Effect of a Nutritional Support System to Increase Survival and Reduce Mortality in Patients with COVID-19 in Stage III and Comorbidities: A Blinded Randomized Controlled Clinical Trial
Abstract
:1. Introduction
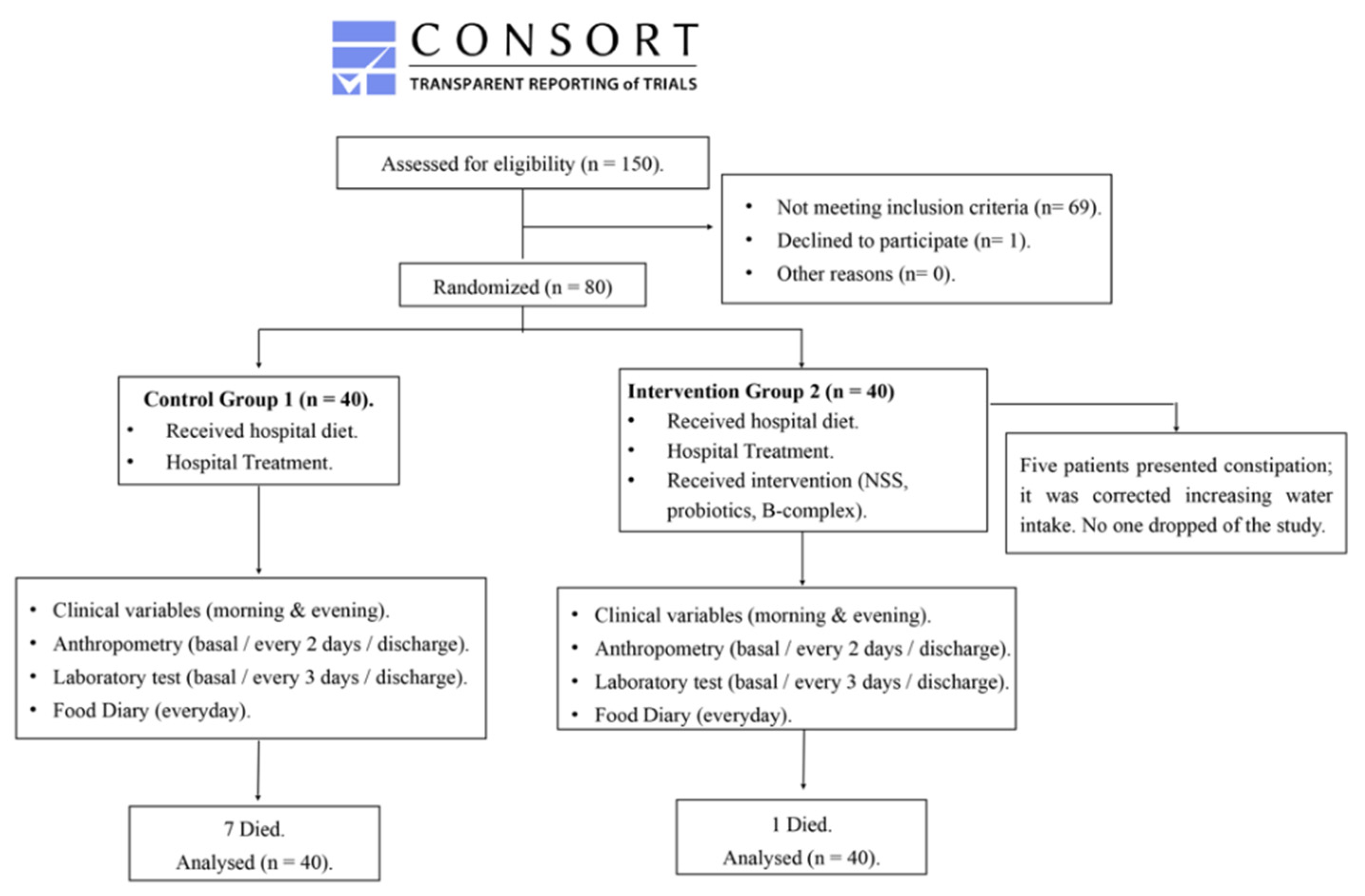
2. Materials and Methods
2.1. Subject Selection
2.2. Trial Design and Supervision
- (1)
- B-complex: 10 mg of cyanocobalamin, 100 mg of thiamin, and 100 mg of pyridoxine administered intramuscularly every 24 h for the first 5 days.
- (2)
- NSS powder: one envelope orally after morning meals and another after evening meals, diluted in 400 mL of water each, during the whole intervention for a maximum of 21 days. Each envelope contained: Spirulina Maxima 2.5 g, folic acid 5 mg, glutamine 5 g, vegetable protein 10 g, constituted by two foods without processing or fragmenting in amino acids, brewer’s yeast, and amaranth, ascorbic acid 1 g, zinc 20 mg, selenium 100 mcg, cholecalciferol 2000 IU, resveratrol 200 mg, Omega-3 fatty acids 1 g, L-Arginine 750 mg, inulin 20 g, and magnesium 400 mg.
- (3)
- Probiotics: Saccharomyces boulardii (SB) 500 mg daily for 6 days orally.
