WORKbiota: A Systematic Review about the Effects of Occupational Exposure on Microbiota and Workers’ Health
Abstract
1. Introduction
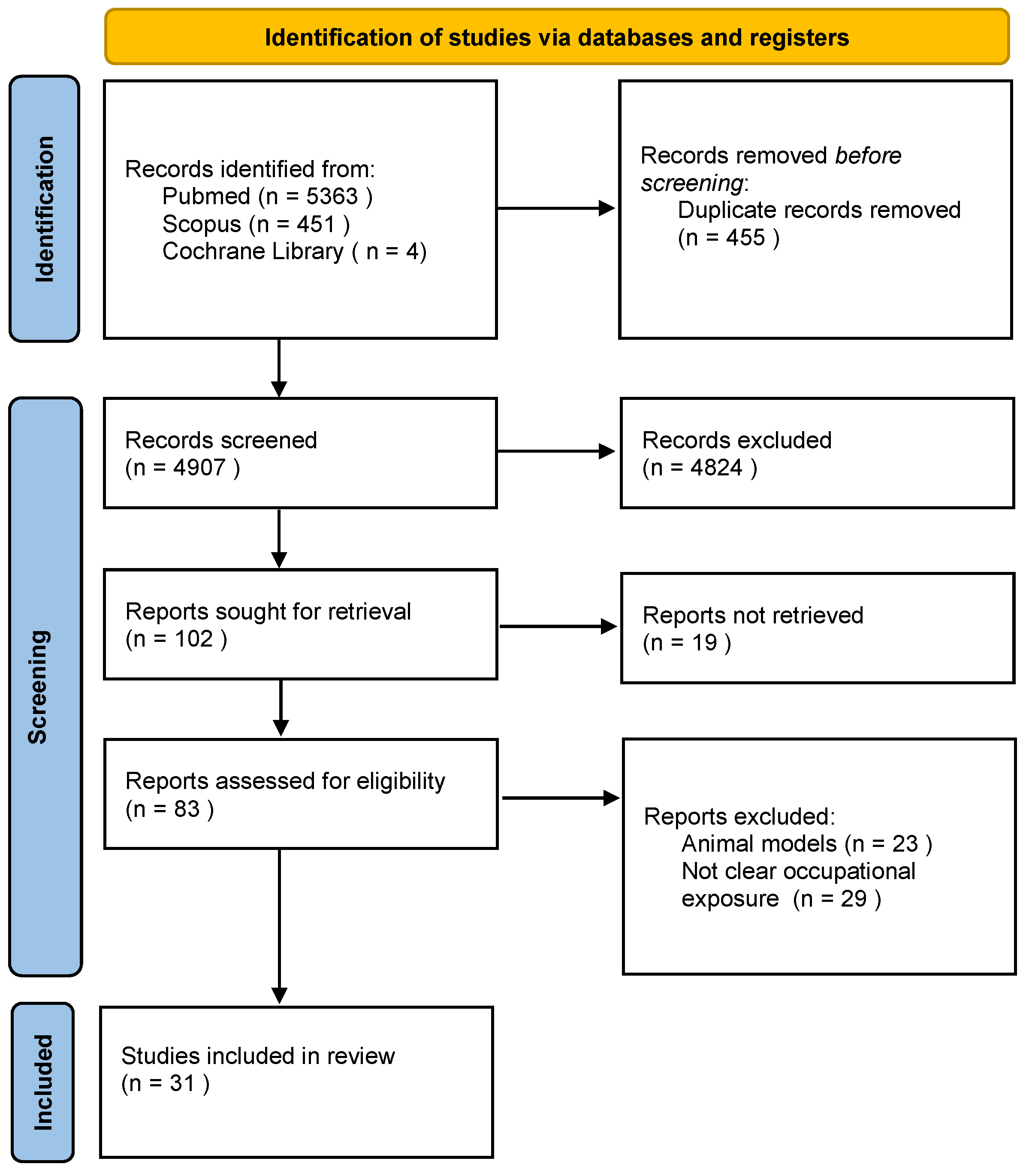
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility, Inclusion, and Exclusion Criteria
- Analysis of the baseline composition of the workers’ microbiota.
- Evaluation of comparisons between the microbiota of exposed workers and subjects not exposed to a particular occupational environment (animals included).
- Description of short- and long-term effects on the human microbiota due to occupational exposure.
- Studies conducted exclusively on animal models.
- Studies involving analysis of the microbiota in individuals not exposed to occupational risk factors or whose occupational exposure was not described in the preliminary recruitment phase of study participants.
