Association between Handgrip Strength and Cognitive Function in Older Adults: Korean Longitudinal Study of Aging (2006–2018)
Abstract
1. Introduction
2. Methods
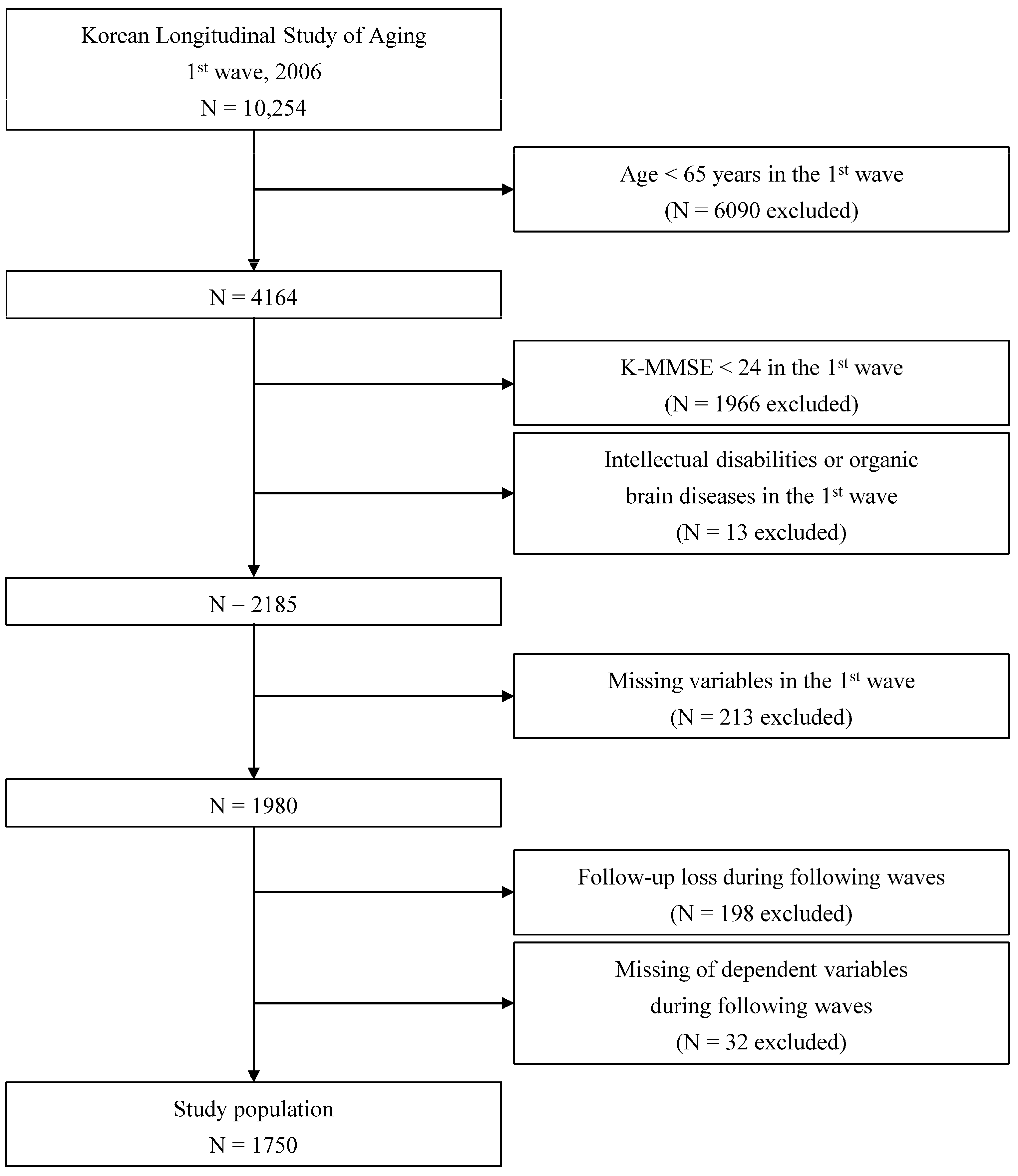
2.1. Survey Overview and Study Population
2.2. Assessment of Cognitive Function
2.3. Assessment of Handgrip Strength
2.4. Assessment of Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
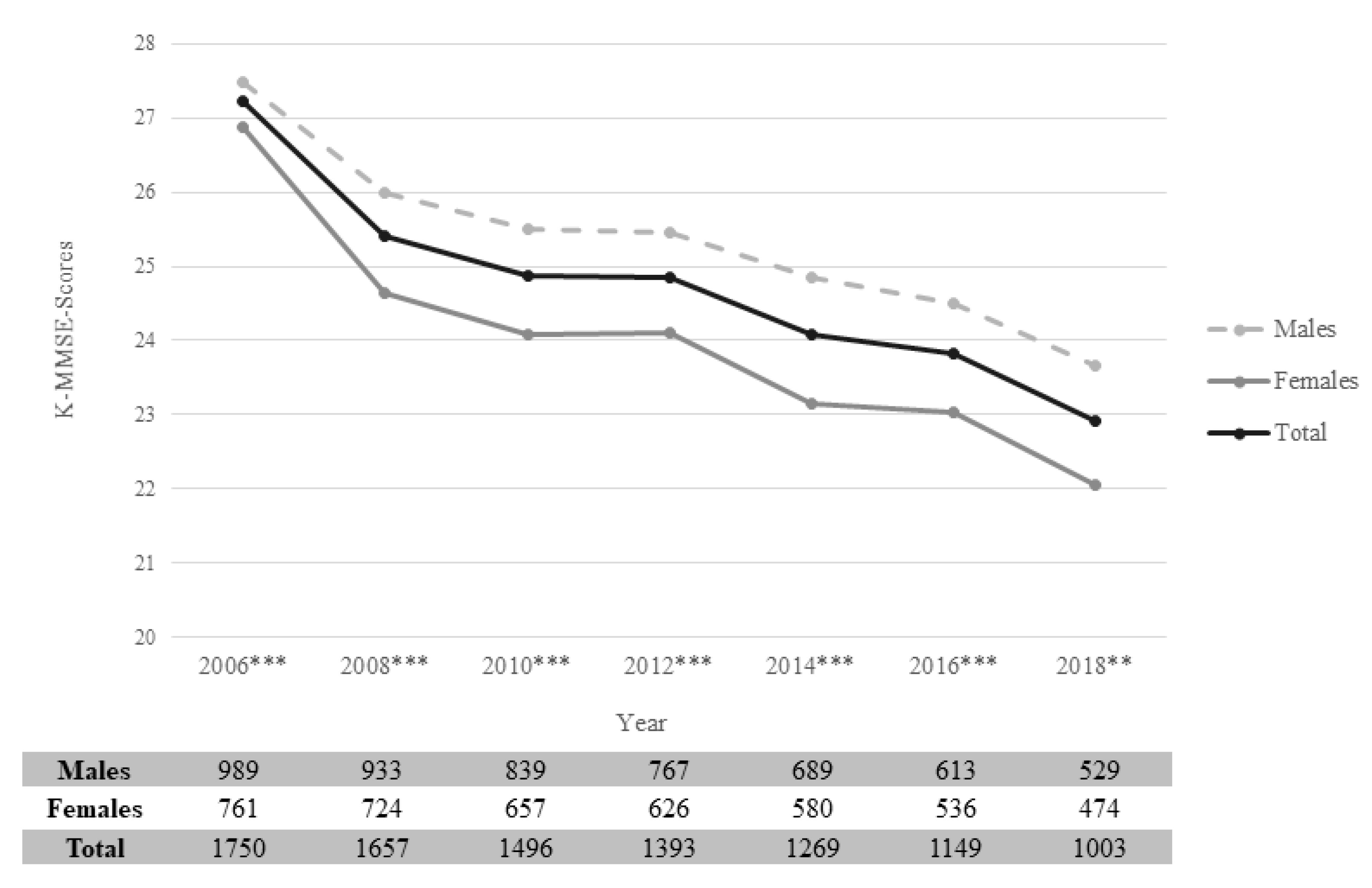
3.2. Cognitive Function
3.3. Association between Covariates and Cognitive Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prince, M.J.; Prina, M.; Guerchet, M. World Alzheimer Report 2013: Journey of Caring: An Analysis of Long-Term Care for Dementia; Alzheimer’s Disease International: London, United Kingdom, 2013. [Google Scholar]
- de Jager, C.A.; Budge, M.M.; Clarke, R. Utility of TICS-M for the assessment of cognitive function in older adults. Int. J. Geriatr. Psychiatry 2003, 18, 318–324. [Google Scholar] [CrossRef]
- Deary, I.J.; Corley, J.; Gow, A.J.; Harris, S.E.; Houlihan, L.M.; Marioni, R.E.; Penke, L.; Rafnsson, S.B.; Starr, J.M. Age-associated cognitive decline. Br. Med. Bull. 2009, 92, 135–152. [Google Scholar] [CrossRef]
- Prince, M.; Guerchet, M.; Prina, M. The Epidemiology and Impact of Dementia: Current State and Future Trends; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Organisation for Economic Co-operation and Development. Working Better with Age: Korea; OECD Publishing: Paris, France, 2018. [Google Scholar]
- Makimoto, K.; Kang, Y.; Kobayashi, S.; Liao, X.Y.; Panuthai, S.; Sung, H.C.; Suzuki, M.; Terada, S.; Yamakawa, M. Prevalence of behavioural and psychological symptoms of dementia in cognitively impaired elderly residents of long-term care facilities in East Asia: A cross-sectional study. Psychogeriatrics 2019, 19, 171–180. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare. Korean Dementia Observatory 2016; National Institute of Dementia: Seongnam, Korea, 2016. [Google Scholar]
- Rantanen, T.; Guralnik, J.M.; Foley, D.; Masaki, K.; Leveille, S.; Curb, J.D.; White, L. Midlife hand grip strength as a predictor of old age disability. JAMA 1999, 281, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Syddall, H.; Cooper, C.; Martin, F.; Briggs, R.; Aihie Sayer, A. Is grip strength a useful single marker of frailty? Age Ageing 2003, 32, 650–656. [Google Scholar] [CrossRef]