2.3. Clinical and Laboratory Monitoring
2.4. Statistical Analysis
2.5. Sample Size
2.6. Ethical Aspects
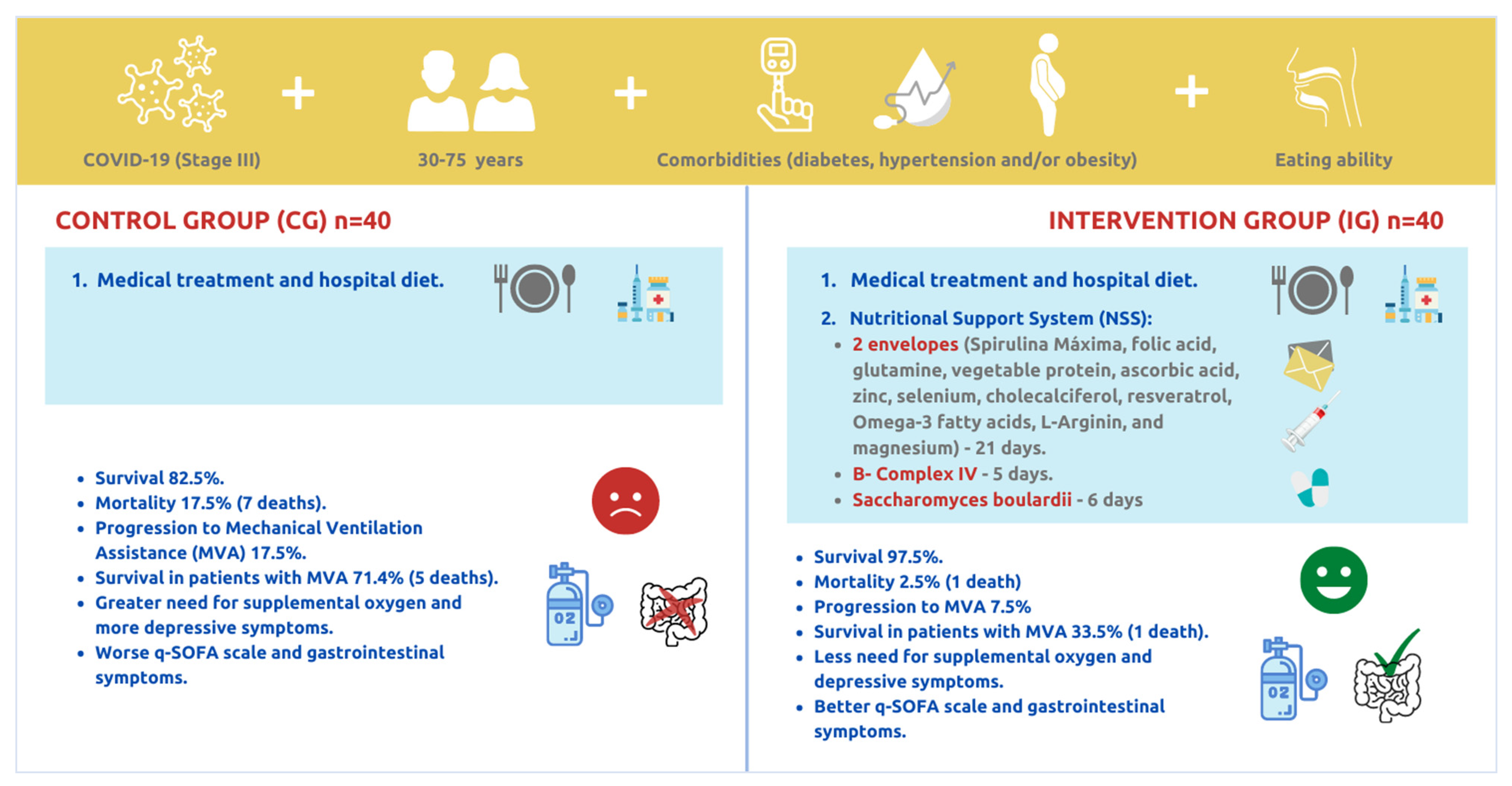
3. Results
3.1. Baseline Results
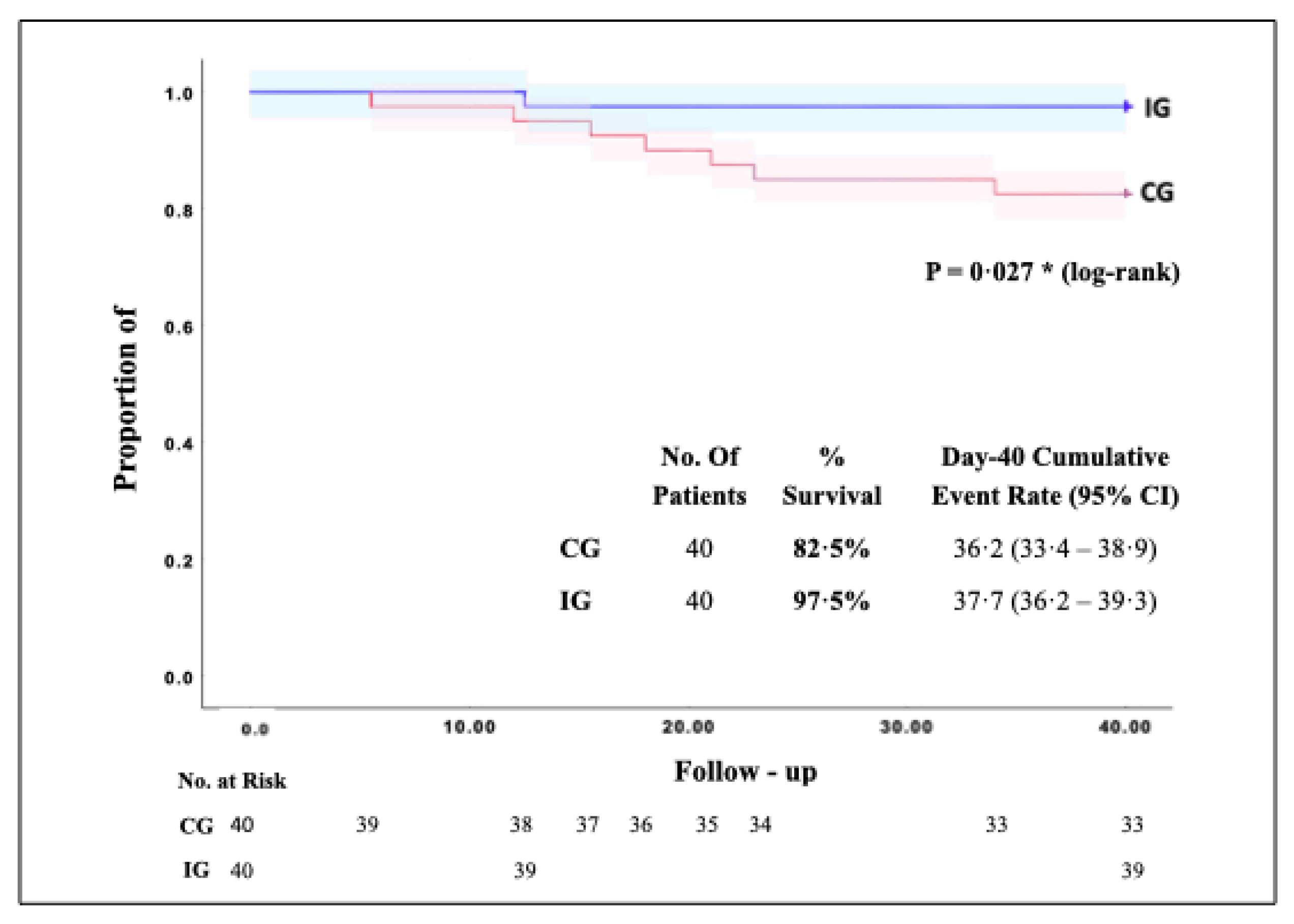
3.2. Survival and Mortality of the Studied Patients
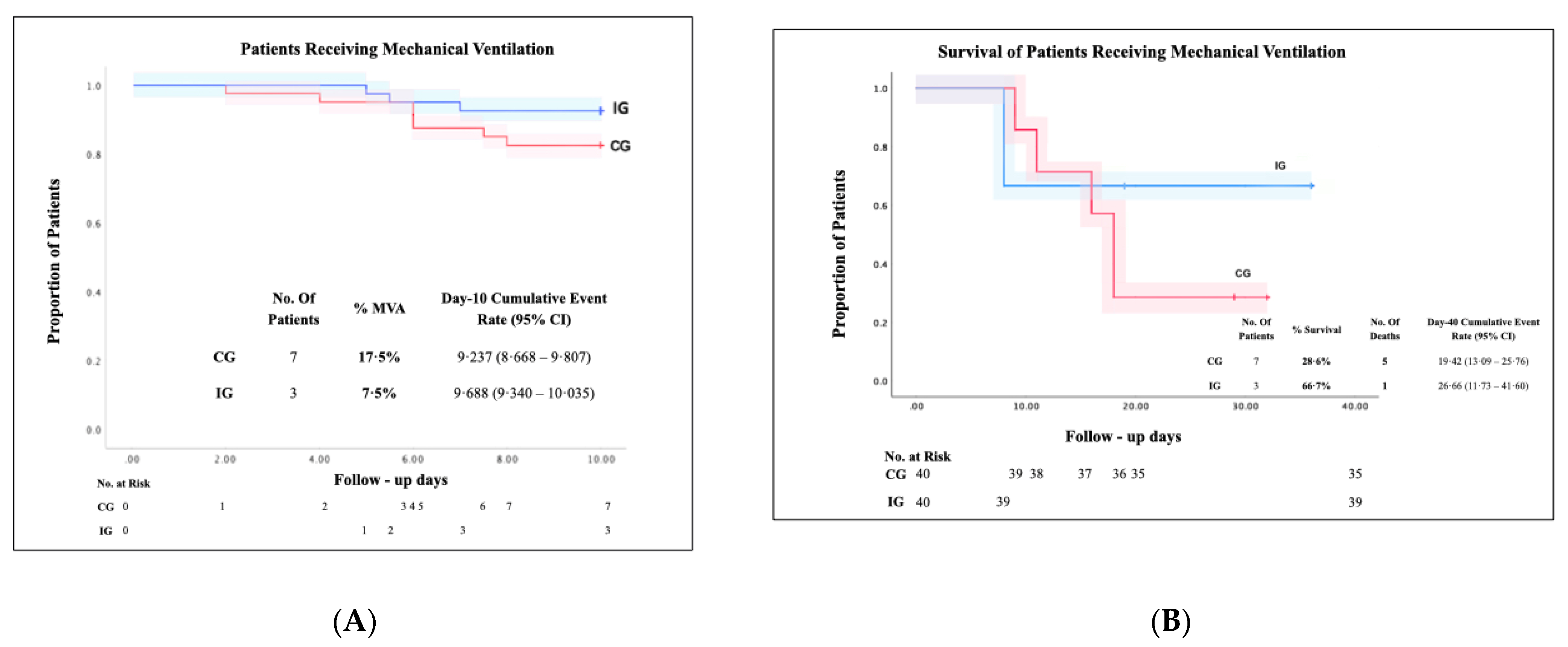
3.3. Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, S.; Hu, N.; Lou, J.; Chen, K.; Kang, X.; Xiang, Z.; Chen, H.; Wang, D.; Liu, N.; Liu, D.; et al. Characteristics of COVID-19 infection in Beijing. J. Infect. 2020, 80, 401–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leguía Valentín, E.D.; Niño Montero, J.S.; Quino Florentini, M.G. Coronavirus causante del síndrome respiratorio de Oriente Medio (MERS-CoV). Rev. Med. Carrion. 2019, 1, 1–15. Available online: http://cuerpomedico.hdosdemayo.gob.pe/index.php/revistamedicacarrionica/article/view/300/208 (accessed on 2 September 2021).
- Covid19.who.int. WHO Coronavirus (COVID-19) Dashboard. 2021. Available online: https://covid19.who.int/ (accessed on 2 September 2021).
- Salud, S. Coronavirus COVID19 Comunicado Técnico Diario. 2021. Available online: https://www.gob.mx/salud/documentos/coronavirus-covid-19-comunicado-tecnico-diario-238449 (accessed on 2 September 2021).
- COVID-19 Tablero México. COVID-19 Tablero México. 2021. Available online: https://datos.covid-19.conacyt.mx/ (accessed on 2 September 2021).
- Salud.edomex.gob.mx. Estadísticas COVID-19|Centro Estatal de Vigilancia Epidemiológica y Control de Enfermedades. 2021. Available online: https://salud.edomex.gob.mx/cevece/estadisticas_covid19 (accessed on 2 September 2021).
- Talanquer, M. La Letalidad Hospitalaria por COVID-19 en México: Desigualdades Institucionales. 2021. Available online: https://datos.nexos.com.mx/la-letalidad-hospitalaria-por-covid-19-en-mexico-desigualdades-institucionales/ (accessed on 2 September 2021).
- Wang, Y.; Liu, Y.; Liu, L.; Wang, X.; Luo, N.; Li, L. Clinical Outcomes in 55 Patients with Severe Acute Respiratory Syndrome Coronavirus 2 Who Were Asymptomatic at Hospital Admission in Shenzhen, China. J. Infect. Dis. 2020, 221, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Bhimraj, A.; Morgan, R.L.; Shumaker, A.H.; Lavergne, V.; Baden, L.; Cheng, V.C.-C.; Edwards, K.M.; Gandhi, R.; Muller, W.J.; O’Horo, J.C.; et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with (COVID-19). IDSA 2021, 1–44. [Google Scholar] [CrossRef]
- Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Krznaric, Z.; Nitzan, D.; Pirlich, M.; Singer, P.; endorsed by the ESPEN Council. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin. Nutr. 2020, 39, 1631–1638. [Google Scholar] [CrossRef]
- Louca, P.; Murray, B.; Klaser, K.; Graham, M.S.; Mazidi, M.; Leeming, E.R.; Thompson, E.; Bowyer, R.; Drew, D.A.; Nguyen, L.H.; et al. Modest effects of dietary supplements during the COVID-19 pandemic: Insights from 445 850 users of the COVID-19 Symptom Study app. BMJ Nutr. Prev. Health 2021, 4, 149–157. [Google Scholar] [CrossRef]
- Castro, M.; Rodríguez, F. Levaduras: Probióticos y prebióticos para mejorar la producción animal. Ciencia y Tecnología Agropecuaria 2005, 6, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Anand, S.; Mande, S.S. Diet, Microbiota and Gut-Lung Connection. Front. Microbiol. 2018, 9, 2147. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Pujadas, E.; Chaudhry, F.; McBride, R.; Richter, F.; Zhao, S.; Wajnberg, A.; Nadkarni, G.; Glicksberg, B.S.; Houldsworth, J.; Cordon-Cardo, C. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir. Med. 2020, 8, e70. [Google Scholar] [CrossRef]
- Al Sulaiman, K.; Aljuhani, O.; Al Dossari, M.; Alshahrani, A.; Alharbi, A.; Algarni, R.; Al Jeraisy, M.; Al Harbi, S.; Al Katheri, A.; Al Eidan, F.; et al. Evaluation of thiamine as adjunctive therapy in COVID-19 critically ill patients: A two-center propensity score matched study. Crit. Care 2021, 25, 1–8. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Mikkelsen, K.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Stojanovska, L.; Apostolopoulos, V. Be well: A potential role for vitamin B in COVID-19. Maturitas 2021, 144, 108–111. [Google Scholar] [CrossRef]
- Ratha, S.K.; Renuka, N.; Rawat, I.; Bux, F. Prospective options of algae-derived nutraceuticals as supplements to combat COVID-19 and human coronavirus diseases. Nutrition 2021, 83, 111089. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Elias, J.; Espinosa-Tanguma, R. The Folate Concentration and/or Folic Acid Metabolites in Plasma as Factor for COVID-19 Infection. Front. Pharmacol. 2020, 11, 1062. [Google Scholar] [CrossRef]
