Non-Epithelial Ovarian Cancers: How Much Do We Really Know?
Abstract
1. Introduction
2. Ovarian GCT
2.1. Dysgerminomas
2.2. Yolk Sac Tumours
2.3. Treatment of GCT
3. Ovarian SCST
3.1. Ovarian GrCT
3.2. Sertoli-Leydig Cell Tumours
4. Small Cell Ovarian Carcinomas
5. mRNA Profiles of NEOC
6. Clinical Trials and Novel Approaches of NEOC
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Bennetsen, A.K.K.; Baandrup, L.; Aalborg, G.L.; Kjaer, S.K. Non-epithelial ovarian cancer in Denmark—Incidence and survival over nearly 40 years. Gynecol. Oncol. 2020, 157, 693–699. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Morice, P.; Lorusso, D.; Prat, J.; Oaknin, A.; Pautier, P.; Colombo, N. ESMO Guidelines Committee. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv1–iv18. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Karathanasi, A.; Zakynthinakis-Kyriakou, N.; Tsiouris, A.K.; Chatziantoniou, A.A.; Kanellos, F.S.; Tatsi, K. Ovarian carcinosarcoma: Current developments and future perspectives. Crit. Rev. Oncol. Hematol. 2019, 134, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, E.P.; Labidi-Galy, S.I.; Moschetta, M.; Uccello, M.; Kalaitzopoulos, D.R.; Perez-Fidalgo, J.A.; Boussios, S. Endometriosis-associated ovarian carcinomas: Insights into pathogenesis, diagnostics, and therapeutic targets-a narrative review. Ann. Transl. Med. 2020, 8, 1712. [Google Scholar] [CrossRef]
- Veneris, J.T.; Mahajan, P.; Frazier, A.L. Contemporary management of ovarian germ cell tumors and remaining controversies. Gynecol. Oncol. 2020, 158, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, A.M.; Rezvani, M.; Elsayes, K.M.; Baskin, H., Jr.; Mourad, A.; Foster, B.R.; Jarboe, E.A.; Menias, C.O. Ovarian malignant germ cell tumors: Cellular classification and clinical and imaging features. Radiographics 2014, 34, 777–801. [Google Scholar] [CrossRef]
- Euscher, E.D. Germ Cell Tumors of the Female Genital Tract. Surg. Pathol. Clin. 2019, 12, 621–649. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer. J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Adhikari, L.; Hassell, L.A. Ovary General: WHO Classification. Available online: https://www.pathologyoutlines.com/topic/ovarytumorwhoclassif.html (accessed on 5 December 2021).
- Kaur, B. Pathology of malignant ovarian germ cell tumours. Diagn. Histopathol. 2020, 26, 289–297. [Google Scholar] [CrossRef]
- Low, J.J.; Ilancheran, A.; Ng, J.S. Malignant ovarian germ-cell tumours. Best Pract. Res. Clin. Obstet. Gynaecol. 2012, 26, 347–355. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, D.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Analysis of outcomes and prognostic factors after fertility-sparing surgery in malignant ovarian germ cell tumors. Gynecol. Oncol. 2017, 145, 513–518. [Google Scholar] [CrossRef]
- Dellino, M.; Silvestris, E.; Loizzi, V.; Paradiso, A.; Loiacono, R.; Minoia, C.; Daniele, A.; Cormio, G. Germinal ovarian tumors in reproductive age women: Fertility-sparing and outcome. Medicine 2020, 99, e22146. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Moschetta, M.; Tatsi, K.; Tsiouris, A.K.; Pavlidis, N. A review on pregnancy complicated by ovarian epithelial and non-epithelial malignant tumors: Diagnostic and therapeutic perspectives. J. Adv. Res. 2018, 12, 1–9. [Google Scholar] [CrossRef]
