Ultra-Endurance Participation and Acute Kidney Injury: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Acute Kidney Injury and Ultra-Endurance Events
4. Non-Steroidal Anti-Inflammatory Drugs
5. Hydration
6. Exertional Rhabdomyolysis
7. Exercise-Associated Hyponatremia
8. Gastrointestinal Dysfunction
9. Beetroot/Nitrates
10. Females
11. Hormones
12. Ultra-Endurance Performance
13. Future Considerations
14. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scheer, V.; Tiller, N.B.; Doutreleau, S.; Khodaee, M.; Knechtle, B.; Pasternak, A.; Rojas-Valverde, D. Potential Long-Term Health Problems Associated with Ultra-Endurance Running: A Narrative Review. Sport Med. 2021, 52, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Rubio-arias, J.Á.; Ávila-Gandía, V.; López-román, F.J.; Soto-méndez, F.; Alcaraz, P.E.; Ramos-campo, D.J. Muscle damage and inflammation biomarkers after two ultra-endurance mountain races of different distances: 54 km vs 111 km. Physiol. Behav. 2019, 205, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Lecina, M.; López, I.; Castellar, C.; Pradas, F. Extreme ultra-trail race induces muscular damage, risk for acute kidney injury and hyponatremia: A case report. Int. J. Environ. Res. Public Health 2021, 18, 11323. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Valverde, D.; Sánchez-Ureña, B.; Crowe, J.; Timón, R.; Olcina, G.J. Exertional rhabdomyolysis and acute kidney injury in endurance sports: A systematic review. Eur. J. Sport Sci. 2021, 21, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Riebe, D.; Franklin, B.A.; Thompson, P.D.; Garber, C.E.; Whitfield, G.P.; Magal, M.; Pescatello, L.S. Updating ACSM’s recommendations for exercise preparticipation health screening. Med. Sci. Sport. Exerc. 2015, 47, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.; Verma, G.; Pata, R.; Martin, T.; Perazella, M.; Parikh, C. Kidney Injury and Repair Biomarkers in Marathon Runners. Am. J. Kidney Dis. 2017, 70, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Scheer, V.; Rojas-Valverde, D. Long-term health issues in ultraendurance runners: Should we be concerned? BMJ Open Sport Exerc. Med. 2021, 7, e001131. [Google Scholar] [CrossRef]
- Hodgson, L.E.; Walter, E.; Venn, R.M.; Galloway, R.; Pitsiladis, Y.; Sardat, F.; Forni, L.G. Acute kidney injury associated with endurance events—Is it a cause for concern? A systematic review. BMJ Open Sport Exerc. Med. 2017, 3, e000093. [Google Scholar] [CrossRef]
- Rojas-Valverde, D.; Olcina, G.; Sánchez-Ureña, B.; Pino-Ortega, J.; Martínez-Guardado, I.; Timón, R. Proteinuria and bilirubinuria as potential risk indicators of acute kidney injury during running in outpatient settings. Medicina 2020, 56, 562. [Google Scholar] [CrossRef]
- Hewing, B.; Schattke, S.; Spethmann, S.; Sanad, W.; Schroeckh, S.; Schimke, I.; Halleck, F.; Peters, H.; Brechtel, L.; Lock, J.; et al. Cardiac and renal function in a large cohort of amateur marathon runners. Cardiovasc. Ultrasound 2015, 13, 13. [Google Scholar] [CrossRef][Green Version]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.A.; Lilja, M.; Mandić, M.; Gustafsson, T.; Larsen, F.J.; Lundberg, T.R. Resistance training with co-ingestion of anti-inflammatory drugs attenuates mitochondrial function. Front. Physiol. 2017, 8, 1074. [Google Scholar] [CrossRef] [PubMed]
- Omeragic, E.; Marjanovic, A.; Djedjibegovic, J.; Turalic, A.; Dedic, M.; Niksic, H.; Lugusic, A.; Sober, M. Prevalence of use of permitted pharmacological substances for recovery among athletes. Pharmacia 2021, 68, 35–42. [Google Scholar] [CrossRef]