- Serseg, T.; Benarous, K.; Yousfi, M. Hispidin and Lepidine E: Two Natural Compounds and Folic Acid as Potential Inhibitors of 2019-novel Coronavirus Main Protease (2019-nCoVMpro), Molecular Docking and SAR Study. Curr. Comput. Aided Drug Des. 2021, 17, 469–479. [Google Scholar] [CrossRef]
- Cengiz, M.; Uysal, B.B.; Ikitimur, H.; Ozcan, E.; Islamoğlu, M.S.; Aktepe, E.; Yavuzer, H.; Yavuzer, S. Effect of oral l-Glutamine supplementation on Covid-19 treatment. Clin. Nutr. Exp. 2020, 33, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Singh, M.; Kirtipal, N.; Kang, S.G. SARS-CoV-2 and Glutamine: SARS-CoV-2 Triggered Pathogenesis via Metabolic Reprograming of Glutamine in Host Cells. Front. Mol. Biosci. 2021, 7, 627842. [Google Scholar] [CrossRef]
- Vanhove, K.; Derveaux, E.; Graulus, G.-J.; Mesotten, L.; Thomeer, M.; Noben, J.-P.; Guedens, W.; Adriaensens, P. Glutamine Addiction and Therapeutic Strategies in Lung Cancer. Int. J. Mol. Sci. 2019, 20, 252. [Google Scholar] [CrossRef] [Green Version]
- Dy, A.B.C.; Tanyaratsrisakul, S.; Voelker, D.R.; Ledford, J.G. The Emerging Roles of Surfactant Protein-A in Asthma. J. Clin. Cell. Immunol. 2018, 9, 553. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; O’Leary, E.M.; Witt, L.J.; Tian, Y.; Gökalp, G.A.; Meliton, A.Y.; Dulin, N.O.; Mutlu, G.M. Glutamine Metabolism Is Required for Collagen Protein Synthesis in Lung Fibroblasts. Am. J. Respir. Cell Mol. Biol. 2019, 61, 597–606. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Del Campo, S.S.F.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef] [PubMed]
- Maneira, C.; Bermejo, P.M.; Pereira, G.A.G.; de Mello, F.D.S.B. Exploring G protein-coupled receptors and yeast surface display strategies for viral detection in baker’s yeast: SARS-CoV-2 as a case study. FEMS Yeast Res. 2021, 21, foab004. [Google Scholar] [CrossRef]
- De Sousa Cavalcante, L.; Costa-Silva, T.A.; Souza, T.A.; Ienne, S.; Monteiro, G. Chemogenomic study of gemcitabine using Saccharomyces cerevisiae as model cell—Molecular insights about chemoresistance. Braz. J. Microbiol. 2020, 51, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.A., III; Truwit, J.D.; Hite, R.D.; Morris, P.E.; Dewilde, C.; Priday, A.; Fisher, B.; Thacker, L.R., 2nd; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients with Sepsis and Severe Acute Respiratory Failure. JAMA 2019, 322, 1261. [Google Scholar] [CrossRef]
- Mejia, L.; Alvarado, A. Vitamina C como antioxidante en el manejo del SARS-CoV-2. Rev. Colomb. Endocrinol. Diabetes Metab. 2020, 7, 99–101. [Google Scholar] [CrossRef]
- Vollbracht, C.; Kraft, K. Feasibility of Vitamin C in the Treatment of Post Viral Fatigue with Focus on Long COVID, Based on a Systematic Review of IV Vitamin C on Fatigue. Nutrients 2021, 13, 1154. [Google Scholar] [CrossRef]
- Pormohammad, A.; Monych, N.K.; Turner, R.J. Zinc and SARS-CoV-2: A molecular modeling study of Zn interactions with RNA-dependent RNA-polymerase and 3C-like proteinase enzymes. Int. J. Mol. Med. 2020, 47, 326–334. [Google Scholar] [CrossRef]
- Doboszewska, U.; Wlaź, P.; Nowak, G.; Młyniec, K. Targeting zinc metalloenzymes in COVID-19. Br. J. Pharmacol. 2020, 177, 4887–4898. [Google Scholar] [CrossRef] [PubMed]
- Bermano, G.; Méplan, C.; Mercer, D.K.; Hesketh, J.E. Selenium and viral infection: Are there lessons for COVID-19? Br. J. Nutr. 2021, 125, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Rakib, A.; Nain, Z.; Sami, S.A.; Mahmud, S.; Islam, A.; Ahmed, S.; Siddiqui, A.B.F.; Babu, S.M.O.F.; Hossain, P.; Shahriar, A.; et al. A molecular modelling approach for identifying antiviral selenium-containing heterocyclic compounds that inhibit the main protease of SARS-CoV-2: An in-silico investigation. Brief. Bioinform. 2021, 22, 1476–1498. [Google Scholar] [CrossRef]
- Staples, S.; Wall, S.B.; Li, R.; Tipple, T.E. Selenium-independent antioxidant and anti-inflammatory effects of thioredoxin reductase inhibition in alveolar macrophages. Life Sci. 2020, 259, 118285. [Google Scholar] [CrossRef]
- Malik, M.; Suboc, T.M.; Tyagi, S.; Salzman, N.; Wang, J.; Ying, R.; Tanner, M.J.; Kakarla, M.; Baker, J.E.; Widlansky, M.E. Lactobacillus plantarum 299v Supplementation Improves Vascular Endothelial Function and Reduces Inflammatory Biomarkers in Men with Stable Coronary Artery Disease. Circ. Res. 2018, 123, 1091–1102. [Google Scholar] [CrossRef]