- Matz, M.; Coleman, M.P.; Sant, M.; Chirlaque, M.D.; Visser, O.; Gore, M.; Allemani, C.; the CONCORD Working Group. The histology of ovarian cancer: Worldwide distribution and implications for international survival comparisons (CONCORD-2). Gynecol. Oncol. 2017, 144, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Vicus, D.; Beiner, M.E.; Klachook, S.; Le, L.W.; Laframboise, S.; Mackay, H. Pure dysgerminoma of the ovary 35 years on: A single institutional experience. Gynecol. Oncol. 2010, 117, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kang, S.B. Ovarian dysgerminoma: Color Doppler ultrasonographic findings and comparison with CT and MR imaging findings. J. Ultrasound Med. 1995, 14, 843–848. [Google Scholar] [CrossRef]
- Guerriero, S.; Testa, A.C.; Timmerman, D.; Van Holsbeke, C.; Ajossa, S.; Fischerova, D.; Franchi, D.; Leone, F.P.; Domali, E.; Alcazar, J.L.; et al. Imaging of gynecological disease (6): Clinical and ultrasound characteristics of ovarian dysgerminoma. Ultrasound Obstet. Gynecol. 2011, 37, 596–602. [Google Scholar] [CrossRef]
- Tîrnovanu, M.C.; Florea, I.D.; Tănase, A.; Toma, B.F.; Cojocaru, E.; Ungureanu, C.; Lozneanu, L. Un-common Metastasis of Ovarian Dysgerminoma: A Case Report and Review of the Literature. Medicina 2021, 57, 534. [Google Scholar] [CrossRef]
- Chan, J.K.; Tewari, K.S.; Waller, S.; Cheung, M.K.; Shin, J.Y.; Osann, K.; Kapp, D.S. The influence of conservative surgical practices for malignant ovarian germ cell tumors. J. Surg. Oncol. 2008, 98, 111–116. [Google Scholar] [CrossRef]
- Kurman, R.J.; Norris, H.J. Endodermal sinus tumor of the ovary: A clinical and pathologic analysis of 71 cases. Cancer 1976, 38, 2404–2419. [Google Scholar] [CrossRef]
- Boussios, S.; Attygalle, A.; Hazell, S.; Moschetta, M.; McLachlan, J.; Okines, A.; Banerjee, S. Malignant Ovarian Germ Cell Tumors in Postmenopausal Patients: The Royal Marsden Experience and Literature Review. Anticancer Res. 2015, 35, 6713–6722. [Google Scholar] [PubMed]
- Chao, W.T.; Liu, C.H.; Lai, C.R.; Chen, Y.J.; Chuang, C.M.; Wang, P.H. Alpha-fetoprotein-producing ovarian clear cell adenocarcinoma with fetal gut differentiation: A rare case report and literature review. J. Ovarian Res. 2018, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Rakusić, Z.; Krpan, A.M.; Mareković, Z.; Juretić, A.; Gasparov, S. Retroperitoneal and metachronous testicular germ cell tumors with different histology and teratoma growing syndrome—A case report. Coll. Antropol. 2011, 35, 937–940. [Google Scholar]
- Li, Y.; Zheng, Y.; Lin, J.; Xu, G.; Cai, A.; Chen, R.; Wu, M. Radiological-pathological correlation of yolk sac tumor in 20 patients. Acta Radiol. 2016, 57, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Umezu, T.; Kajiyama, H.; Terauchi, M.; Shibata, K.; Ino, K.; Nawa, A.; Kikkawa, F. Long-term outcome and prognostic factors for yolk sac tumor of the ovary. Nagoya J. Med. Sci. 2008, 70, 29–34. [Google Scholar]
- McBee, W.C., Jr.; Brainard, J.; Sawady, J.; Rose, P.G. Yolk sac tumor of the ovary associated with endometrioid carcinoma with metastasis to the vagina: A case report. Gynecol. Oncol. 2007, 105, 244–247. [Google Scholar] [CrossRef]
- Chen, L.H.; Yip, K.C.; Wu, H.J.; Yong, S.B. Yolk Sac Tumor in an Eight-Year-Old Girl: A Case Report and Literature Review. Front. Pediatr. 2019, 7, 169. [Google Scholar] [CrossRef]
- Turkmen, O.; Karalok, A.; Basaran, D.; Kimyon, G.C.; Tasci, T.; Ureyen, I.; Tulunay, G.; Turan, T. Fertility-Sparing Surgery Should Be the Standard Treatment in Patients with Malignant Ovarian Germ Cell Tumors. J. Adolesc. Young Adult Oncol. 2017, 6, 270–276. [Google Scholar] [CrossRef]