- Lipman, G.S.; Shea, K.; Christensen, M.; Phillips, C.; Burns, P.; Higbee, R.; Koskenoja, V.; Eifling, K.; Krabak, B.J. Ibuprofen versus placebo effect on acute kidney injury in ultramarathons: A randomised controlled trial. Emerg. Med. J. 2017, 34, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Dumke, C.L.; Oley, K.; McAnulty, S.R.; Davis, J.M.; Murphy, E.A.; Utter, A.C.; Lind, R.H.; McAnulty, L.S.; et al. Ibuprofen use, endotoxemia, inflammation, and plasma cytokines during ultramarathon competition. Brain Behav. Immun. 2006, 20, 578–584. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Fogard, K. Factors related to successful completion of a 161-km ultramarathon. Int. J. Sports Physiol. Perform. 2011, 6, 25–37. [Google Scholar] [CrossRef]
- Martínez, S.; Aguiló, A.; Moreno, C.; Lozano, L.; Tauler, P. Use of non-steroidal anti-inflammatory drugs among participants in a mountain ultramarathon event. Sports 2017, 5, 11. [Google Scholar] [CrossRef]
- Gorski, T.; Cadore, E.L.; Pinto, S.S.; da Silva, E.M.; Correa, C.S.; Beltrami, F.G.; Kruel, L.M. Use of NSAIDs in triathletes: Prevalence, level of awareness and reasons for use. Br. J. Sports Med. 2011, 45, 85–90. [Google Scholar] [CrossRef]
- Steckling, F.M.; Lima, F.D.; Farinha, J.B.; Rosa, P.C.; Royes, L.F.F.; Cuevas, M.J.; Bresciani, G.; Soares, F.A.; González-Gallego, J.; Barcelos, R.P. Diclofenac attenuates inflammation through TLR4 pathway and improves exercise performance after exhaustive swimming. Scand. J. Med. Sci. Sports 2020, 30, 264–271. [Google Scholar] [CrossRef]
- Lundberg, T.R.; Howatson, G. Analgesic and anti-inflammatory drugs in sports: Implications for exercise performance and training adaptations. Scand. J. Med. Sci. Sports 2018, 28, 2252–2262. [Google Scholar] [CrossRef]
- Lilja, M.; Mandić, M.; Apró, W.; Melin, M.; Olsson, K.; Rosenborg, S.; Gustafsson, T.; Lundberg, T.R. High doses of anti-inflammatory drugs compromise muscle strength and hypertrophic adaptations to resistance training in young adults. Acta Physiol. 2018, 222, e12948. [Google Scholar] [CrossRef] [PubMed]
- Nderitu, P.; Doos, L.; Jones, P.W.; Davies, S.J.; Kadam, U.T. Non-steroidal anti-inflammatory drugs and chronic kidney disease progression: A systematic review. Fam. Pract. 2013, 30, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.K.; Chiu, Y.H.; Tsai, Y.F.; Tai, L.C.; Hou, P.C.; How, C.K.; Yang, C.C.; Kao, W.F. Clinical impact of speed variability to identify ultramarathon runners at risk for acute kidney injury. PLoS ONE 2015, 10, e0133146. [Google Scholar] [CrossRef] [PubMed]
- Bongers, C.C.; Alsady, M.; Nijenhuis, T.; Tulp, A.D.; Eijsvogels, T.M.; Deen, P.M.; Hopman, M.T. Impact of acute versus prolonged exercise and dehydration on kidney function and injury. Physiol. Rep. 2018, 6, e13734. [Google Scholar] [CrossRef]
- Lipman, G.S.; Krabak, B.J.; Rundell, S.D.; Shea, K.M.; Badowski, N.; Little, C. Incidence and prevalence of acute kidney injury during multistage ultramarathons. Clin. J. Sport Med. 2016, 26, 314–319. [Google Scholar] [CrossRef]
- Kao, W.F.; Hou, S.K.; Chiu, Y.H.; Chou, S.L.; Kuo, F.C.; Wang, S.H.; Chen, J.J. Effects of 100-km ultramarathon on acute kidney injury. Clin. J. Sport Med. 2015, 25, 49–54. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Hoffman, M.D.; Stellingwerff, T. Research in Sports Medicine Considerations for ultra-endurance activities: Part 1- nutrition. Res. Sport Med. 2019, 27, 166–181. [Google Scholar] [CrossRef]
- Vitale, K.; Getzin, A. Nutrition and supplement update for the endurance athlete: Review and recommendations. Nutrients 2019, 11, 1289. [Google Scholar] [CrossRef]