- Ling, S.F.; Broad, E.; Murphy, R.; Pappachan, J.M.; Pardesi-Newton, S.; Kong, M.F.; Jude, E.B. High-Dose Cholecalciferol Booster Therapy is Associated with a Reduced Risk of Mortality in Patients with COVID-19: A Cross-Sectional Multi-Centre Observational Study. Nutrients 2020, 12, 3799. [Google Scholar] [CrossRef] [PubMed]
- Abdrabbo, M.; Birch, C.M.; Brandt, M.; Cicigoi, K.A.; Coffey, S.J.; Dolan, C.C.; Dvorak, H.; Gehrke, A.C.; Gerzema, A.E.L.; Hansen, A.; et al. Vitamin D and COVID-19: A review on the role of vitamin D in preventing and reducing the severity of COVID-19 infection. Protein Sci. 2021, 30, 2206–2220. [Google Scholar] [CrossRef]
- Ramdani, L.H.; Bachari, K. Potential therapeutic effects of Resveratrol against SARS-CoV-2. Acta Virol. 2020, 64, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Weill, P.; Plissonneau, C.; Legrand, P.; Rioux, V.; Thibault, R. May omega-3 fatty acid dietary supplementation help reduce severe complications in Covid-19 patients? Biochimie 2020, 179, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Reizine, F.; Lesouhaitier, M.; Gregoire, M.; Pinceaux, K.; Gacouin, A.; Maamar, A.; Painvin, B.; Camus, C.; Le Tulzo, Y.; Tattevin, P.; et al. SARS-CoV-2-Induced ARDS Associates with MDSC Expansion, Lymphocyte Dysfunction, and Arginine Shortage. J. Clin. Immunol. 2021, 41, 515–525. [Google Scholar] [CrossRef]
- Van Kempen, T.A.T.G.; Deixler, E. SARS-CoV-2: Influence of phosphate and magnesium, moderated by vitamin D, on energy (ATP) metabolism and on severity of COVID-19. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E2–E6. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Siedlecka, K.; Ruszkowski, J.; Fic, M.; Folwarski, M.; Makarewicz, W. Saccharomyces boulardii CNCM I-745: A Non-bacterial Microorganism Used as Probiotic Agent in Supporting Treatment of Selected Diseases. Curr. Microbiol. 2020, 77, 1987–1996. [Google Scholar] [CrossRef]
- Salama, C.; Han, J.; Yau, L.; Reiss, W.G.; Kramer, B.; Neidhart, J.D.; Criner, G.J.; Kaplan-Lewis, E.; Baden, R.; Pandit, L.; et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N. Engl. J. Med. 2021, 384, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—preliminary report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Ge, Y.; Shi, Y.; Lv, P.; Zhang, J.; Fu, G.; Zhou, Y.; Jiang, K.; Lin, N.; et al. Evaluation of Nutrition Risk and Its Association With Mortality Risk in Severely and Critically Ill COVID-19 Patients. JPEN J. Parenter. Enter. Nutr. 2021, 45, 32–42. [Google Scholar] [CrossRef]
- Adebayo, A.; Varzideh, F.; Wilson, S.; Gambardella, J.; Eacobacci, M.; Jankauskas, S.S.; Donkor, K.; Kansakar, U.; Trimarco, V.; Mone, P.; et al. l-Arginine y COVID-19: An Update. Nutrients 2021, 13, 3951. [Google Scholar] [CrossRef]
- Kory, P.; Meduri, G.U.; Iglesias, J.; Varon, J.; Marik, P.E. Clinical and Scientific Rationale for the “MATH+” Hospital Treatment Protocol for COVID-19. J. Intensiv. Care Med. 2021, 36, 135–156. [Google Scholar] [CrossRef]
- Anker, M.S.; Landmesser, U.; von Haehling, S.; Butler, J.; Coats, A.J.; Anker, S.D. Weight loss, malnutrition, and cachexia in COVID-19: Facts and numbers. J. Cachexia Sarcopenia Muscle 2021, 12, 9–13. [Google Scholar] [CrossRef]
- Djokic, G.; Vojvodic, P.; Korcok, D.; Agic, A.; Rankovic, A.; Djordjevic, V.; Vojvodic, A.; Vlaskovic-Jovicevic, T.; Peric-Hajzler, Z.; Vojvodic, J.; et al. The Effects of Magnesium—Melatonin—Vit B Complex Supplementation in Treatment of Insomnia. Open Access Maced. J. Med. Sci. 2019, 7, 3101–3105. [Google Scholar] [CrossRef] [Green Version]
- Sidawi, T.; Garau, J.; COVID-19 Mortality. Trends in the evolution of the pandemic. J. Health Sci. 2021, 36, 42–49, p-ISSN: 1579-5853; e-ISSN: 2255-0569. [Google Scholar] [CrossRef]
- Zhu, Z.; Cai, T.; Fan, L.; Lou, K.; Hua, X.; Huang, Z.; Gao, G. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. J. Infect. Dis. 2020, 95, 332–339. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955. [Google Scholar] [CrossRef]
- Hao, Q.; Dong, B.R.; Wu, T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2015, CD006895. [Google Scholar] [CrossRef] [PubMed]
- Infusino, F.; Marazzato, M.; Mancone, M.; Fedele, F.; Mastroianni, C.M.; Severino, P.; Ceccarelli, G.; Santinelli, L.; Cavarretta, E.; Marullo, A.G.M.; et al. Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review. Nutrients 2020, 12, 1718. [Google Scholar] [CrossRef]