- Johansen, G.; Dahm-Kähler, P.; Staf, C.; Flöter Rådestad, A.; Rodriguez-Wallberg, K.A. Fertility-sparing surgery for treatment of non-epithelial ovarian cancer: Oncological and reproductive outcomes in a prospective nationwide population-based cohort study. Gynecol. Oncol. 2019, 155, 287–293. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, D.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Outcomes of Surgery Alone and Surveillance Strategy in Young Women with Stage I Malignant Ovarian Germ Cell Tumors. Int. J. Gynecol. Cancer 2016, 26, 859–864. [Google Scholar] [CrossRef]
- Pectasides, D.; Pectasides, E.; Kassanos, D. Germ cell tumors of the ovary. Cancer Treat. Rev. 2008, 34, 427–441. [Google Scholar] [CrossRef]
- Kang, H.; Kim, T.J.; Kim, W.Y.; Choi, C.H.; Lee, J.W.; Kim, B.G.; Bae, D.S. Outcome and reproductive function after cumulative high-dose combination chemotherapy with bleomycin, etoposide and cisplatin (BEP) for patients with ovarian endodermal sinus tumor. Gynecol. Oncol. 2008, 111, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.O.; Berwick, M.; Verschraegen, C.F.; Wiggins, C.; Lansing, L.; Muller, C.Y.; Qualls, C.R. Incidence and survival rates for female malignant germ cell tumors. Obstet. Gynecol. 2006, 107, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Lanfredini, N.; Tana, R. Menstrual function and childbearing potential after fertility-sparing surgery and platinum-based chemotherapy for malignant ovarian germ cell tumours. Gynecol. Endocrinol. 2014, 30, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, D.M.; Miller, A.M.; Champion, V.L.; Monahan, P.O.; Zhao, Q.; Cella, D.; Williams, S.D.; Gynecologic Oncology Group. Reproductive and sexual function after platinum-based chemo-therapy in long-term ovarian germ cell tumor survivors: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2007, 25, 2792–2797. [Google Scholar] [CrossRef] [PubMed]
- De La Motte Rouge, T.; Pautier, P.; Duvillard, P.; Rey, A.; Morice, P.; Haie-Meder, C.; Kerbrat, P.; Culine, S.; Troalen, F.; Lhommé, C. Survival and reproductive function of 52 women treated with surgery and bleomycin, etoposide, cisplatin (BEP) chemotherapy for ovarian yolk sac tumor. Ann. Oncol. 2008, 19, 1435–1441. [Google Scholar] [CrossRef]
- Uccello, M.; Boussios, S.; Samartzis, E.P.; Moschetta, M. Systemic anti-cancer treatment in malignant ovarian germ cell tumours (MOGCTs): Current management and promising approaches. Ann. Transl. Med. 2020, 8, 1713. [Google Scholar] [CrossRef]
- Boussios, S.; Mikropoulos, C.; Samartzis, E.; Karihtala, P.; Moschetta, M.; Sheriff, M.; Karathanasi, A.; Sadauskaite, A.; Rassy, E.; Pavlidis, N. Wise Management of Ovarian Cancer: On the Cutting Edge. J. Pers. Med. 2020, 10, 41. [Google Scholar] [CrossRef]
- Boussios, S.; Moschetta, M.; Zarkavelis, G.; Papadaki, A.; Kefas, A.; Tatsi, K. Ovarian sex-cord stromal tumours and small cell tumours: Pathological, genetic and management aspects. Crit. Rev. Oncol. Hematol. 2017, 120, 43–51. [Google Scholar] [CrossRef]
- Boyce, E.A.; Costaggini, I.; Vitonis, A.; Feltmate, C.; Muto, M.; Berkowitz, R.; Cramer, D.; Horowitz, N.S. The epidemiology of ovarian granulosa cell tumors: A case-control study. Gynecol. Oncol. 2009, 115, 221–225. [Google Scholar] [CrossRef]
- Boussios, S.; Moschetta, M.; Karathanasi, A.; Tsiouris, A.K.; Kanellos, F.S.; Tatsi, K.; Katsanos, K.H.; Christodoulou, D.K. Malignant peritoneal mesothelioma: Clinical aspects, and therapeutic perspectives. Ann. Gastroenterol. 2018, 31, 659–669. [Google Scholar] [CrossRef]