- Sasaki, H.; Kasagi, F.; Yamada, M.; Fujita, S. Grip strength predicts cause-specific mortality in middle-aged and elderly persons. Am. J. Med. 2007, 120, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Acha, A.; Snih, S.A.; Raji, M.A.; Kuo, Y.-F.; Markides, K.S.; Ottenbacher, K.J. Handgrip Strength and Cognitive Decline in Older Mexican Americans. J. Gerontol. Ser. A 2006, 61, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Wilson, R.S.; Boyle, P.A.; Bienias, J.L.; Bennett, D.A. Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology 2007, 29, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Praetorius Björk, M.; Johansson, B.; Hassing, L.B. I forgot when I lost my grip—Strong associations between cognition and grip strength in level of performance and change across time in relation to impending death. Neurobiol. Aging 2016, 38, 68–72. [Google Scholar] [CrossRef]
- Taekema, D.G.; Ling, C.H.Y.; Kurrle, S.E.; Cameron, I.D.; Meskers, C.G.M.; Blauw, G.J.; Westendorp, R.G.J.; de Craen, A.J.M.; Maier, A.B. Temporal relationship between handgrip strength and cognitive performance in oldest old people. Age Ageing 2012, 41, 506–512. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, J. Prospective Association of Handgrip Strength with Risk of New-Onset Cognitive Dysfunction in Korean Adults: A 6-Year National Cohort Study. Tohoku J. Exp. Med. 2018, 244, 83–91. [Google Scholar] [CrossRef]
- Kim, G.R.; Sun, J.; Han, M.; Nam, C.M.; Park, S. Evaluation of the directional relationship between handgrip strength and cognitive function: The Korean Longitudinal Study of Ageing. Age Ageing 2019, 48, 426–432. [Google Scholar] [CrossRef]
- Tothova, V.; Bartlova, S.; Dolak, F.; Kaas, J.; Kimmer, D.; Manhalova, J.; Martinek, L.; Olisarova, V. Quality of life in patients with chronic diseases. Neuro Endocrinol. Lett. 2014, 35 (Suppl. 1), 11–18. [Google Scholar]
- Laws, K.R.; Irvine, K.; Gale, T.M. Sex differences in cognitive impairment in Alzheimer’s disease. World J. Psychiatry 2016, 6, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-T.; Beiser, A.S.; Breteler, M.M.B.; Fratiglioni, L.; Helmer, C.; Hendrie, H.C.; Honda, H.; Ikram, M.A.; Langa, K.M.; Lobo, A.; et al. The changing prevalence and incidence of dementia over time—Current evidence. Nat. Rev. Neurol. 2017, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.T.; Iulita, M.F.; Cavedo, E.; Chiesa, P.A.; Schumacher Dimech, A.; Santuccione Chadha, A.; Baracchi, F.; Girouard, H.; Misoch, S.; Giacobini, E.; et al. Sex differences in Alzheimer disease—The gateway to precision medicine. Nat. Rev. Neurol. 2018, 14, 457–469. [Google Scholar] [CrossRef]