- Munshi, R.; Hussein, M.H.; Toraih, E.A.; Elshazli, R.M.; Jardak, C.; Sultana, N.; Youssef, M.R.; Omar, M.; Attia, A.S.; Fawzy, M.S.; et al. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J. Med. Virol. 2021, 93, 733–740. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, R.; Lei, L.; Liu, H.; Wang, Y.; Wang, Y.; Qian, H.; Dai, T.; Zhang, T.; Lai, Y.; et al. Frontline Science: COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J. Leukoc. Biol. 2021, 109, 13–22. [Google Scholar] [CrossRef]
- Rogero, M.M.; Leão, M.D.C.; Santana, T.M.; Pimentel, M.V.D.M.; Carlini, G.C.; da Silveira, T.F.; Gonçalves, R.C.; Castro, I.A. Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radic. Biol. Med. 2020, 156, 190–199. [Google Scholar] [CrossRef]
- Van der Beek, C.M.; Canfora, E.E.; Kip, A.M.; Gorissen, S.H.M.; Olde Damink, S.W.M.; van Eijk, H.M.; Holst, J.J.; Blaak, E.E.; Dejong, C.H.C.; Lenaerts, K. The prebiotic inulin improves substrate metabolism and promotes short-chain fatty acid production in overweight to obese men. Metabolism 2018, 87, 25–35. [Google Scholar] [CrossRef]
- Birkeland, E.; Gharagozlian, S.; Birkeland, K.I.; Valeur, J.; Måge, I.; Rud, I.; Aas, A.M. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: A randomized controlled trial. Eur. J. Nutr. 2020, 59, 3325–3338. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.; Nair, D.T. Vitamin B12 may inhibit RNA-dependent-RNA polymerase activity of nsp12 from the SARS-CoV-2 virus. IUBMB Life 2020, 72, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Virgens, I.P.A.; Santana, N.M.; Lima, S.C.V.C.; Fayh, A.P.T. Can COVID-19 be a risk for cachexia for patients during intensive care? Narrative review and nutritional recommendations. Br. J. Nutr. 2020, 126, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Can Melatonin Be a Potential “Silver Bullet” in Treating COVID-19 Patients? Diseases 2020, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, C.E.; Martin, J.A.; Manriquez, F.V.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Focus on the essentials: Tryptophan metabolism and the microbiome-gut-brain axis. Curr. Opin. Pharmacol. 2019, 48, 137–145. [Google Scholar] [CrossRef] [PubMed]
| Specific Nutrients in the NSS | Dose | Function |
|---|---|---|
| Cyanocobalamin | 10 mg | B complex has demonstrated effects on immune response, proinflammatory cytokine levels, respiratory function, endothelial integrity, and hypercoagulability. B complex binding to the active site of the nsp 12 and 3C-like protein of SARS-CoV-2, blocking viral RNA transcription [15]. Thiamine inhibits carbonic anhydrase isoenzymes in vitro and could limit hypoxia and decrease hospitalization in early stages of COVID-19 [16]. Pyridoxine upregulates IL-10. Cobalamin modulates gut microbiota and low levels elevate methylmalonic acid and homocysteine, increasing inflammation, reactive oxygen species, and oxidative stress. Methylcobalamin forms adenosylcobalamin for mitochondrial energy production [17]. |
| Thiamin | 100 mg | |
| Pyridoxine | 100 mg | |
| Spirulina Maxima | 5 g | Spirulina Maxima (SM) is a natural biological source of ACE inhibitory peptides, which are used to enhance ACE2 activity for the treatment of tissue injury of several organs in severe cases of coronavirus infection. ACE inhibitory peptides modulate oxidative stress, cytokine release syndrome, and tissue injury in SARS-CoV2 and other coronavirus infections through regulation of ACE2 activity [18]. C-phycocyanin, an extract of SM blocks in vitro hemagglutination of viruses such as influenza [15]. |
| Folic acid | 5 mg | Folic acid (FA) inhibits the furin protease that SARS-CoV-2 needs to enter the host cell; FA components (histidine and lepidine) block SARS-CoV-2 transcription and replication by forming hydrogen bonds with the SARS-CoV-2 enzyme 3CL hydrolase, counteracting 3CLpro (Mpro) which counteracts the host innate immune response [19]. FA inhibits RNA expression of various viruses at the post-transcriptional level [20]. |
| Glutamine | 10 g | Glutamine (Gln) stimulates the immune system by inhibiting inflammatory responses [21]. In COVID-19 the depletion of Gln reserves represses the increase in CD8+ T lymphocytes, IL-2 and IFN-γ [22]. Gln stimulates collagen production in fibroblasts and stimulates lung myofibroblast differentiation in patients infected with EBV, HVC, HV8, and SARS-CoV-2 [23]. Gln increases ventilatory response and is employed to maximize alveolar exchange in lung cancer and asthma [24]. Administering 30 g/day of Gln improves oxygen saturation and reduces progression to MVA, hospital stay, and intensive care unit (ICU) admission [25,26]. |