2.3. Quality Assessment and Risk of Bias Assessment
3. Results
3.1. Original Articles
3.1.1. Tools for Microbiota Sampling and Analysis
3.1.2. Occupational Exposure and Workers’ Categories
3.1.3. Works Involving Contact with Animals
3.1.4. Healthcare Workers
3.1.5. Metalworking Fluid Workers
3.1.6. Workers Exposed to Dust
3.1.7. Workers Exposed to Pesticides
3.1.8. Shift Workers
3.1.9. Military Personnel
3.1.10. Sailors
3.1.11. Tunnel Workers
3.1.12. Diving Sub-Sea Workers
3.2. Reviews
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Neish, A.S.; Jones, R.M. Redox Signaling Mediates Symbiosis between the Gut Microbiota and the Intestine. Gut Microbes 2014, 5, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Biodiversity and Functional Genomics in the Human Microbiome. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3534939/ (accessed on 22 October 2021).
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Salzano, F.A.; Marino, L.; Salzano, G.; Botta, R.M.; Cascone, G.; D’Agostino Fiorenza, U.; Selleri, C.; Casolaro, V. Microbiota Composition and the Integration of Exogenous and Endogenous Signals in Reactive Nasal Inflammation. J. Immunol. Res. 2018, 2018, 2724951. [Google Scholar] [CrossRef]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin Microbiota–Host Interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef]
- Hevia, A.; Milani, C.; López, P.; Cuervo, A.; Arboleya, S.; Duranti, S.; Turroni, F.; González, S.; Suárez, A.; Gueimonde, M.; et al. Intestinal Dysbiosis Associated with Systemic Lupus Erythematosus. mBio 2014, 5, e01548-14. [Google Scholar] [CrossRef]
- Mu, Q.; Zhang, H.; Liao, X.; Lin, K.; Liu, H.; Edwards, M.R.; Ahmed, S.A.; Yuan, R.; Li, L.; Cecere, T.E.; et al. Control of Lupus Nephritis by Changes of Gut Microbiota. Microbiome 2017, 5, 73. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Siljander, H.; Vatanen, T.; Hyötyläinen, T.; Hämäläinen, A.-M.; Peet, A.; Tillmann, V.; Pöhö, P.; Mattila, I.; et al. The Dynamics of the Human Infant Gut Microbiome in Development and in Progression towards Type 1 Diabetes. Cell Host Microbe 2015, 17, 260–273. [Google Scholar] [CrossRef]
- Lynch, S.V.; Wood, R.A.; Boushey, H.; Bacharier, L.B.; Bloomberg, G.R.; Kattan, M.; O’Connor, G.T.; Sandel, M.T.; Calatroni, A.; Matsui, E.; et al. Effects of Early-Life Exposure to Allergens and Bacteria on Recurrent Wheeze and Atopy in Urban Children. J. Allergy Clin. Immunol. 2014, 134, 593–601.e12. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.-C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early Infancy Microbial and Metabolic Alterations Affect Risk of Childhood Asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the Intestinal Microbiome in Inflammatory Bowel Disease and Treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef] [PubMed]
- Savin, Z.; Kivity, S.; Yonath, H.; Yehuda, S. Smoking and the Intestinal Microbiome. Arch. Microbiol. 2018, 200, 677–684. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.-P. The Firmicutes/Bacteroidetes Ratio of the Human Microbiota Changes with Age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Duncan, S.H.; Lobley, G.E.; Holtrop, G.; Ince, J.; Johnstone, A.M.; Louis, P.; Flint, H.J. Human Colonic Microbiota Associated with Diet, Obesity and Weight Loss. Int. J. Obes. 2005 2008, 32, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations: Cell. Available online: https://www.cell.com/fulltext/S0092-8674(16)31524-0 (accessed on 22 October 2021).
- Rhythmicity of the Intestinal Microbiota Is Regulated by Gender and the Host Circadian Clock|PNAS. Available online: https://www.pnas.org/content/112/33/10479 (accessed on 23 October 2021).
- Kondo, T. A Cyanobacterial Circadian Clock Based on the Kai Oscillator. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 47–55. [Google Scholar] [CrossRef][Green Version]
- Bacterial Bioluminescence Regulates Expression of a Host Cryptochrome Gene in the Squid-Vibrio Symbiosis | MBio. Available online: https://journals.asm.org/doi/10.1128/mBio.00167-13 (accessed on 23 October 2021).