- Van Meurs, H.S.; Bleeker, M.C.; van der Velden, J.; Overbeek, L.I.; Kenter, G.G.; Buist, M.R. The incidence of endometrial hyperplasia and cancer in 1031 patients with a granulosa cell tumor of the ovary: Long-term follow-up in a population-based cohort study. Int. J. Gynecol. Cancer 2013, 23, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Outwater, E.K.; Wagner, B.J.; Mannion, C.; McLarney, J.K.; Kim, B. Sex cord-stromal and steroid cell tumors of the ovary. Radiographics 1998, 18, 1523–1546. [Google Scholar] [CrossRef]
- Khosla, D.; Dimri, K.; Pandey, A.K.; Mahajan, R.; Trehan, R. Ovarian granulosa cell tumor: Clinical features, treatment, outcome, and prognostic factors. N. Am. J. Med. Sci. 2014, 6, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Pectasides, D.; Pectasides, E.; Psyrri, A. Granulosa cell tumor of the ovary. Cancer Treat. Rev. 2008, 34, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Petraglia, F.; Luisi, S.; Pautier, P.; Sabourin, J.C.; Rey, R.; Lhomme, C.; Bidart, J.M. Inhibin B is the major form of inhibin/activin family secreted by granulosa cell tumors. J. Clin. Endocrinol. Metab. 1998, 83, 1029–1032. [Google Scholar] [CrossRef]
- Shah, S.P.; Köbel, M.; Senz, J.; Morin, R.D.; Clarke, B.A.; Wiegand, K.C.; Leung, G.; Zayed, A.; Mehl, E.; Kalloger, S.E.; et al. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N. Engl. J. Med. 2009, 360, 2719–2729. [Google Scholar] [CrossRef] [PubMed]
- Schumer, S.T.; Cannistra, S.A. Granulosa cell tumor of the ovary. J. Clin. Oncol. 2003, 21, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Zarkavelis, G.; Seraj, E.; Zerdes, I.; Tatsi, K.; Pentheroudakis, G. Non-epithelial Ovarian Cancer: Elucidating Uncommon Gynaecological Malignancies. Anticancer Res. 2016, 36, 5031–5042. [Google Scholar] [CrossRef] [PubMed]
- Van Meurs, H.S.; van Lonkhuijzen, L.R.; Limpens, J.; van der Velden, J.; Buist, M.R. Hormone therapy in ovarian granulosa cell tumors: A systematic review. Gynecol. Oncol. 2014, 134, 196–205. [Google Scholar] [CrossRef]
- Zhang, M.; Cheung, M.K.; Shin, J.Y.; Kapp, D.S.; Husain, A.; Teng, N.N.; Berek, J.S.; Osann, K.; Chan, J.K. Prognostic factors responsible for survival in sex cord stromal tumors of the ovary-an analysis of 376 women. Gynecol. Oncol. 2007, 104, 396–400. [Google Scholar] [CrossRef]
- Morice, P.; Pautier, P.; Fanchin, R.; Haie-Meder, C.; Chauveaud-Lambling, A.; Frydman, R.; Frydman, N. Therapy Insight: Fertility in women after cancer treatment. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 819–826. [Google Scholar] [CrossRef]
- Young, R.H.; Dickersin, G.R.; Scully, R.E. Juvenile granulosa cell tumor of the ovary. A clinicopathological analysis of 125 cases. Am. J. Surg. Pathol. 1984, 8, 575–596. [Google Scholar] [CrossRef] [PubMed]
- Young, R.H. Sex cord-stromal tumors of the ovary and testis: Their similarities and differences with consideration of selected problems. Mod. Pathol. 2005, 18, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.M. Recent advances in the pathology and classification of ovarian sex cord-stromal tumors. Int. J. Gynecol. Pathol. 2006, 25, 199–215. [Google Scholar] [CrossRef]
- De Kock, L.; Terzic, T.; McCluggage, W.G.; Stewart, C.J.R.; Shaw, P.; Foulkes, W.D.; Clarke, B.A. DICER1 Mutations Are Consistently Present in Moderately and Poorly Differentiated Sertoli-Leydig Cell Tumors. Am. J. Surg. Pathol. 2017, 41, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Al-Agha, O.M.; Huwait, H.F.; Chow, C.; Yang, W.; Senz, J.; Kalloger, S.E.; Huntsman, D.G.; Young, R.H.; Gilks, C.B. FOXL2 is a sensitive and specific marker for sex cord-stromal tumors of the ovary. Am. J. Surg. Pathol. 2011, 35, 484–494. [Google Scholar] [CrossRef]