- Spruit, M.A.; Sillen, M.J.H.; Groenen, M.T.J.; Wouters, E.F.M.; Franssen, F.M.E. New Normative Values for Handgrip Strength: Results from the UK Biobank. J. Am. Med. Dir. Assoc. 2013, 14, 775.e5–775.e11. [Google Scholar] [CrossRef] [PubMed]
- Puh, U. Age-related and sex-related differences in hand and pinch grip strength in adults. Int. J. Rehabil. Res. 2010, 33, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Park, S.K.; Lee, D.R.; Lee, J. The Relationship between Handgrip Strength and Cognitive Function in Elderly Koreans over 8 Years: A Prospective Population-Based Study Using Korean Longitudinal Study of Ageing. Korean J. Fam. Med. 2018, 40, 9. [Google Scholar] [CrossRef]
- Kim, J.-H. Effect of grip strength on mental health. J. Affect. Disord. 2019, 245, 371–376. [Google Scholar] [CrossRef]
- Korea Labor Institute. User Guide for 2006 Korean Longitudinal Study of Ageing; Korea Labor Institute: Seoul, Korea, 2007. [Google Scholar]
- Kang, Y.; Na, D.L.; Hahn, S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J. Korean Neurol. Assoc. 1997, 15, 300–308. [Google Scholar]
- Son, D.H.; Yoo, J.W.; Cho, M.R.; Lee, Y.J. Relationship between handgrip strength and pulmonary function in apparently healthy older women. J. Am. Geriatr. Soc. 2018, 66, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Andresen, E.M.; Malmgren, J.A.; Carter, W.B.; Patrick, D.L. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am. J. Prev. Med. 1994, 10, 77–84. [Google Scholar] [CrossRef]
- Ko, K.D.; Cho, Y.T.; Cho, S.I.; Sung, J.H.; Cho, B.L.; Son, K.Y.; Choi, H.C. Association of health risk behaviors with mental health among elderly Koreans. Ann. Geriatr. Med. Res. 2012, 16, 66–73. [Google Scholar]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157. [Google Scholar] [CrossRef]
- Fritz, N.E.; McCarthy, C.J.; Adamo, D.E. Handgrip strength as a means of monitoring progression of cognitive decline—A scoping review. Ageing Res. Rev. 2017, 35, 112–123. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Gu, N.; Yang, B.; Wang, J.; Li, C. A prospective study on the association between grip strength and cognitive function among middle-aged and elderly Chinese participants. Front. Aging Neurosci. 2019, 11, 250. [Google Scholar] [CrossRef]