- Paulose, J.K.; Wright, J.M.; Patel, A.G.; Cassone, V.M. Human Gut Bacteria Are Sensitive to Melatonin and Express Endogenous Circadian Rhythmicity. PLoS ONE 2016, 11, e0146643. [Google Scholar] [CrossRef]
- Murakami, M.; Tognini, P.; Liu, Y.; Eckel-Mahan, K.L.; Baldi, P.; Sassone-Corsi, P. Gut Microbiota Directs PPARγ-Driven Reprogramming of the Liver Circadian Clock by Nutritional Challenge. EMBO Rep. 2016, 17, 1292–1303. [Google Scholar] [CrossRef]
- Wang, Y.; Kuang, Z.; Yu, X.; Ruhn, K.A.; Kubo, M.; Hooper, L.V. The Intestinal Microbiota Regulates Body Composition through NFIL3 and the Circadian Clock. Science 2017, 357, 912–916. [Google Scholar] [CrossRef]
- Knutsson, A.; Bøggild, H. Gastrointestinal Disorders among Shift Workers. Scand. J. Work. Environ. Health 2010, 36, 85–95. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Bailey, M.T. Influence of Stressor-Induced Nervous System Activation on the Intestinal Microbiota and the Importance for Immunomodulation. Adv. Exp. Med. Biol. 2014, 817, 255–276. [Google Scholar] [CrossRef] [PubMed]
- Galley, J.D.; Nelson, M.C.; Yu, Z.; Dowd, S.E.; Walter, J.; Kumar, P.S.; Lyte, M.; Bailey, M.T. Exposure to a Social Stressor Disrupts the Community Structure of the Colonic Mucosa-Associated Microbiota. BMC Microbiol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Golubeva, A.V.; Crampton, S.; Desbonnet, L.; Edge, D.; O’Sullivan, O.; Lomasney, K.W.; Zhdanov, A.V.; Crispie, F.; Moloney, R.D.; Borre, Y.E.; et al. Prenatal Stress-Induced Alterations in Major Physiological Systems Correlate with Gut Microbiota Composition in Adulthood. Psychoneuroendocrinology 2015, 60, 58–74. [Google Scholar] [CrossRef]
- Microbiota and Host Determinants of Behavioural Phenotype in Maternally Separated Mice|Nature Communications. Available online: https://www.nature.com/articles/ncomms8735 (accessed on 22 October 2021).
- National Academies of Sciences, Engineering, and Medicine; Division on Earth and Life Studies; Board on Life Sciences; Board on Environmental Studies and Toxicology; Committee on Advancing Understanding of the Implications of Environmental-Chemical Interactions with the Human Microbiome. Environmental Chemicals, the Human Microbiome, and Health Risk: A Research Strategy; National Academies Press (US): Washington, DC, USA, 2017; ISBN 978-0-309-46869-5. [Google Scholar]
- Liebers, V.; Raulf-Heimsoth, M.; Brüning, T. Health Effects Due to Endotoxin Inhalation (Review). Arch. Toxicol. 2008, 82, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Olenchock, S.A.; Christiani, D.C.; Mull, J.C.; Ye, T.T.; Lu, P.L. Endotoxins in Baled Cottons and Airborne Dusts in Textile Mills in the People’s Republic of China. Appl. Environ. Microbiol. 1983, 46, 817–820. [Google Scholar] [CrossRef]
- Schierl, R.; Egger, U.; Schneider, F.; Eichelser, R.; Neser, S.; Nowak, D. Endotoxin Concentration in Modern Animal Houses in Southern Bavaria. Ann. Agric. Environ. Med. 2007, 14, 129–136. [Google Scholar]
- La Torre, G.; Esposito, A.; Sciarra, I.; Chiappetta, M. Definition, Symptoms and Risk of Techno-Stress: A Systematic Review. Int. Arch. Occup. Environ. Health 2019, 92, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Ottawa Hospital Research Institute. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 23 October 2021).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ahmed, N.; Mahmoud, N.F.; Solyman, S.; Hanora, A. Human Nasal Microbiome as Characterized by Metagenomics Differs Markedly Between Rural and Industrial Communities in Egypt. Omics J. Integr. Biol. 2019, 23, 573–582. [Google Scholar] [CrossRef]
- Grant, E.T.; Kyes, R.C.; Kyes, P.; Trinh, P.; Ramirez, V.; Tanee, T.; Pinlaor, P.; Dangtakot, R.; Rabinowitz, P.M. Fecal Microbiota Dysbiosis in Macaques and Humans within a Shared Environment. PLoS ONE 2019, 14, e0210679. [Google Scholar] [CrossRef]
- Hang, J.; Zavaljevski, N.; Yang, Y.; Desai, V.; Ruck, R.C.; Macareo, L.R.; Jarman, R.G.; Reifman, J.; Kuschner, R.A.; Keiser, P.B. Composition and Variation of Respiratory Microbiota in Healthy Military Personnel. PLoS ONE 2017, 12, e0188461. [Google Scholar] [CrossRef]
- Islam, M.Z.; Johannesen, T.B.; Lilje, B.; Urth, T.R.; Larsen, A.R.; Angen, Ø.; Larsen, J. Investigation of the Human Nasal Microbiome in Persons with Long- and Short-Term Exposure to Methicillin-Resistant Staphylococcus Aureus and Other Bacteria from the Pig Farm Environment. PLoS ONE 2020, 15, e0232456. [Google Scholar] [CrossRef]