- Colombo, N.; Parma, G.; Zanagnolo, V.; Insinga, A. Management of ovarian stromal cell tumors. J. Clin. Oncol. 2007, 25, 2944–2951. [Google Scholar] [CrossRef]
- Plotnikova, O.; Baranova, A.; Skoblov, M. Comprehensive Analysis of Human microRNA-mRNA Interactome. Front. Genet. 2019, 10, 933. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J.; Halsall, D.J.; Hook, C.E.; Williams, D.M.; Nicholson, J.C.; Coleman, N. Identification of microRNAs From the miR-371~373 and miR-302 clusters as potential serum biomarkers of malignant germ cell tumors. Am. J. Clin. Pathol. 2011, 135, 119–125. [Google Scholar] [CrossRef]
- Croce, C.M. Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 2009, 10, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Ozturk, M.A.; Moschetta, M.; Karathanasi, A.; Zakynthinakis-Kyriakou, N.; Katsanos, K.H.; Christodoulou, D.K.; Pavlidis, N. The Developing Story of Predictive Biomarkers in Colorectal Cancer. J. Pers. Med. 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Saxby, H.; Mikropoulos, C.; Boussios, S. An Update on the Prognostic and Predictive Serum Biomarkers in Metastatic Prostate Cancer. Diagnostics 2020, 10, 549. [Google Scholar] [CrossRef] [PubMed]
- Revythis, A.; Shah, S.; Kutka, M.; Moschetta, M.; Ozturk, M.A.; Pappas-Gogos, G.; Ioannidou, E.; Sheriff, M.; Rassy, E.; Boussios, S. Unraveling the Wide Spectrum of Melanoma Biomarkers. Diagnostics 2021, 11, 1341. [Google Scholar] [CrossRef]
- Moran, S.; Martinez-Cardús, A.; Boussios, S.; Esteller, M. Precision medicine based on epigenomics: The paradigm of carcinoma of unknown primary. Nat. Rev. Clin. Oncol. 2017, 14, 682–694. [Google Scholar] [CrossRef]
- Cocquet, J.; Pailhoux, E.; Jaubert, F.; Servel, N.; Xia, X.; Pannetier, M.; De Baere, E.; Messiaen, L.; Cotinot, C.; Fellous, M.; et al. Evolution and expression of FOXL2. J. Med. Genet. 2002, 39, 916–921. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoon, S.; Park, M.; Park, H.O.; Ko, J.J.; Lee, K.; Bae, J. Differential apoptotic activities of wild-type FOXL2 and the adult-type granulosa cell tumor-associated mutant FOXL2 (C134W). Oncogene 2011, 30, 1653–1663. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.H.; Kim, H.M.; Park, H.O.; Ha, N.C.; Kim, T.H.; Park, M.; Lee, K.; Bae, J. FOXL2 posttranslational modifications mediated by GSK3β determine the growth of granulosa cell tumours. Nat. Commun. 2014, 5, 2936. [Google Scholar] [CrossRef]
- Heravi-Moussavi, A.; Anglesio, M.S.; Cheng, S.W.; Senz, J.; Yang, W.; Prentice, L.; Fejes, A.P.; Chow, C.; Tone, A.; Kalloger, S.E.; et al. Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N. Engl. J. Med. 2012, 366, 234–242. [Google Scholar] [CrossRef]
- Takamizawa, J.; Konishi, H.; Yanagisawa, K.; Tomida, S.; Osada, H.; Endoh, H.; Harano, T.; Yatabe, Y.; Nagino, M.; Nimura, Y.; et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004, 64, 3753–3756. [Google Scholar] [CrossRef]
- Zhong, X.; Li, N.; Liang, S.; Huang, Q.; Coukos, G.; Zhang, L. Identification of microRNAs regulating reprogramming factor LIN28 in embryonic stem cells and cancer cells. J. Biol. Chem. 2010, 285, 41961–41971. [Google Scholar] [CrossRef]
- Murray, M.J.; Saini, H.K.; Siegler, C.A.; Hanning, J.E.; Barker, E.M.; van Dongen, S.; Ward, D.M.; Raby, K.L.; Groves, I.J.; Scarpini, C.G.; et al. LIN28 Expression in malignant germ cell tumors downregulates let-7 and increases oncogene levels. Cancer Res. 2013, 73, 4872–4884. [Google Scholar] [CrossRef]