- Nikolaidis, P.T.; Veniamakis, E.; Rosemann, T.; Knechtle, B. Nutrition in ultra-endurance: State of the art. Nutrients 2018, 10, 1995. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Jentjens, R.L.P.G.; Moseley, L. Nutritional considerations in triathlon. Sport Med. 2005, 35, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International society of sports nutrition position stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Chinnaiyan, K.M.; Gallagher, M.J.; Colar, J.M.; Geddes, T.; Gold, J.M.; Trivax, J.E. Changes in renal markers and acute kidney injury after marathon running. Nephrology 2011, 16, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Bruso, J.R.; Hoffman, M.D.; Rogers, I.R.; Lee, L.; Towle, G.; Hew-butler, T. Rhabdomyolysis and hyponatremia: A cluster of five cases at the 161-km 2009 Western States Endurance Run. Wilderness Environ. Med. 2010, 21, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Boulter, J.; Noakes, T.D.; Hew-Butler, T. Acute renal failure in four Comrades Marathon runners ingesting the same electrolyte supplement: Coincidence or causation? S. Afr. Med. J. 2011, 101, 876–878. [Google Scholar]
- Stasi, A.; Intini, A.; Divella, C.; Franzin, R.; Montemurno, E.; Grandaliano, G.; Ronco, C.; Fiaccadori, E.; Pertosa, G.B.; Gesualdo, L.; et al. Emerging role of Lipopolysaccharide binding protein in sepsis-induced acute kidney injury. Nephrol. Dial. Transplant. 2017, 32, 24–31. [Google Scholar] [CrossRef]
- Lim, C.L.; Suzuki, K. Systemic inflammation mediates the effects of endotoxemia in the mechanisms of heat stroke. Biol. Med. 2016, 9, 1. [Google Scholar] [CrossRef]
- Makris, K.; Spanou, L. Acute kidney injury: Definition, pathophysiology and clinical phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar]
- Lipman, G.S.; Krabak, B.J.; Waite, B.L.; Logan, S.B.; Menon, A.; Chan, G.K. A prospective cohort study of acute kidney injury in multi-day ultramarathon runners. Wilderness Environ. Med. 2014, 22, 358. [Google Scholar] [CrossRef]
- Shin, K.; Park, K.D.; Ahn, J.; Park, Y.; Kim, Y.-J. Comparison of changes in biochemical markers for skeletal muscles, hepatic metabolism, and renal function after three types of long-distance running: Observational study. Medicine 2016, 95, e3657. [Google Scholar] [CrossRef]
- Lopes, J.A.; Jorge, S. The RIFLE and AKIN classifications for acute kidney injury: A critical and comprehensive review. Clin. Kidney J. 2013, 6, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron. Clin. Pract. 2012, 120, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Khodaee, M.; Irion, B.; Spittler, J.; Saeedi, A.; Hoffman, M.D. Characteristics of runners meeting acute kidney injury criteria following a 161-km ultramarathon. Transl. Sport Med. 2021, 4, 733–740. [Google Scholar] [CrossRef]
- Rojas-Valverde, D.; Martínez-Guardado, I.; Sánchez-Ureña, B.; Timón, R.; Scheer, V.; Pino-Ortega, J.; Olcina, G. Outpatient assessment of mechanical load, heat strain and dehydration as causes of transitional acute kidney injury in endurance trail runners. Int. J. Environ. Res. Public Health 2021, 18, 10217. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Belli, T.; Macedo, D.V.; De Araujo, G.G.; Dos Reis, I.G.M.; Scariot, P.P.M.; Lazarim, F.L.; Nunes, L.A.S.; Brenzikofer, R.; Gobatto, C.A. Mountain ultramarathon induces early increases of muscle damage, inflammation, and risk for acute renal injury. Front. Physiol. 2018, 9, 1368. [Google Scholar] [CrossRef]
- Jouffroy, R.; Lebreton, X.; Mansencal, N.; Anglicheau, D. Acute kidney injury during an ultra-distance race. PLoS ONE 2019, 14, e0222544. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-gomar, F.; Salvagno, G.L.; Aloe, R.; Schena, F.; Guidi, G.C. Variation of serum and urinary neutrophil gelatinase associated lipocalin (NGAL) after strenuous physical exercise. Clin. Chem. Lab. Med. 2012, 50, 1585–1589. [Google Scholar] [CrossRef]
- Noakes, T.D.; Carter, J. Biochemical parameters in athletes before and after having run 160 kilometres. S. Afr. Med. J. 1976, 50, 1562–1566. [Google Scholar]