- Kates, A.E.; Dalman, M.; Torner, J.C.; Smith, T.C. The Nasal and Oropharyngeal Microbiomes of Healthy Livestock Workers. PLoS ONE 2019, 14, e0212949. [Google Scholar] [CrossRef]
- Khan, M.F.; Wang, H. Environmental Exposures and Autoimmune Diseases: Contribution of Gut Microbiome. Front. Immunol. 2020, 10, 3094. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, J.G.; Aebi, S.; Oppliger, A.; Hilty, M. The Indoor-Air Microbiota of Pig Farms Drives the Composition of the Pig Farmers’ Nasal Microbiota in a Season-Dependent and Farm-Specific Manner. Appl. Environ. Microbiol. 2019, 85, e03038-18. [Google Scholar] [CrossRef]
- Lai, P.S.; Allen, J.G.; Hutchinson, D.S.; Ajami, N.J.; Petrosino, J.F.; Winters, T.; Hug, C.; Wartenberg, G.R.; Vallarino, J.; Christiani, D.C. Impact of Environmental Microbiota on Human Microbiota of Workers in Academic Mouse Research Facilities: An Observational Study. PLoS ONE 2017, 12, e0180969. [Google Scholar] [CrossRef]
- Lai, P.S.; Christiani, D.C. Impact of Occupational Exposure on Human Microbiota. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.-H.; Liu, Y.-W.; Ji, Z.-H.; Fu, T.; Yan, M.; Shao, Z.-J.; Long, Y. Alterations in the Intestinal Microbiome and Mental Health Status of Workers in an Underground Tunnel Environment. BMC Microbiol. 2021, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Mbareche, H.; Veillette, M.; Pilote, J.; Létourneau, V.; Duchaine, C. Bioaerosols Play a Major Role in the Nasopharyngeal Microbiota Content in Agricultural Environment. Int. J. Environ. Res. Public Health 2019, 16, 1375. [Google Scholar] [CrossRef] [PubMed]
- Mortaş, H.; Bilici, S.; Karakan, T. The Circadian Disruption of Night Work Alters Gut Microbiota Consistent with Elevated Risk for Future Metabolic and Gastrointestinal Pathology. Chronobiol. Int. 2020, 37, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Biswas, D. Environmental Influences of High-Density Agricultural Animal Operation on Human Forearm Skin Microflora. Microorganisms 2020, 8, 1481. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.C.; Paterson, J.L.; Ferguson, S.A.; Stanley, D.; Wright, K.P.; Dawson, D. The Shift Work and Health Research Agenda: Considering Changes in Gut Microbiota as a Pathway Linking Shift Work, Sleep Loss and Circadian Misalignment, and Metabolic Disease. Sleep Med. Rev. 2017, 34, 3–9. [Google Scholar] [CrossRef]
- Reynolds, A.C.; Broussard, J.; Paterson, J.L.; Wright, K.P.; Ferguson, S.A. Sleepy, Circadian Disrupted and Sick: Could Intestinal Microbiota Play an Important Role in Shift Worker Health? Mol. Metab. 2016, 6, 12–13. [Google Scholar] [CrossRef]
- Rocha, L.A.; Ferreira de Almeida e Borges, L.; Gontijo Filho, P.P. Changes in Hands Microbiota Associated with Skin Damage Because of Hand Hygiene Procedures on the Health Care Workers. Am. J. Infect. Control 2009, 37, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, M.; Aiello, A.; Larson, E.; Chenoweth, C.; Foxman, B. Healthcare Workers’ Hand Microbiome May Mediate Carriage of Hospital Pathogens. Pathogens 2013, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Ye, Z.; Sandberg, S.; Reyes, I.; Fritsche, T.R.; Keifer, M. The Nasal Microbiota of Dairy Farmers Is More Complex than Oral Microbiota, Reflects Occupational Exposure, and Provides Competition for Staphylococci. PLoS ONE 2017, 12, e0183898. [Google Scholar] [CrossRef]
- Stanaway, I.B.; Wallace, J.C.; Shojaie, A.; Griffith, W.C.; Hong, S.; Wilder, C.S.; Green, F.H.; Tsai, J.; Knight, M.; Workman, T.; et al. Human Oral Buccal Microbiomes Are Associated with Farmworker Status and Azinphos-Methyl Agricultural Pesticide Exposure. Appl. Environ. Microbiol. 2017, 83, e02149-16. [Google Scholar] [CrossRef]
- Sun, J.; Huang, T.; Chen, C.; Cao, T.-T.; Cheng, K.; Liao, X.-P.; Liu, Y.-H. Comparison of Fecal Microbial Composition and Antibiotic Resistance Genes from Swine, Farm Workers and the Surrounding Villagers. Sci. Rep. 2017, 7, 4965. [Google Scholar] [CrossRef]
- Environmental Remodeling of Human Gut Microbiota and Antibiotic Resistome in Livestock Farms|Nature Communications. Available online: https://www.nature.com/articles/s41467-020-15222-y (accessed on 22 October 2021).