- Hoei-Hansen, C.E.; Kraggerud, S.M.; Abeler, V.M.; Kaern, J.; Rajpert-De Meyts, E.; Lothe, R.A. Ovarian dysgerminomas are characterised by frequent KIT mutations and abundant expression of pluripotency markers. Mol. Cancer 2007, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Kraggerud, S.M.; Hoei-Hansen, C.E.; Alagaratnam, S.; Skotheim, R.I.; Abeler, V.M.; Rajpert-De Meyts, E.; Lothe, R.A. Molecular characteristics of malignant ovarian germ cell tumors and comparison with testicular counterparts: Implications for pathogenesis. Endocr. Rev. 2013, 34, 339–376. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J.; Saini, H.K.; van Dongen, S.; Palmer, R.D.; Muralidhar, B.; Pett, M.R.; Piipari, M.; Thornton, C.M.; Nicholson, J.C.; Enright, A.J.; et al. The two most common histological subtypes of malignant germ cell tumour are distinguished by global microRNA profiles, associated with differential transcription factor expression. Mol. Cancer 2010, 9, 290. [Google Scholar] [CrossRef]
- Palmer, R.D.; Murray, M.J.; Saini, H.K.; van Dongen, S.; Abreu-Goodger, C.; Muralidhar, B.; Pett, M.R.; Thornton, C.M.; Nicholson, J.C.; Enright, A.J.; et al. Malignant germ cell tumors display common microRNA profiles resulting in global changes in expression of messenger RNA targets. Cancer Res. 2010, 70, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.K.; Li, X.; Mu, N.; Hrydziuszko, O.; Garcia-Majano, B.; Larsson, C.; Lui, W.O. MicroRNA expression profiles in non-epithelial ovarian tumors. Int. J. Oncol. 2018, 52, 55–66. [Google Scholar] [CrossRef]
- Gozuacik, D.; Akkoc, Y.; Ozturk, D.G.; Kocak, M. Autophagy-Regulating microRNAs and Cancer. Front. Oncol. 2017, 7, 65. [Google Scholar] [CrossRef]
- Wainwright, E.N.; Jorgensen, J.S.; Kim, Y.; Truong, V.; Bagheri-Fam, S.; Davidson, T.; Svingen, T.; Fernandez-Valverde, S.L.; McClelland, K.S.; Taft, R.J.; et al. SOX9 regulates microRNA miR-202-5p/3p expression during mouse testis differentiation. Biol. Reprod. 2013, 89, 34. [Google Scholar] [CrossRef]
- Sontakke, S.D.; Mohammed, B.T.; McNeilly, A.S.; Donadeu, F.X. Characterization of microRNAs differentially expressed during bovine follicle development. Reproduction 2014, 148, 271–283. [Google Scholar] [CrossRef]
- Bannister, S.C.; Smith, C.A.; Roeszler, K.N.; Doran, T.J.; Sinclair, A.H.; Tizard, M.L. Manipulation of estrogen synthesis alters MIR202* expression in embryonic chicken gonads. Biol. Reprod. 2011, 85, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.; Bard, J.E.; Nowak, N.J.; Buck, M.J.; Kandel, E.S. FOXO1 regulates expression of a microRNA cluster on X chromosome. Aging 2013, 5, 347–356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gross, D.N.; Wan, M.; Birnbaum, M.J. The role of FOXO in the regulation of metabolism. Curr. Diab. Rep. 2009, 9, 208–214. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Y.A.; Pangas, S.A.; Adams, J.; Zhou, W.; Castrillon, D.H.; Wilhelm, D.; Richards, J.S. FOXO1/3 and PTEN Depletion in Granulosa Cells Promotes Ovarian Granulosa Cell Tumor Development. Mol. Endocrinol. 2015, 29, 1006–1024. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, U.; Casadei, C.; Bergamini, A.; Attademo, L.; Cormio, G.; Lorusso, D.; Pignata, S.; Mangili, G. Therapeutic Challenges for Cisplatin-Resistant Ovarian Germ Cell Tumors. Cancers 2019, 11, 1584. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, N.J.; Chan, H.; Toner, G.; Stockler, M.R.; Martin, A.; Yip, S.; Wong, N.; Yeung, A.; Mazhar, D.; Pashankar, F.; et al. Pro-tocol for the P3BEP trial (ANZUP 1302): An international randomised phase 3 trial of accelerated versus standard BEP chemotherapy for adult and paediatric male and female patients with intermediate and poor-risk metastatic germ cell tumours. BMC Cancer 2018, 18, 854. [Google Scholar] [CrossRef] [PubMed]