| Vegetable protein (Brewer’s yeast-Saccharomyces cerevisiae SC and Amaranth) | 20 g | The balance of proteins of high biological value (PHBV) increases antibody production, modulates inflammatory response, stimulates GALT, MALT, and BALT [27]. Brewer’s yeast provides Saccharomyces cerevisiae (SC) and has an immunomodulatory effect by stimulating GPCR/YSD, prevents ACE2 receptor binding to SARS-CoV-2 protein S, and modulates the activation of MAPKs, inhibiting the production of IL-1β, IL-6, and TNF-α [26]. SC in vitro promotes muscle amino acid reserve recovery during SARS-CoV-2 infection, and also regulates the Cop9 signalosome pathway (CSN), a protein complex that regulates Cullin-RING ubiquitin ligase (CRL) activity, thus preventing protein degradation [28]. |
| Ascorbic acid | 2 g | During SARS-CoV-2 infection, ascorbic acid (AA) modulates ROS production, reduces glyceraldehyde 3-phosphate dehydrogenase (GAPDH) enzyme activity, resulting in the prevention of cell death [29], pneumocytes preservation, and attenuates the activation of immune response [30,31]. AA inhibits immune cell lactate and preserves innate immunity of alveolar epithelial type II cells in SARS-CoV-2 infection [32]. |
| Zinc | 40 mg | Zinc (Zn) inhibits SARS-CoV-2 replication in vitro through inhibition of RNA-dependent RNA polymerase (RdRp) and 3C like viral proteinase (3CLpro). Zn upregulates TNF-α and IL-1β levels, modulates cytokine storm in COVID-19 [32]. In SARS-CoV-2 infection Zn enhances the synthesis of metalloenzymes, ACE and ACE 2, that when disabled have beneficial therapeutic effects on the disease progression [33]. |
| Selenium | 200 mcg | Selenium (Se) integrates glutathione peroxidases, selenoprotein F, K, S, and thioredoxin reductases (TXNRD), which promote differentiation and proliferation of innate and adaptive immunity [34]. Se participates in alveolar distention together with the surfactant factor. TXNRD1 upregulates Nrf2 in lung epithelial cells and decreases the expression of NF-κB, IL-1β, IL-6, and TNF-α, thereby regulating the immune and inflammatory response in COVID-19 [35]. Se increases the proliferation of T and NK lymphocytes; 200 µg/day decrease viral transcription and replication, inhibiting the major protease (Mpro) of SARS-CoV-2 [36]. |
| Cholecalciferol | 4000 IU | Cholecalciferol (D3) activates chemotaxis, decreasing the expression of IL-1, 2, 6, 12, IFN-γ, and TNF-α activation, inhibits antigen-presenting cells, therefore has anti-proliferative effect of T cells [37]. In COVID-19, D3 increases the proliferation and differentiation of keratinocytes, endothelial cells, osteoblasts, and lymphocytes; increases the production of IL-10, IL-4, IL-5, and transforms growth factor-β; D3 activates the TH1 response [38]. Its antioxidant effect increases Nrf2 factor activity, activates the Nrf2-Keap1 (Kelch-like ECH-associated protein 1) pathway and maintains redox balance in COVID-19. The effect on the regulation of RAAS (renin-angiotensin-aldosterone system), promotes the inhibition of renin gene transcription, blocking the CREB-CBP/p300 complex, which intervenes in the homeostasis of superoxide dismutase (SOD), Glucose 6-phosphate dehydrogenase, glutathione reductase (GR), and glutathione peroxidase (GP), inhibit genes that attenuate SAH, systemic inflammation, and renal and cardiovascular lesions [39]. |
| Resveratrol | 400 mg | Resveratrol inhibits NF-κB and activation of SIRT1 and p53 signaling pathways. Activation of SIRT1 increases NAD levels and enhances mitochondrial function, regulating the inflammatory response and dysfunctional physiological processes. The activation of SIRT1 and Superoxide Dismutase (SOD) by Resveratrol increased ACE2 function and decreased inflammation [40]. |
| Omega-3 fatty acids | 2 g | Omega-3 PUFA constitute membrane phospholipids and regulate membrane fluidity and protein complex assembly in lipid bilayers; modulate the expression, stability, and enzymatic activities of ACE2 and TMPRSS2. Omega-3 LC-PUFA, DHA regulates lipid raft formation [41]. ACE2 and TMPRSS2 are described to be present mainly in lipid pools. Lipid rafts have been shown to be involved in SARS-CoV entry into Vero E6 cells; 80% of the surfactant factor is constituted by phospholipids, mainly omega-3 PUFAs that contribute to adequate alveolar distension by regulating the production of type II pneumocytes and surfactant factor [32]. |