- Swanson, G.R.; Siskin, J.; Gorenz, A.; Shaikh, M.; Raeisi, S.; Fogg, L.; Forsyth, C.; Keshavarzian, A. Disrupted Diurnal Oscillation of Gut-Derived Short Chain Fatty Acids in Shift Workers Drinking Alcohol: Possible Mechanism for Loss of Resiliency of Intestinal Barrier in Disrupted Circadian Host. Transl. Res. J. Lab. Clin. Med. 2020, 221, 97–109. [Google Scholar] [CrossRef]
- Tan, S.C.; Chong, C.W.; Yap, I.K.S.; Thong, K.L.; Teh, C.S.J. Comparative Assessment of Faecal Microbial Composition and Metabonome of Swine, Farmers and Human Control. Sci. Rep. 2020, 10, 8997. [Google Scholar] [CrossRef]
- Walters, W.A.; Reyes, F.; Soto, G.M.; Reynolds, N.D.; Fraser, J.A.; Aviles, R.; Tribble, D.R.; Irvin, A.P.; Kelley-Loughnane, N.; Gutierrez, R.L.; et al. Epidemiology and Associated Microbiota Changes in Deployed Military Personnel at High Risk of Traveler’s Diarrhea. PLoS ONE 2020, 15, e0236703. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.G.; Kapoor, B.; Cummings, K.J.; Stanton, M.L.; Nett, R.J.; Kreiss, K.; Abraham, J.L.; Colby, T.V.; Franko, A.D.; Green, F.H.Y.; et al. Evidence for Environmental-Human Microbiota Transfer at a Manufacturing Facility with Novel Work-Related Respiratory Disease. Am. J. Respir. Crit. Care Med. 2020, 202, 1678–1688. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Zhu, Y.-S.; Guo, C.; Xia, Y.; Guo, Z.-M.; Li, Q.-L.; Lu, J.-H. A Comparative Study of Associated Microbiota Between Pig Farm and Pig Slaughterhouse in Guangdong, China. Curr. Microbiol. 2020, 77, 3310–3320. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Pan, Z.; Jin, C.; Ni, Y.; Fu, Z.; Jin, Y. Gut Microbiota: An Underestimated and Unintended Recipient for Pesticide-Induced Toxicity. Chemosphere 2019, 227, 425–434. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Jin, H.; Lv, R.; Shi, H.; De, G.; Yang, B.; Sun, Z.; Zhang, H. Probiotics Maintain the Intestinal Microbiome Homeostasis of the Sailors during a Long Sea Voyage. Gut Microbes 2020, 11, 930–943. [Google Scholar] [CrossRef]
- Zheng, N.; Li, S.-H.; Dong, B.; Sun, W.; Li, H.-R.; Zhang, Y.-L.; Li, P.; Fang, Z.-W.; Chen, C.-M.; Han, X.-Y.; et al. Comparison of the Gut Microbiota of Short-Term and Long-Term Medical Workers and Non-Medical Controls: A Cross-Sectional Analysis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Kang, L.; Xiao, X.; Jia, L.; Zhang, Q.; Deng, M. “Gut Microbiota-Circadian Clock Axis” in Deciphering the Mechanism Linking Early-Life Nutritional Environment and Abnormal Glucose Metabolism. Int. J. Endocrinol. 2019, 2019, 5893028. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, L.; Sun, G.; Li, Y.; Huang, R. Alterations in the Gut Microbiota of Patients with Silica-Induced Pulmonary Fibrosis. J. Occup. Med. Toxicol. Lond. Engl. 2019, 14, 5. [Google Scholar] [CrossRef]
- Cummings, K.J.; Stanton, M.L.; Nett, R.J.; Segal, L.N.; Kreiss, K.; Abraham, J.L.; Colby, T.V.; Franko, A.D.; Green, F.H.Y.; Sanyal, S.; et al. Severe Lung Disease Characterized by Lymphocytic Bronchiolitis, Alveolar Ductitis, and Emphysema (BADE) in Industrial Machine-Manufacturing Workers. Am. J. Ind. Med. 2019, 62, 927–937. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, G.; Ji, H.; Peng, B.; Huang, Z.; Jin, W.; Chen, X.; Guan, H.; Tang, G.; Zhang, H.; et al. Changes in the Gut Microbiota during and after Commercial Helium-Oxygen Saturation Diving in China. Occup. Environ. Med. 2019, 76, 801–807. [Google Scholar] [CrossRef]
- Teichman, E.M.; O’Riordan, K.J.; Gahan, C.G.M.; Dinan, T.G.; Cryan, J.F. When Rhythms Meet the Blues: Circadian Interactions with the Microbiota-Gut-Brain Axis. Cell Metab. 2020, 31, 448–471. [Google Scholar] [CrossRef]
- Li, Y.; Hao, Y.; Fan, F.; Zhang, B. The Role of Microbiome in Insomnia, Circadian Disturbance and Depression. Front. Psychiatry 2018, 9, 669. [Google Scholar] [CrossRef]
- History of Medicine: Origin of the Term Microbiome and Why It Matters—The UWA Profiles and Research Repository. Available online: https://research-repository.uwa.edu.au/en/publications/history-of-medicine-origin-of-the-term-microbiome-and-why-it-matt (accessed on 23 October 2021).