- El Helali, A.; Kwok, G.S.T.; Tse, K.Y. Adjuvant and post-surgical treatment in non-epithelial ovarian cancer. Best. Pract. Res. Clin. Obstet. Gynaecol. 2021, 78, 74–85. [Google Scholar] [CrossRef]
- Burton, E.R.; Brady, M.; Homesley, H.D.; Rose, P.G.; Nakamura, T.; Kesterson, J.P.; Rotmensch, J.; Tate Thigpen, J.; Van Le, L. A phase II study of paclitaxel for the treatment of ovarian stromal tumors: An NRG Oncology/Gynecologic Oncology Group Study. Gynecol. Oncol. 2016, 140, 48–52. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Geerts, I.; Vergote, I.; Neven, P.; Billen, J. The role of inhibins B and antimüllerian hormone for diagnosis and follow-up of granulosa cell tumors. Int. J. Gynecol. Cancer 2009, 19, 847–855. [Google Scholar] [CrossRef]
- Herzog, T.J.; Arguello, D.; Reddy, S.K.; Gatalica, Z. PD-1, PD-L1 expression in 1599 gynecological cancers: Implications for immunotherapy. Gynecol. Oncol. 2015, 137, 204–205. [Google Scholar] [CrossRef]
- Fankhauser, C.D.; Curioni-Fontecedro, A.; Allmann, V.; Beyer, J.; Tischler, V.; Sulser, T.; Moch, H.; Bode, P.K. Frequent PD-L1 expression in testicular germ cell tumors. Br. J. Cancer 2015, 113, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Adra, N.; Einhorn, L.H.; Althouse, S.K.; Ammakkanavar, N.R.; Musapatika, D.; Albany, C.; Vaughn, D.; Hanna, N.H. Phase II trial of pembrolizumab in patients with platinum refractory germ-cell tumors: A Hoosier Cancer Research Network Study GU14-206. Ann. Oncol. 2018, 29, 209–214. [Google Scholar] [CrossRef]
- Mego, M.; Svetlovska, D.; Chovanec, M.; Rečkova, M.; Rejlekova, K.; Obertova, J.; Palacka, P.; Sycova-Mila, Z.; De Giorgi, U.; Mardiak, J. Phase II study of avelumab in multiple re-lapsed/refractory germ cell cancer. Invest. New Drugs 2019, 37, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Giannatempo, P.; Raggi, D.; Mariani, L.; Colecchia, M.; Farè, E.; Monopoli, F.; Calareso, G.; Ali, S.M.; Ross, J.S.; et al. An Open-label Randomized Phase 2 study of Dur-valumab Alone or in Combination with Tremelimumab in Patients with Advanced Germ Cell Tumors (APACHE): Results from the First Planned Interim Analysis. Eur. Urol. 2019, 75, 201–203. [Google Scholar] [CrossRef] [PubMed]
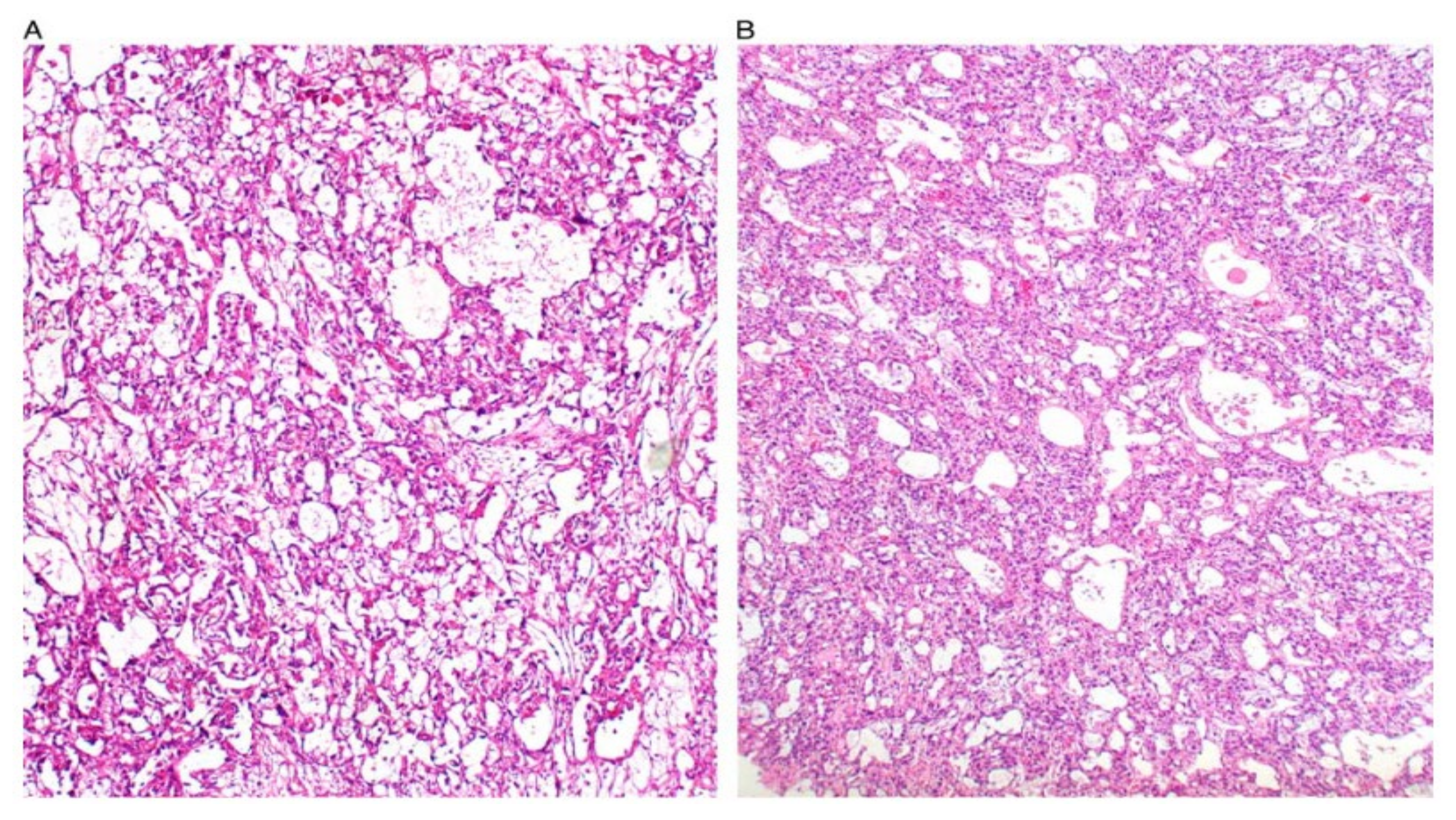

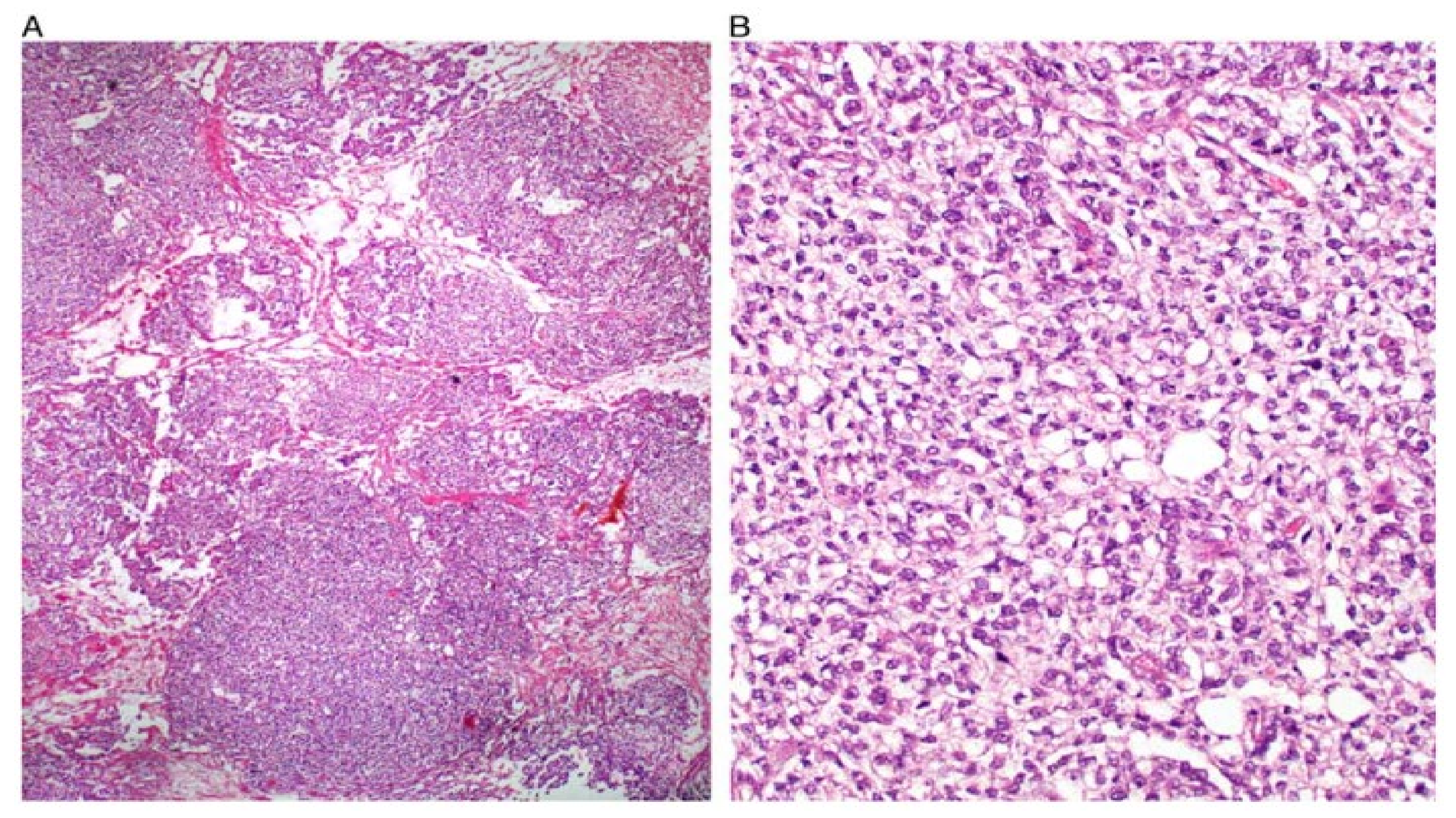

| Primitive GCT |
|
| Mature teratoma |
| Immature teratoma |
Monodermal and somatic-type tumours arising from dermoid cysts
|
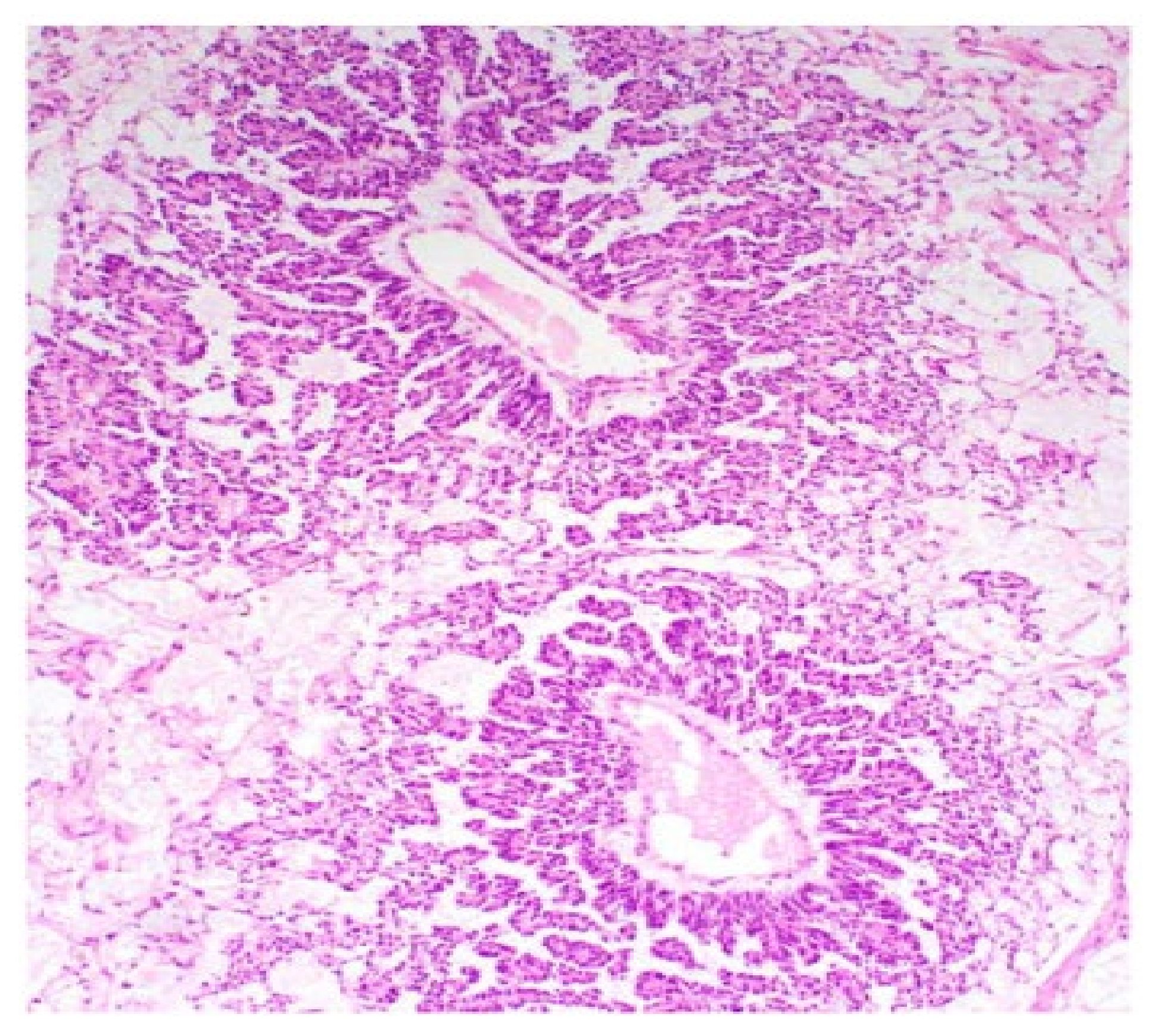
| Histological Appearance of YST | Brief Description |
|---|---|
| Microcystic/reticular | Loose meshwork of cysts and tumour cells that have a ‘signet-ring’ morphology |
| Endodermal sinus (festoon) | Anastomosing network of labyrinthine-like spaces lined with tumour cells and Schiller-Duval bodies (glomeruloid structures) |
| Solid | Sheets of polygonal tumour cells |
| Alveolar-glandular | Large cystic/irregular alveolar spaces which are lined by single or multiple layers of columnar cells |
| Parietal | Tumour cells surround bands of PAS positive hyaline globules |
| Papillary | Pleomorphic tumour cells line papillae containing connective tissue fibrovascular cores |
| Polyvesicular vitelline | Cysts or vesicles lined by flat/columnar/cuboidal tumour cells |