| Arginine | 1.5 g | Arginine (Arg) decreases cell apoptosis caused by SARS-CoV-2, it also matures CD3+ lymphocytes and regulates the production of CD8 T lymphocytes; When Arg reserves are decreased in COVID-19, it reduces the immune response through TLR4/MAPK signaling; in addition, the viral spike protein in SARS-CoV-2 contains arginine residues that modulate receptor binding to the virus membrane [42]. Arginine increases the ventilatory response and is used to increase alveolar exchange in lung cancer and asthma [23,24]. |
| Magnesium. | 800 mg | In SARS-CoV-2, magnesium (Mg) activates protein kinases, stimulates T-cell receptors and production by generating ATP, controls cell membrane inflammation, and has vasodilatory and antithrombotic effects. Is a modulator of the release of acetylcholine and histamine in the inflammatory cascade in viral infections [43] |
| Saccharomyces boulardii (SB) | 500 mg | Saccharomyces boulardii (SB) has immunological effect, activates Th1 and Th2 response, improves endocrine regulation and increases chemotaxis. At intestinal level it significantly increases the concentration of IgA (important in SARS-CoV-2 diarrhea); also inhibits the synthesis of IL-8; reduces the activation of MAPK Erk1/2, JNK/SAPK and the nuclear translocation of NF-kB [44]. Increases short-chain fatty acids (SCFA) synthesis that regulate the immune response, inflammation and activates the gut-lung axis [41]. |
| Inuline | 20 g | Consumption of inulin-type fructans has been associated with regulation of the immune system, modulation of GI peptides, production of SCFA and modulation of triglyceride metabolism. SCFA are a source of energy for colonocytes, stimulating the synthesis of IL-10 by macrophages, neutrophils and T cells, as well as inhibiting the synthesis of TNF- α and IL-6 [27]. |
| (A) | |||
|---|---|---|---|
| Characteristics | CG | IG | p-Value |
| n = 40 | n = 40 | ||
| Mean ± SD age—years | 53.9 ± 10.3 | 51.5 ± 11.4 | 0.351 |
| Female gender—no. (%) | 13 (32.5) | 15 (37.5) | 0.407 |
| Male gender—no. (%) | 27 (67.5) | 25 (62.5) | 0.407 |
| Risk factors and coexisting conditions—no. (%) | |||
| Overweight | 38 (95) | 36 (90) | 0.338 |
| Obesity | 14 (32) | 13 (32.5) | 1 |
| DM 2 | 13 ± 0.325 | 11 ± 0.275 | 0.404 |
| Cardiovascular disease | 17 (42.5) | 10 (25) | 0.078 |
| Hyperlipidemia | 11 (27.5) | 7 (17.5) | 0.422 |
| Gastrointestinal Disease | 14 (35) | 13 (32.5) | 1 |
| Total Risk Factors | 2.92 ± 1.42 | 2.57 ± 1.35 | 0.261 |
| COVID-19 Symptoms—no. (%) | |||
| Dyspnea | 24 (60) | 26 (65) | 0.409 |
| Nausea and vomit | 6 (15) | 7 (17.5) | 0.5 |
| Hyposmia | 12 (30) | 15 (37.5) | 0.637 |
| Dysgeusia | 18 (45) | 20 (50) | 0.412 |
| Headache | 26 (65) | 29 (72.5) | 0.315 |
| Myalgia | 32 (80) | 30 (75) | 0.395 |
| Diarrhea | 18 (45) | 12 (30) | 0.124 |
| Anorexia | 20 (50) | 21 (52.5) | 0.500 |
| Total of symptoms | 7.05 ± 2.11 | 6.8 ± 2.23 | 0.608 |
| Gastrointestinal Clinic | |||
| Bristol—no. (%) | 9 (50%) | 5 (27.8%) | 0.153 |
| No. of defecations—Mean ± SD | 0.54 ± 0.6 | 0.52 ± 0.73 | 0.717 |
| Abdominal distension—no. (%) | 28 (70%) | 28 (70%) | 0.596 |
| Vital Signs Mean ± SD | |||
| Breathing Frequency—bpm | 21.18 ± 3.01 | 21.48 ± 3.01 | 0.378 |
| Oxygen Saturation—% | 92.73 ± 4.17 | 94 ± 3.18 | 0.144 |
| Heart Rate—bpm | 70.7 ± 15.4 | 75.5 ± 9.88 | 0.105 |
| Temperature—°C | 36.27 ± 0.73 | 36.3 ± 0.62 | 0.935 |
| Oxygen flow—L/min | 5.9 ± 3.82 | 6 ± 3.29 | 0.571 |
| Qsofa—pts | 0.425 ± 0.59 | 0.65 ± 0.62 | 0.100 |
| (B) | |||
| Characteristics | CG | IG | p-Value |
| n = 40 | n = 40 | ||
| Nutritional Status—Mean ± SD | |||
| MNA®—pts | 11.13 ± 2.26 | 11.38 ± 1.65 | 0.828 |
| BMI—kg/m2 | 29.35 ± 3.89 | 29.98 ± 4.07 | 0.403 |
| Hydric balance—mL/day | −203.4 ± 966 | −301.5 ± 1167 | 0.806 |
| Medication—no. (%) | |||
| Antihypertensive | 13 (32.5) | 9 (22.5) | 0.227 |
| Antidiabetics | 9 (22.5) | 11 (27.5) | 0.398 |
| Antilipids | 3 (7.5) | 3 (7.50) | 0.662 |
| Antibiotics | 1 (2.50) | 4 (10) | 0.359 |
| Antiacids | 6 (15) | 6 (15) | 0.662 |
| NSAIDs | 8 (20) | 12 (30) | 0.220 |
| Laboratory studies—Mean ± SD | |||
| Hb—g/dL | 15.53 ± 2.222 | 15.54 ± 2.088 | 0.987 |
| MCHC—G/Dl | 33.39 ± 1.4 | 33.46 ± 1.17 | 0.753 |
| Platelets—103/µL | 222.2 ± 53.93 | 248.4 ± 139.9 | 0.790 |
| Leukocytes—103/µL | 8.97 ± 4.15 | 8.46 ± 4.36 | 0.400 |
| Neutrophils—% | 83.5 ± 8.87 | 80.78 ± 9.29 | 0.172 |
| Glycemia—mg/dL | 135.4 ± 59.39 | 134.8 ± 58.83 | 0.872 |
| Total cholesterol—mg/dL | 142.8 ± 42.82 | 135 ± 23.53 | 0.335 |