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Robles Alonso, V.; Guarner, F. Linking the Gut Microbiota to Human Health. Br. J. Nutr. 2013, 109 (Suppl. S2), S21–S26. [Google Scholar] [CrossRef] [PubMed]
- Sarkodie, E.K.; Zhou, S.; Baidoo, S.A.; Chu, W. Influences of Stress Hormones on Microbial Infections. Microb. Pathog. 2019, 131, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Kaczmarek, J.L.; Thompson, S.V.; Holscher, H.D. Complex Interactions of Circadian Rhythms, Eating Behaviors, and the Gastrointestinal Microbiota and Their Potential Impact on Health. Nutr. Rev. 2017, 75, 673–682. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Bauer, L.L.; Gourineni, V.; Pelkman, C.L.; Fahey, G.C.; Swanson, K.S. Agave Inulin Supplementation Affects the Fecal Microbiota of Healthy Adults Participating in a Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. J. Nutr. 2015, 145, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Caporaso, J.G.; Hooda, S.; Brulc, J.M.; Fahey, G.C.; Swanson, K.S. Fiber Supplementation Influences Phylogenetic Structure and Functional Capacity of the Human Intestinal Microbiome: Follow-up of a Randomized Controlled Trial. Am. J. Clin. Nutr. 2015, 101, 55–64. [Google Scholar] [CrossRef]
- Kaczmarek, J.L.; Musaad, S.M.; Holscher, H.D. Time of Day and Eating Behaviors Are Associated with the Composition and Function of the Human Gastrointestinal Microbiota. Am. J. Clin. Nutr. 2017, 106, 1220–1231. [Google Scholar] [CrossRef]
- Sinha, R.; Abu-Ali, G.; Vogtmann, E.; Fodor, A.A.; Ren, B.; Amir, A.; Schwager, E.; Crabtree, J.; Ma, S.; Microbiome Quality Control Project Consortium; et al. Assessment of Variation in Microbial Community Amplicon Sequencing by the Microbiome Quality Control (MBQC) Project Consortium. Nat. Biotechnol. 2017, 35, 1077–1086. [Google Scholar] [CrossRef]
- Salter, S.J.; Cox, M.J.; Turek, E.M.; Calus, S.T.; Cookson, W.O.; Moffatt, M.F.; Turner, P.; Parkhill, J.; Loman, N.J.; Walker, A.W. Reagent and Laboratory Contamination Can Critically Impact Sequence-Based Microbiome Analyses. BMC Biol. 2014, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E.; Morton, J.T.; Zech Xu, Z.; Kightley, E.P.; Thompson, L.R.; Hyde, E.R.; Gonzalez, A.; et al. Deblur Rapidly Resolves Single-Nucleotide Community Sequence Patterns. mSystems 2017, 2, e00191-16. [Google Scholar] [CrossRef]
- Kraemer, J.G.; Ramette, A.; Aebi, S.; Oppliger, A.; Hilty, M. Influence of Pig Farming on the Human Nasal Microbiota: Key Role of Airborne Microbial Communities. Appl. Environ. Microbiol. 2018, 84, e02470-17. [Google Scholar] [CrossRef]
- Angelakis, E.; Yasir, M.; Bachar, D.; Azhar, E.I.; Lagier, J.-C.; Bibi, F.; Jiman-Fatani, A.A.; Alawi, M.; Bakarman, M.A.; Robert, C.; et al. Gut Microbiome and Dietary Patterns in Different Saudi Populations and Monkeys. Sci. Rep. 2016, 6, 32191. [Google Scholar] [CrossRef]
- Brooks, B.; Olm, M.R.; Firek, B.A.; Baker, R.; Thomas, B.C.; Morowitz, M.J.; Banfield, J.F. Strain-Resolved Analysis of Hospital Rooms and Infants Reveals Overlap between the Human and Room Microbiome. Nat. Commun. 2017, 8, 1814. [Google Scholar] [CrossRef]
- Ticinesi, A.; Milani, C.; Lauretani, F.; Nouvenne, A.; Mancabelli, L.; Lugli, G.A.; Turroni, F.; Duranti, S.; Mangifesta, M.; Viappiani, A.; et al. Gut Microbiota Composition Is Associated with Polypharmacy in Elderly Hospitalized Patients. Sci. Rep. 2017, 7, 11102. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.-U.; Chen, G.Y.; Núñez, G. Role of the Gut Microbiota in Immunity and Inflammatory Disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Tlaskalová-Hogenová, H.; Štěpánková, R.; Kozáková, H.; Hudcovic, T.; Vannucci, L.; Tučková, L.; Rossmann, P.; Hrnčíř, T.; Kverka, M.; Zákostelská, Z.; et al. The Role of Gut Microbiota (Commensal Bacteria) and the Mucosal Barrier in the Pathogenesis of Inflammatory and Autoimmune Diseases and Cancer: Contribution of Germ-Free and Gnotobiotic Animal Models of Human Diseases. Cell. Mol. Immunol. 2011, 8, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Mika, M.; Korten, I.; Qi, W.; Regamey, N.; Frey, U.; Casaulta, C.; Latzin, P.; Hilty, M.; SCILD study group. The Nasal Microbiota in Infants with Cystic Fibrosis in the First Year of Life: A Prospective Cohort Study. Lancet Respir. Med. 2016, 4, 627–635. [Google Scholar] [CrossRef]