- Horvat, P.; Kubinova, R.; Pajak, A.; Tamosiunas, A.; Schottker, B.; Pikhart, H.; Peasey, A.; Kozela, M.; Jansen, E.; Singh-Manoux, A.; et al. Blood-Based Oxidative Stress Markers and Cognitive Performance in Early Old Age: The HAPIEE Study. Dement. Geriatr. Cogn. Disord. 2016, 42, 297–309. [Google Scholar] [CrossRef]
- Scicchitano, B.M.; Pelosi, L.; Sica, G.; Musaro, A. The physiopathologic role of oxidative stress in skeletal muscle. Mech. Ageing Dev. 2018, 170, 37–44. [Google Scholar] [CrossRef]
- Cunningham, C.; Hennessy, E. Co-morbidity and systemic inflammation as drivers of cognitive decline: New experimental models adopting a broader paradigm in dementia research. Alzheimers Res. Ther. 2015, 7, 33. [Google Scholar] [CrossRef]
- Jo, E.; Lee, S.-R.; Park, B.-S.; Kim, J.-S. Potential mechanisms underlying the role of chronic inflammation in age-related muscle wasting. Aging Clin. Exp. Res. 2012, 24, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, H.H.; Rapp, S.R.; Williamson, J.D.; Lovato, J.; Absher, J.R.; Gass, M.; Henderson, V.W.; Johnson, K.C.; Kostis, J.B.; Sink, K.M.; et al. The relationship between cognitive function and physical performance in older women: Results from the women’s health initiative memory study. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 300–306. [Google Scholar] [CrossRef]
- Erickson, K.I.; Weinstein, A.M.; Lopez, O.L. Physical activity, brain plasticity, and Alzheimer’s disease. Arch. Med. Res. 2012, 43, 615–621. [Google Scholar] [CrossRef]
- Dye, R.V.; Miller, K.J.; Singer, E.J.; Levine, A.J. Hormone replacement therapy and risk for neurodegenerative diseases. Int. J. Alzheimer’s Disease 2012, 2012, 258454. [Google Scholar] [CrossRef]
- Okamoto, S.; Kobayashi, E.; Murayama, H.; Liang, J.; Fukaya, T.; Shinkai, S. Decomposition of gender differences in cognitive functioning: National Survey of the Japanese elderly. BMC Geriatr. 2021, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Hwang, E. Gender differences in the cognitive function and nutritional status in older age: A representative nationwide data of Korean elders. J. Korean Public Health Nur. 2017, 31, 209–219. [Google Scholar]