- Irving, R.A.; Noakes, T.D.; Smit, R.V.Z. Metabolic and renal changes in two athletes during a world 24 hour relay record performance. Br. J. Sports Med. 1989, 23, 227–232. [Google Scholar] [CrossRef]
- Irving, R.A.; Noakes, T.D.; Raine, R.I.; Van Zyl Smit, R. Transient oliguria with renal tubular dysfunction after a 90 km running race. Med. Sci. Sports Exerc. 1990, 22, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Irving, R.A.; Noakes, T.D.; Burger, S.C.; Myburgh, K.H.; Querido, D.; van Zyl Smit, R. Plasma volume and renal function during and after ultramarathon running. Med. Sci. Sports Exerc. 1990, 22, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Neumayr, G.; Pfister, R.; Hoertnagl, H.; Mitterbauer, G.; Getzner, W.; Ulmer, H.; Gaenzer, H.; Joannidis, M. The effect of marathon cycling on renal function. Int. J. Sports Med. 2003, 24, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Neumayr, G.; Pfister, R.; Hoertnagl, H.; Mitterbauer, G.; Prokop, W.; Joannidis, M. Renal function and plasma volume following ultramarathon cycling. Int. J. Sports Med. 2005, 26, 2–8. [Google Scholar] [CrossRef]
- Christensen, D.L.; Espino, D.; Infante-Ramírez, R.; Brage, S.; Terzic, D.; Goetze, J.P.; Kjaergaard, J. Normalization of elevated cardiac, kidney, and hemolysis plasma markers within 48 h in Mexican Tarahumara runners following a 78 km race at moderate altitude. Am. J. Hum. Biol. 2014, 26, 836–843. [Google Scholar] [CrossRef]
- Chlíbková, D.; Knechtle, B.; Rosemann, T.; Tomášková, I.; Novotný, J.; Žákovská, A.; Uher, T. Rhabdomyolysis and exercise-associated hyponatremia in ultra-bikers and ultra-runners. J. Int. Soc. Sports Nutr. 2015, 12, 29. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Weiss, R.H. Does acute kidney injury from an ultramarathon increase the risk for greater subsequent injury? Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2016, 26, 417–422. [Google Scholar] [CrossRef]
- Zyl-smit RVan Mills, P.; Vogelpoel, L. Unrecognised acute renal failure following the comrades marathon. S. Afr. Med. J. 2000, 90, 39–40. [Google Scholar]
- Poussel, M.; Touzé, C.; Allado, E.; Frimat, L.; Hily, O.; Thilly, N.; Rousseau, H.; Vauthier, J.C.; Chenuel, B. Ultramarathon and renal function: Does exercise-induced acute kidney injury really exist in common conditions? Front. Sport Act Living 2020, 1, 71. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Stuempfle, K.J.; Fogard, K.; Hew-butler, T.; Winger, J.; Weiss, R.H. Urine dipstick analysis for identification of runners susceptible to acute kidney injury following an ultramarathon. J. Sports Sci. 2013, 31, 20–31. [Google Scholar] [CrossRef]
- Andres, S.; Ziegenhagen, R.; Trefflich, I.; Pevny, S.; Schultrich, K.; Braun, H.; Schänzer, W.; Hirsch-Ernst, K.I.; Schäfer, B.; Lampen, A. Creatine and creatine forms intended for sports nutrition. Mol. Nutr. Food Res. 2017, 61, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kashani, K.; Rosner, M.H.; Ostermann, M. Creatinine: From physiology to clinical application. Eur. J. Int. Med. 2020, 72, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Cavalier, E.; Pottel, H. Serum creatinine: Not so simple! Nephron 2017, 136, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Inker, L.A. Assessment of glomerular filtration rate in health and disease: A state of the art review. Clin. Pharmacol. Ther. 2017, 102, 405–419. [Google Scholar] [CrossRef]
- Botev, R.; Mallié, J.P.; Wetzels, J.F.; Couchoud, C.; Schück, O. The clinician and estimation of glomerular filtration rate by creatinine-based formulas: Current limitations and quo vadis. Clin. J. Am. Soc. Nephrol. 2011, 6, 937–950. [Google Scholar] [CrossRef]