- Bogaert, D.; Keijser, B.; Huse, S.; Rossen, J.; Veenhoven, R.; van Gils, E.; Bruin, J.; Montijn, R.; Bonten, M.; Sanders, E. Variability and Diversity of Nasopharyngeal Microbiota in Children: A Metagenomic Analysis. PLoS ONE 2011, 6, e17035. [Google Scholar] [CrossRef]
- Kumari, P.; Woo, C.; Yamamoto, N.; Choi, H.-L. Variations in Abundance, Diversity and Community Composition of Airborne Fungi in Swine Houses across Seasons. Sci. Rep. 2016, 6, 37929. [Google Scholar] [CrossRef]
- Kumari, P.; Choi, H.L. Seasonal Variability in Airborne Biotic Contaminants in Swine Confinement Buildings. PLoS ONE 2014, 9, e112897. [Google Scholar] [CrossRef]
- De Boeck, I.; Wittouck, S.; Wuyts, S.; Oerlemans, E.F.M.; van den Broek, M.F.L.; Vandenheuvel, D.; Vanderveken, O.; Lebeer, S. Comparing the Healthy Nose and Nasopharynx Microbiota Reveals Continuity As Well As Niche-Specificity. Front. Microbiol. 2017, 8, 2372. [Google Scholar] [CrossRef]
- Roman, P.; Cardona, D.; Sempere, L.; Carvajal, F. Microbiota and Organophosphates. Neurotoxicology 2019, 75, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Ruzafa, L.; Cruz, F.; Roman, P.; Cardona, D. Gut Microbiota and Neurological Effects of Glyphosate. Neurotoxicology 2019, 75, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-X.; Wang, Y.-P. Gut Microbiota-Brain Axis. Chin. Med. J. 2016, 129, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Eslamfam, S.; Fang, L.; Qiao, S.; Ma, X. Maintenance of Gastrointestinal Glucose Homeostasis by the Gut-Brain Axis. Curr. Protein Pept. Sci. 2017, 18, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, Y.; Liang, H.; Chen, S.; Pan, S.; Chang, L.; Li, S.; Zhang, Y.; Liu, X.; Xu, Y.; et al. Sleep Deprivation and a Non-24-h Working Schedule Lead to Extensive Alterations in Physiology and Behavior. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 6969–6979. [Google Scholar] [CrossRef]
- Liu, R.T. The Microbiome as a Novel Paradigm in Studying Stress and Mental Health. Am. Psychol. 2017, 72, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the Gut-Brain Axis: Regulation by the Microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef]
- Molina-Torres, G.; Rodriguez-Arrastia, M.; Roman, P.; Sanchez-Labraca, N.; Cardona, D. Stress and the Gut Microbiota-Brain Axis. Behav. Pharmacol. 2019, 30, 187–200. [Google Scholar] [CrossRef]
- Fabbrizzi, A.; Amedei, A.; Lavorini, F.; Renda, T.; Fontana, G. The Lung Microbiome: Clinical and Therapeutic Implications. Intern. Emerg. Med. 2019, 14, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
| Scale | Examined Study | Questions | Scores Range |
|---|---|---|---|
| Insa | Narrative Reviews | N.7 (yes/no) | 0–7 pt |
| New Castle Ottawa | Case-Control | Selection N.4, Comparability N.1, Exposure N.3 (yes/no) | 0–8 pt |
| New Castle Ottawa | Cross-Sectional | Selection N.4, Comparability N.1, Outcome N.2 (yes/no) | 0–10 pt |
| New Castle Ottawa | Cohort Studies | Selection N.4, Comparability N.1, Outcome N.3 (yes/no) | 0–8 pt |
| Author | Year | Type of Study | Country | Score |
|---|---|---|---|---|
| Ahmed N. [39] | 2019 | Cross-sectional | Egypt | 6 |
| Grant E. [40] | 2019 | Cross-sectional | Thailand | 5 |
| Hang J. [41] | 2017 | Longitudinal | USA | 4 |
| Islam Z. [42] | 2020 | Longitudinal | Denmark | 5 |
| Kates AE. [43] | 2019 | Cross-sectional | USA | 8 |