| Hepatoid | Aggregates, cords or clusters of polygonal cells which resemble hepatocytes |
| Myxomatous | Myxoid stroma containing tumour cells with tubular/cords/glandular-like structures |
| miRNA | Target |
|---|---|
| miR-199a-5p | Beclin 1 (BECN1) Podocalyxin-like (PODXL) MAF BZIP transcription factor B (MAFB) |
| miR-199a | Nuclear factor κB kinase subunit beta (IKKβ) |
| miR-202-3p | Forkhead Box L2 (FOXL2) |
| miR-506~514 | Forkhead Box O1 (FOXO1) |
| Trial Reference | Type of Trial | Target NEOC Patient Group Included | Interventions | Primary Endpoint | Recruitment Status |
|---|---|---|---|---|---|
| NCT04876456 | Phase II | Refractory GCT | Cabozantinib | Clinical response according to RECIST | Recruiting |
| NCT04804007 | Phase II | Relapsed GCT treated with HDCT + AuSCT | Etoposide vs. observation | 12-month PFS | Recruiting |
| NCT04602377 | Phase II | Advanced SCCOHT | Pembrolizumab + PAVEP for 6 weeks, followed by pembrolizumab alone vs. Pembrolizumab alone | Clinical response according to RECIST | Recruiting |
| NCT04602377 | Phase II | GrCT | Onapristone ER vs. Onapristone ER + Anastrozole | Clinical response according to RECIST | Recruiting |
| NCT03067181 | Phase III | Childhood and adult GCT | Active surveillance vs. Randomised trial of carboplatin vs. Cisplatin | OS and event-free survival 2 years post-enrolment | Recruiting |
| NCT02834013 | Phase II | GCT | Nivolumab alone for PD-L1 amplified cohort vs. Nivolumab + ipilimumab for all other cohorts | Clinical response according to RECIST | Recruiting |
| NCT02429700 | Phase III | SCST | Paclitaxel vs. BEP | 5-year PFS | Recruiting |
| NCT01042522 | Randomised phase II | GrCT, SLCT and SCST | Paclitaxel and carboplatin vs. BEP and cisplatin | 10-year PFS | Active, not recruiting |
| NCT00788125 | Phase I/II | GCT | Dasatinib with ifosfamide + carboplatin + etoposide | Maximum tolerated dose and toxicity of dasatinib | Active, not recruiting |
| NCT00788125 | Phase I | HER overexpressed adult GCT | pNGVL3-hICD vaccine + sargramostim | Immune response and safety of vaccine | Active, not recruiting |
| NCT00432094 | Phase II | Childhood + adult relapsed GCT | 2 AuSCT with non-cross-resistant conditioning regimens vs. 1 AuSCT only | 1-year OS | Active, not recruiting |
| NCT02582697 | Phase III | Metastatic GCT | Standard BEP vs. Accelerated BEP | 5-year PFS | Recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheung, A.; Shah, S.; Parker, J.; Soor, P.; Limbu, A.; Sheriff, M.; Boussios, S. Non-Epithelial Ovarian Cancers: How Much Do We Really Know? Int. J. Environ. Res. Public Health 2022, 19, 1106. https://doi.org/10.3390/ijerph19031106
Cheung A, Shah S, Parker J, Soor P, Limbu A, Sheriff M, Boussios S. Non-Epithelial Ovarian Cancers: How Much Do We Really Know? International Journal of Environmental Research and Public Health. 2022; 19(3):1106. https://doi.org/10.3390/ijerph19031106
Chicago/Turabian StyleCheung, Alison, Sidrah Shah, Jack Parker, Pavandeep Soor, Anu Limbu, Matin Sheriff, and Stergios Boussios. 2022. "Non-Epithelial Ovarian Cancers: How Much Do We Really Know?" International Journal of Environmental Research and Public Health 19, no. 3: 1106. https://doi.org/10.3390/ijerph19031106
APA StyleCheung, A., Shah, S., Parker, J., Soor, P., Limbu, A., Sheriff, M., & Boussios, S. (2022). Non-Epithelial Ovarian Cancers: How Much Do We Really Know? International Journal of Environmental Research and Public Health, 19(3), 1106. https://doi.org/10.3390/ijerph19031106