- Zhang, Z. Gender Differentials in Cognitive Impairment and Decline of the Oldest Old in China. J. Gerontol. Ser. B 2006, 61, S107–S115. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.-M. Factors affecting cognitive function according to gender in community-dwelling elderly individuals. Epidemiol. Health 2017, 39, e2017054. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Boyle, P.A.; Yu, L.; Shah, R.C.; Wilson, R.S.; Bennett, D.A. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 2012, 78, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Sattler, C.; Erickson, K.I.; Toro, P.; Schroder, J. Physical fitness as a protective factor for cognitive impairment in a prospective population-based study in Germany. J. Alzheimers Dis. 2011, 26, 709–718. [Google Scholar] [CrossRef]
- Byers, A.L.; Yaffe, K. Depression and risk of developing dementia. Nat. Rev. Neurol. 2011, 7, 323. [Google Scholar] [CrossRef]
- Kaup, A.R.; Byers, A.L.; Falvey, C.; Simonsick, E.M.; Satterfield, S.; Ayonayon, H.N.; Smagula, S.F.; Rubin, S.M.; Yaffe, K. Trajectories of Depressive Symptoms in Older Adults and Risk of Dementia Trajectories of Depressive Symptoms in Older Adults and Risk of Dementia Trajectories of Depressive Symptoms in Older Adults and Risk of Dementia. JAMA Psychiatry 2016, 73, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Skoog, I.; Lernfelt, B.; Landahl, S.; Palmertz, B.; Andreasson, L.A.; Nilsson, L.; Persson, G.; Oden, A.; Svanborg, A. 15-year longitudinal study of blood pressure and dementia. Lancet 1996, 347, 1141–1145. [Google Scholar] [CrossRef]
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, X.; Shi, X.; Zheng, Z.; Zhang, A.; Guo, J.; Fang, Y. Chronic Obstructive Pulmonary Disease as a Risk Factor for Cognitive Dysfunction: A Meta-Analysis of Current Studies. J. Alzheimers Dis. 2016, 52, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M.; Janelsins, M.C.; van Wijngaarden, E. Cognitive function in cancer survivors: Analysis of the 1999–2002 National Health and Nutrition Examination Survey. Support Care Cancer 2016, 24, 2155–2162. [Google Scholar] [CrossRef][Green Version]