- Kork, F.; Balzer, F.; Krannich, A.; Bernardi, M.H.; Eltzschig, H.K.; Jankowski, J.; Spies, C. Back-calculating baseline creatinine overestimates prevalence of acute kidney injury with poor sensitivity. Acta Physiol. 2017, 219, 615–626. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Gulbis, B.; De Bruyn, E.; Baudry, S.; Carpentier, A. Limitations of serum values to estimate glomerular filtration rate during exercise. Br. J. Sports Med. 2013, 47, 1166–1170. [Google Scholar] [CrossRef]
- Little, C.E.; Lipman, G.S.; Migliaccio, D.; Young, D.S.; Krabak, B.J. Accuracy of estimated creatinine in multistage ultramarathon runners. Wilderness Environ. Med. 2019, 30, 129–133. [Google Scholar] [CrossRef]
- Dharnidharka, V.R.; Kwon, C.; Stevens, G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am. J. Kidney Dis. 2002, 40, 221–226. [Google Scholar] [CrossRef]
- Rabb, H.; Griffin, M.D.; McKay, D.B.; Swaminathan, S.; Pickkers, P.; Rosner, M.H.; Kellum, J.A.; Ronco, C. Inflammation in AKI: Current understanding, key questions, and knowledge gaps. J. Am. Soc. Nephrol. 2016, 27, 371–379. [Google Scholar] [CrossRef]
- Tangri, N.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Beck, G.J.; Greene, T.; Coresh, J.; Levey, A.S. Changes in dietary protein intake has no effect on serum cystatin C levels independent of the glomerular filtration rate. Kidney Int. 2011, 79, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Becker, C.; Inker, L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 2022, 313, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Mingels, A.; Jacobs, L.; Kleijnen, V.; Wodzig, W.; van Dieijen-Visser, M. Cystatin C a marker for renal function after exercise. Int. J. Sports Med. 2009, 30, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.R.; Parikh, C.R. Biomarkers of acute and chronic kidney disease. Annu. Rev. Physiol. 2019, 81, 309–333. [Google Scholar] [CrossRef]
- Mishra, J.; Dent, C.; Tarabishi, R.; Mitsnefes, M.M.; Ma, Q.; Kelly, C.; Ruff, S.M.; Zahedi, K.; Shao, M.; Bean, J.; et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005, 365, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Wharam, P.C.; Speedy, D.B.; Noakes, T.D.; Thompson, J.M.D.; Reid, S.A.; Holtzhausen, L.-M. NSAID use increases the risk of developing hyponatremia during an Ironman triathlon. Med. Sci. Sports Exerc. 2006, 38, 618–622. [Google Scholar] [CrossRef]
- Whelton, A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: Physiologic foundations and clinical implications. Am. J. Med. 1999, 106, 13S–24S. [Google Scholar] [CrossRef]
- Murray, M.D.; Brater, D.C. Renal toxicity of the nonsteroidal anti-inflammatory drugs. Annu. Rev. Pharmacol. Toxicol. 1993, 33, 435–465. [Google Scholar] [CrossRef]
- Miller, S.B. Prostaglandins in health and disease: An overview. In Seminars in Arthritis and Rheumatism; WB Saunders: Philadelphia, PA, USA, 2006; Volume 36, pp. 37–49. [Google Scholar] [CrossRef]
- Shimizu, Y.; Takaori, K.; Maeda, S. Exercise-induced acute renal failure in a trainee cyclist without hypouricemia: Successful athletic career post-treatment. J. Gen. Fam. Med. 2017, 18, 432–435. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Stellingwerff, T.; Costa, R.J. Considerations for ultra-endurance activities: Part 2—Hydration. Res. Sport Med. 2019, 27, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Valverde, D.; Sánchez-Ureña, B.; Pino-Ortega, J.; Gómez-Carmona, C.; Gutiérrez-Vargas, R.; Timón, R.; Olcina, G. External workload indicators of muscle and kidney mechanical injury in endurance trail running. Int. J. Environ. Res. Public Health 2019, 16, 3909. [Google Scholar] [CrossRef] [PubMed]
