STH, Pham DT, Pham HT, Tran DK, Le SH, et al. The Distribution of Dengue Virus Serotype in Quang Nam Province (Vietnam) during the Outbreak in 2018. International Journal of Environmental Research and Public Health. 2022; 19(3):1285. https://doi.org/10.3390/ijerph19031285
Chicago/Turabian StylePhan, Duong Q., Linh D. N. Nguyen, Son T. Pham, Tai Nguyen, Phuong T. T. Pham, Suong T. H. Nguyen, Dien T. Pham, Huong T. Pham, Duy K. Tran, Sa H. Le, and et al. 2022. "The Distribution of Dengue Virus Serotype in Quang Nam Province (Vietnam) during the Outbreak in 2018" International Journal of Environmental Research and Public Health 19, no. 3: 1285. https://doi.org/10.3390/ijerph19031285
APA StylePhan, D. Q., Nguyen, L. D. N., Pham, S. T., Nguyen, T., Pham, P. T. T., Nguyen, S. T. H., Pham, D. T., Pham, H. T., Tran, D. K., Le, S. H., Pham, T. T., Nguyen, K. C. D., Dipalma, G., Inchingolo, A. D., Piscitelli, P., Miani, A., Salvatore, S., Cantore, S., Aityan, S. K., ... Pham, V. H. (2022). The Distribution of Dengue Virus Serotype in Quang Nam Province (Vietnam) during the Outbreak in 2018. International Journal of Environmental Research and Public Health, 19(3), 1285. https://doi.org/10.3390/ijerph19031285