- Cronk, B.B.; Johnson, D.K.; Burns, J.M.; Alzheimer’s Disease Neuroimaging Initiative. Body mass index and cognitive decline in mild cognitive impairment. Alzheimer Dis. Assoc. Disord. 2010, 24, 126. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, J.; Zhang, N.; Fu, P.; Jing, Z.; Yu, C.; Zhao, D.; Hao, W.; Zhou, C. Body mass index and mild cognitive impairment among rural older adults in China: The moderating roles of gender and age. BMC Psychiatry 2021, 21, 1–11. [Google Scholar] [CrossRef]
- van der Kooi, A.L.; Snijder, M.B.; Peters, R.J.; van Valkengoed, I.G. The Association of Handgrip Strength and Type 2 Diabetes Mellitus in Six Ethnic Groups: An Analysis of the HELIUS Study. PLoS ONE 2015, 10, e0137739. [Google Scholar] [CrossRef]
| Variables | Males (N = 989) | Females (N = 761) | p | ||||
|---|---|---|---|---|---|---|---|
| Mean | SE | 95% CI | Mean | SE | 95% CI | ||
| Handgrip strength | 29.38 | 0.18 | 29.02–29.74 | 18.09 | 0.15 | 17.81–18.38 | <0.001 |
| K-MMSE | 27.49 | 0.06 | 27.37–27.61 | 26.87 | 0.07 | 26.73–27.01 | <0.001 |
| Variables | Males | Females | p | |
|---|---|---|---|---|
| Handgrip strength | Handgrip 1 (strongest) | 249 (25.2) | 202 (26.5) | 0.176 |
| Handgrip 2 | 271 (27.4) | 181 (23.8) | ||
| Handgrip 3 | 224 (22.6) | 199 (26.2) | ||
| Handgrip 4 (weakest) | 245 (24.8) | 179 (23.5) | ||
| Age (years) | 65–69 | 466 (47.1) | 220 (51.4) | 0.079 |
| 70–74 | 297 (30.0) | 220 (28.9) | ||
| 75–79 | 161 (16.3) | 119 (15.6) | ||
| ≥80 | 65 (6.6) | 31 (4.1) | ||
| Educational attainment | ≤Elementary school | 435 (44.0) | 565 (74.3) | <0.001 |
| Middle school | 157 (15.9) | 86 (11.3) | ||
| High school | 263 (26.6) | 93 (12.2) | ||
| ≥College | 134 (13.5) | 17 (2.2) | ||
| Economic activity | Employed | 343 (34.7) | 91 (12.0) | <0.001 |
| Unemployed | 646 (65.3) | 670 (88.0) | ||
| Equalized household income | Quartile 1: low | 276 (27.9) | 256 (33.6) | 0.039 |
| Quartile 2 | 234 (23.6) | 169 (22.2) | ||
| Quartile 3 | 233 (23.6) | 148 (19.5) | ||
| Quartile 4: high | 246 (24.9) | 188 (24.7) | ||
| Marital status | Married | 910 (92.0) | 423 (55.6) | <0.001 |
| Unmarried (single, divorced, widowed) | 79 (8.0) | 338 (44.4) | ||
| Residential area | Urban | 623 (63.0) | 519 (68.2) | 0.023 |
| Rural | 366 (37.0) | 242 (31.8) | ||
| Alcohol consumption | Never | 284 (28.7) | 656 (86.2) | <0.001 |
| Former drinker | 165 (16.7) | 17 (2.2) | ||
| Current drinker | 540 (54.6) | 88 (11.6) | ||
| Smoking | Never | 420 (42.5) | 729 (95.8) | <0.001 |
| Former smoker | 261 (26.4) | 6 (0.8) | ||
| Current smoker | 308 (31.1) | 26 (3.4) | ||