| Triglycerides—mg/dL | 147.5 ± 58.92 | 132.8 ± 37.39 | 0.533 |
| AST—U/L | 48.06 ± 28.81 | 46.4 ± 49.65 | 0.214 |
| ALT- U/L | 47.69 ± 31.9 | 50.44 ± 50.88 | 0.914 |
| Albumin—g/dL | 3.53 ± 0.44 | 3.57 ± 0.41 | 0.768 |
| Ferritin—ng/mL | 1070 ± 899.3 | 1270 ± 1142 | 0.572 |
| Fibrinogen—mg/dL | 592.2 ± 170.4 | 607.4 ± 162.9 | 0.721 |
| CRP—mg/dL | 157.3 ± 106.7 | 135.3 ± 94.92 | 0.313 |
| D dimer—ng/dL | 291.2 ± 179.9 | 444.9 ± 954.9 | 0.927 |
| Creatinine—mg/dL | 0.88 ± 0.31 | 0.86 ± 0.22 | 0.734 |
| Urea—mg/dL | 33.95 ± 15.84 | 32.95 ± 10.78 | 0.710 |
| BUN—mg/dL | 15.87 ± 7.39 | 15.43 ± 5.02 | 0.690 |
| GFR—mL/min/1.73 m2 | 90.15 ± 20.2 | 93.59 ± 17.4 | 0.673 |
| Procalcitonin—ng/mL | 0.364 ± 0.6 | 0.18 ± 0.188 | 0.356 |
| (A) | |||||||||
| CG | IG | Intergroup p-Value | |||||||
| Clinical Evolution | n | Baseline | Day 3 | p-Value | n | Baseline | Day 3 | p-Value | |
| Oxygen flow—L (intragroup) | 40 | 5.9 ± 3.8 | 6 ± 4.4 | 0.919 | 40 | 6 ± 3.2 | 4.5 ± 3.5 | 0.014 * | |
| qSOFA—pts | 40 | 0.42 ± 0.59 | 0.51 ± 0.57 | 0.608 | 40 | 0.65 ± 0.62 | 0.43 ± 0.49 | 0.040 * | |
| Number of defecations on day 3 | 37 | 0.81 ± 0.90 | 36 | 1.41 ± 1.13 | 0.014 * | ||||
| Distension on day 3 | 31 | 51.60% | 31 | 19.40% | 0.008 * | ||||
| PHQ-9 test—pts (intragroup) | 6 | 3.66 ± 2.5 | 1.50 ± 2.8 | 0.187 | 10 | 5.3 ± 3.4 | 1.9 ± 1.4 | 0.003 * | |
| Oxygen Saturation > 90% on day 3 | 40 | 85% | 40 | 92.50% | 0.241 | ||||
| Hydric Balance on day 3—mL | 17 | 123.4 ± 453.8 | 18 | 456.6 ± 485.5 | 0.043 * | ||||
| Bristol scale on day 3 | 24 | 33.30% | 31 | 41.90% | 0.356 | ||||
| 40-day follow-up | n | Day 40 | n | Day 40 | |||||
| Saturation without Supplementary Oxygen—% | 28 | 90.39 ± 3.4 | 38 | 92.08 ± 2.5 | 0.030 * | ||||
| Need for home Oxygen flow—% | 27 | 85.2% | 39 | 66.70% | 0.078 | ||||
| Time of home Oxygen use—days | 17 | 57.6 ± 24.6 | 23 | 43.8 ± 16.2% | 0.098 | ||||
| Post-COVID syndrome—% | 24 | 37.50% | 34 | 23.50% | 0.195 | ||||
| Weight decrease—% of patients | 11 | 72.70% | 18 | 44.40% | 0.135 | ||||
| Gastrointestinal symptoms—% | 24 | 16.70% | 37 | 8.10% | 0.266 | ||||
| (B) | |||||||||
| Discharge (n = 72) | Death (n = 8) | p-Value | |||||||
| Baseline Values | n | % | n | % | |||||
| Fibrinogen > 700 mg/dL | 13 | 18.50 | 6 | 75.00 | 0.002 * | ||||
| Procalcitonin > 0.5 ng/mL | 4 | 5.5 | 4 | 50 | 0.003 * | ||||
| Blood Urea Nitrogen > 22 mg/dL | 5 | 6.90 | 4 | 50 | 0.004 * | ||||
| RCP > 150 mg/L | 28 | 38.8 | 7 | 87.5 | 0.011 * | ||||
| Neutrophils > 80% | 45 | 62.50 | 8 | 100 | 0.031 * | ||||
| Leukocytes > 10 × 103/μL | 18 | 25.00 | 5 | 62.50 | 0.040 * | ||||
| Urea > 40 mg/dL | 12 | 16.6 | 4 | 50 | 0.047 * | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leal-Martínez, F.; Abarca-Bernal, L.; García-Pérez, A.; González-Tolosa, D.; Cruz-Cázares, G.; Montell-García, M.; Ibarra, A. Effect of a Nutritional Support System to Increase Survival and Reduce Mortality in Patients with COVID-19 in Stage III and Comorbidities: A Blinded Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 1172. https://doi.org/10.3390/ijerph19031172
Leal-Martínez F, Abarca-Bernal L, García-Pérez A, González-Tolosa D, Cruz-Cázares G, Montell-García M, Ibarra A. Effect of a Nutritional Support System to Increase Survival and Reduce Mortality in Patients with COVID-19 in Stage III and Comorbidities: A Blinded Randomized Controlled Clinical Trial. International Journal of Environmental Research and Public Health. 2022; 19(3):1172. https://doi.org/10.3390/ijerph19031172
Chicago/Turabian StyleLeal-Martínez, Fernando, Lorena Abarca-Bernal, Alejandra García-Pérez, Dinnaru González-Tolosa, Georgina Cruz-Cázares, Marco Montell-García, and Antonio Ibarra. 2022. "Effect of a Nutritional Support System to Increase Survival and Reduce Mortality in Patients with COVID-19 in Stage III and Comorbidities: A Blinded Randomized Controlled Clinical Trial" International Journal of Environmental Research and Public Health 19, no. 3: 1172. https://doi.org/10.3390/ijerph19031172
APA StyleLeal-Martínez, F., Abarca-Bernal, L., García-Pérez, A., González-Tolosa, D., Cruz-Cázares, G., Montell-García, M., & Ibarra, A. (2022). Effect of a Nutritional Support System to Increase Survival and Reduce Mortality in Patients with COVID-19 in Stage III and Comorbidities: A Blinded Randomized Controlled Clinical Trial. International Journal of Environmental Research and Public Health, 19(3), 1172. https://doi.org/10.3390/ijerph19031172