| Khan F.M. [44] | 2020 | Narrative review | USA | 5 |
| Kraemer J.G. [45] | 2019 | Longitudinal | Switzerland | 8 |
| Lai P.S. [46] | 2017 | Cross-sectional | USA | 4 |
| Lai P.S. [47] | 2019 | Narrative review | USA | 3 |
| Lu ZH. [48] | 2021 | Longitudinal | China | 7 |
| Mbareche Z. [49] | 2019 | Case-control | Canada | 7 |
| Mortas H. [50] | 2020 | Cross-sectional | Turkey | 5 |
| Peng M. [51] | 2020 | Cross-sectional | USA | 6 |
| Reynolds A.C. [52] | 2016 | Commentary | Australia | 1 |
| Reynolds A.C. [53] | 2016 | Narrative review | Australia | 4 |
| Rocha L.A. [54] | 2009 | Case-control | Brazil | 4 |
| Rosenthal M. [55] | 2014 | Longitudinal | USA | 6 |
| Shukla SK. [56] | 2017 | Case-control | USA | 6 |
| Stanaway I.B. [57] | 2016 | Longitudinal | USA | 7 |
| Sun J. [58] | 2017 | Case-control | China | 6 |
| Sun J. [59] | 2020 | Longitudinal | China | 7 |
| Swanson G.R. [60] | 2020 | Cross-sectional | USA | 7 |
| Tan S.C. [61] | 2020 | Case-control | Malaysia | 7 |
| Walters W.A. [62] | 2020 | Longitudinal | Honduras | 6 |
| Wu BG. [63] | 2020 | Cross-sectional | USA | 7 |
| Wu J. [64] | 2020 | Cross-sectional | China | 7 |
| Yuan Y. [65] | 2019 | Cross-sectional | China | 6 |
| Zhang J. [66] | 2020 | Longitudinal | China | 8 |
| Zheng N. [67] | 2020 | Cohort | China | 8 |
| Zhou L. [68] | 2019 | Narrative review | China | 5 |
| Zhou Y. [69] | 2019 | Case-control | China | 7 |
| Tot = 26 | |
|---|---|
| Biological samples | 26/26 (100%) |
| Fecal sample | 10/26 (38.4%) |
| Nasal swab | 9/26 (34.6%) |
| Oral swab | 6/26 (23%), |
| Skin sample | 5/26 (19.2%) |
| Blood sample | 3/26 (11.5%) |
| Nasopharyngeal swabs | 2/26 (7.7%) |
| Glove juice | 1/26 (3.8%) |
| Environmental samples | 7/26 (27%) |
| Air sample | 7/7 (100%) |
| Fluid sample | 3/7 (42.8%) |
| Tot = 26 | |
|---|---|
| Exposure to biological agents | 15/26 (57.7%) |
| Work with animals | 12/15 (80%) |
| Farmers and slaughters | 10/12 (83.4%) |
| Zookeepers | 1/12 (8.3%) |
| Lab personnel | 1/12 (8.3%) |
| Healthcare workers | 3/15 (20%) |
| Shift workers | 2/26 (7.7%) |
| Exposure to chemical agents | 4/26 (15.3%) |
| Metalworking fluid | 1/4 (25%) |
| Pesticides | 1/4 (25%) |
| Dust (ceramic, silica) | 2/4 (50%) |
| Exposure to stress factors and microclimate | 5/26 (19.3%) |
| Military | 2/5 (40%) |
| Sailors | 1/5 (20%) |
| Diving sub-sea | 1/5 (20%) |
| Tunnel workers | 1/5 (20%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mucci, N.; Tommasi, E.; Chiarelli, A.; Lulli, L.G.; Traversini, V.; Galea, R.P.; Arcangeli, G. WORKbiota: A Systematic Review about the Effects of Occupational Exposure on Microbiota and Workers’ Health. Int. J. Environ. Res. Public Health 2022, 19, 1043. https://doi.org/10.3390/ijerph19031043
Mucci N, Tommasi E, Chiarelli A, Lulli LG, Traversini V, Galea RP, Arcangeli G. WORKbiota: A Systematic Review about the Effects of Occupational Exposure on Microbiota and Workers’ Health. International Journal of Environmental Research and Public Health. 2022; 19(3):1043. https://doi.org/10.3390/ijerph19031043
Chicago/Turabian StyleMucci, Nicola, Eleonora Tommasi, Annarita Chiarelli, Lucrezia Ginevra Lulli, Veronica Traversini, Raymond Paul Galea, and Giulio Arcangeli. 2022. "WORKbiota: A Systematic Review about the Effects of Occupational Exposure on Microbiota and Workers’ Health" International Journal of Environmental Research and Public Health 19, no. 3: 1043. https://doi.org/10.3390/ijerph19031043
APA StyleMucci, N., Tommasi, E., Chiarelli, A., Lulli, L. G., Traversini, V., Galea, R. P., & Arcangeli, G. (2022). WORKbiota: A Systematic Review about the Effects of Occupational Exposure on Microbiota and Workers’ Health. International Journal of Environmental Research and Public Health, 19(3), 1043. https://doi.org/10.3390/ijerph19031043