| Physical activity | Active | 440 (44.5) | 290 (38.1) | 0.007 |
| Inactive | 549 (55.5) | 471 (61.9) | ||
| Chronic diseases | No | 501 (50.7) | 359 (47.2) | 0.037 |
| With one chronic disease | 356 (36.0) | 267 (35.1) | ||
| With two or more chronic diseases | 1323 (13.3) | 135 (17.7) | ||
| Depression | No | 935 (94.5) | 687 (90.3) | <0.001 |
| Yes | 54 (5.5) | 74 (9.7) | ||
| Body mass index (BMI) | Underweight | 44 (4.5) | 36 (4.7) | <0.001 |
| Normal weight | 474 (47.9) | 301 (39.6) | ||
| Overweight | 285 (28.8) | 224 (29.4) | ||
| Obesity | (18.8) | 200 (26.3) | ||
| Variables | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted † | Adjusted ‡ | Unadjusted † | Adjusted ‡ | |||||
| β | SE | β | SE | β | SE | β | SE | |
| Handgrip strength | ||||||||
| Group 1 (strongest) | Ref | Ref | Ref | Ref | ||||
| Group 2 | −0.35 * | 0.15 | −0.21 | 0.15 | −0.32 | 0.19 | −0.20 | 0.18 |
| Group 3 | −0.51 ** | 0.16 | −0.28 | 0.16 | −0.79 *** | 0.19 | −0.65 *** | 0.19 |
| Group 4 (weakest) | −1.04 *** | 0.16 | −0.54 *** | 0.16 | −0.78 *** | 0.19 | −0.53 ** | 0.19 |
| Age (years) | ||||||||
| 65–69 | Ref. | Ref. | ||||||
| 70–74 | −0.01 | 0.11 | 0.10 | 0.13 | ||||
| 75–79 | −0.11 | 0.14 | 0.03 | 0.17 | ||||
| ≥80 | −0.63 *** | 0.19 | −0.68 ** | 0.25 | ||||
| Survey year (wave) | ||||||||
| 1st: 2006 | Ref. | Ref. | ||||||
| 2nd: 2008 | −1.35 *** | 0.12 | −2.06 *** | 0.16 | ||||
| 3rd: 2010 | −1.69 *** | 0.15 | −2.50 *** | 0.20 | ||||
| 4th: 2012 | −1.84 *** | 0.17 | −2.52 *** | 0.22 | ||||
| 5th: 2014 | −2.41 *** | 0.20 | −3.54 *** | 0.24 | ||||
| 6th: 2016 | −2.86 *** | 0.23 | −3.72 *** | 0.27 | ||||
| 7th: 2018 | −3.89 *** | 0.28 | −4.63 *** | 0.31 | ||||
| Educational attainment | ||||||||
| ≤Elementary school | Ref. | Ref. | ||||||
| Middle school | 0.40 * | 0.16 | 0.54 * | 0.21 | ||||
| High school | 0.78 *** | 0.14 | 0.92 *** | 0.21 | ||||
| ≥College | 1.02 *** | 0.18 | 1.62 *** | 0.45 | ||||
| Economic activity | ||||||||
| Unemployed | Ref. | Ref. | ||||||
| Employed | 0.49 *** | 0.11 | 0.26 | 0.17 | ||||
| Equalized household income | ||||||||
| Quartile 1: low | Ref. | Ref. | ||||||
| Quartile 2 | 0.20 | 0.12 | 0.09 | 0.14 | ||||
| Quartile 3 | 0.34 ** | 0.13 | 0.09 | 0.15 | ||||
| Quartile 4: high | 0.03 | 0.13 | −0.09 | 0.15 | ||||
| Marital status | ||||||||
| Married | Ref. | Ref. | ||||||
| Unmarried (single, divorced, widowed) | 0.28 | 0.18 | −0.07 | 0.13 | ||||
| Residential area | ||||||||
| Urban | Ref. | Ref. | ||||||
| Rural | −0.41 *** | 0.12 | −0.58 *** | 0.14 | ||||
| Alcohol consumption | ||||||||
| Never | Ref. | Ref. | ||||||
| Former drinker | −0.34 * | 0.15 | −0.68 * | 0.34 | ||||
| Current drinker | −0.03 | 0.12 | −0.05 | 0.20 | ||||
| Smoking | ||||||||
| Never | Ref. | Ref. | ||||||
| Former smoker | −0.16 | 0.13 | −0.24 | 0.57 | ||||
| Current smoker | 0.07 | 0.13 | 0.55 | 0.36 | ||||
| Physical activity | ||||||||
| Inactive | Ref. | Ref. | ||||||
| Active | 0.35 *** | 0.09 | 0.48 *** | 0.11 | ||||
| Chronic diseases | ||||||||
| No | Ref. | Ref. | ||||||
| with one chronic disease | −0.03 | 0.11 | 0.03 | 0.14 | ||||
| with two or more chronic diseases | −0.36 * | 0.15 | −0.07 | 0.17 | ||||
| Depressive symptoms | ||||||||
| No | Ref. | Ref. | ||||||
| Yes | −1.48 *** | 0.15 | −1.01 *** | 0.15 | ||||
| Body mass index (BMI) | ||||||||
| Underweight | 0.19 | 0.20 | −1.01 *** | 0.24 | ||||
| Normal weight | Ref. | Ref. | ||||||
| Overweight | 0.28 ** | 0.11 | 0.27 * | 0.13 | ||||
| Obesity | 0.01 | 0.14 | 0.24 | 0.15 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Oh, J.W.; Son, N.-H.; Chung, W. Association between Handgrip Strength and Cognitive Function in Older Adults: Korean Longitudinal Study of Aging (2006–2018). Int. J. Environ. Res. Public Health 2022, 19, 1048. https://doi.org/10.3390/ijerph19031048
Lee S, Oh JW, Son N-H, Chung W. Association between Handgrip Strength and Cognitive Function in Older Adults: Korean Longitudinal Study of Aging (2006–2018). International Journal of Environmental Research and Public Health. 2022; 19(3):1048. https://doi.org/10.3390/ijerph19031048
Chicago/Turabian StyleLee, San, Jae Won Oh, Nak-Hoon Son, and Woojin Chung. 2022. "Association between Handgrip Strength and Cognitive Function in Older Adults: Korean Longitudinal Study of Aging (2006–2018)" International Journal of Environmental Research and Public Health 19, no. 3: 1048. https://doi.org/10.3390/ijerph19031048
APA StyleLee, S., Oh, J. W., Son, N.-H., & Chung, W. (2022). Association between Handgrip Strength and Cognitive Function in Older Adults: Korean Longitudinal Study of Aging (2006–2018). International Journal of Environmental Research and Public Health, 19(3), 1048. https://doi.org/10.3390/ijerph19031048