- Knechtle, B.; Nikolaidis, P.T. Physiology and pathophysiology in ultra-marathon running. Front. Physiol. 2018, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M. Exertional rhabdomyolysis and acute renal failure in marathon runners. Sport Med. 2007, 37, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.; Kim, S.; Young, H.; Suk, K. Exercise-induced rhabdomyolysis mechanisms and prevention: A literature review. J. Sport Health Sci. 2016, 5, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Asserraji, M.; Benameur, I.; Maoujoud, O.; El Kharras, A.; Hajbi, H.; Filali, K. Late care in marathon runs leading to exertional heat stroke with multiple organ failure. Asian J. Sports Med. 2014, 5, 136–138. [Google Scholar]
- Armstrong, L.E. Assessing hydration status: The elusive gold standard. J. Am. Coll. Nutr. 2007, 26, 575–584. [Google Scholar] [CrossRef]
- Goulet, E.D.B. Dehydration and endurance performance in competitive athletes. Nutr. Rev. 2012, 70, S132–S136. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Carter, R.; Sawka, M.N. Fluid balance and endurance exercise performance. Curr. Sports Med. Rep. 2003, 2, 202–208. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Goulet, E.D.; Maughan, R.J. Considerations in the use of body mass change to estimate change in hydration status during a 161-kilometer ultramarathon running competition. Sport Med. 2018, 48, 243–250. [Google Scholar] [CrossRef]
- Kao, W.F.; Shyu, C.L.; Yang, X.W.; Hsu, T.F.; Chen, J.J.; Kao, W.C.; Huang, Y.J.; Kuo, F.C.; Huang, C.I.; Lee, C.H. Athletic performance and serial weight changes during 12-and 24-hour ultra-marathons. Clin. J. Sport Med. 2008, 18, 155–158. [Google Scholar] [CrossRef] [PubMed]
- King, R.F.G.J.; Jones, B.; Hara, J.P.O. The availability of water associated with glycogen during dehydration: A reservoir or raindrop? Eur. J. Appl. Physiol. 2018, 118, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.D.; Pasternak, A.; Rogers, I.R.; Khodaee, M.; Hill, J.C.; Townes, D.A.; Scheer, B.V.; Krabak, B.J.; Basset, P.; Lipman, G.S. Medical services at ultra-endurance foot races in remote environments: Medical issues and consensus guidelines. Sport Med. 2014, 44, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- McDermott, B.P.; Anderson, S.A.; Armstrong, L.E.; Casa, D.J.; Cheuvront, S.N.; Cooper, L.; Kenney, W.L.; O’Connor, F.G.; Roberts, W.O. National athletic trainers’ association position statement: Fluid replacement for the physically active. J. Athl. Train. 2017, 52, 877–895. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.D.; Stuempfle, K.J.; Sullivan, K.; Weiss, R.H. Exercise-associated hyponatremia with exertional rhabdomyolysis: Importance of proper treatment. Clin. Nephrol. 2015, 83, 235–242. [Google Scholar] [CrossRef]
- Gill, S.K.; Hankey, J.; Wright, A.; Marczak, S.; Hemming, K.; Allerton, D.M.; Ansley-Robson, P.; Costa, R.J.S. The impact of a 24-h ultra-marathon on circulatory endotoxin and cytokine profile. Int. J. Sports Med. 2015, 36, 688–695. [Google Scholar] [CrossRef]
- Kupchak, B.R.; Kraemer, W.J.; Hoffman, M.D.; Phinney, S.D.; Volek, J.S. The impact of an ultramarathon on hormonal and biochemical parameters in men. Wilderness Environ. Med. 2014, 25, 278–288. [Google Scholar] [CrossRef]
- Mcvane, B.A.; Andreae, M.C.; Fernando, D.B.; Strayer, R.J. Exertional rhabdomyolysis in a long-distance migrant. J. Emerg. Med. 2019, 56, 551–553. [Google Scholar] [CrossRef]
- Atias-Varon, D.; Sherman, H.; Yanovich, R.; Heled, Y. Rhabdomyolysis after crawling military training. Mil. Med. 2017, 182, e1948–e1952. [Google Scholar] [CrossRef]
- Kim, D.; Ko, E.J.; Cho, H.; Park, S.H.; Lee, S.H.; Cho, N.G.; Lee, S.Y.; Jeong, H.Y.; Yang, D.H. Spinning-induced rhabdomyolysis: Eleven case reports and review of the literature. Electrolytes Blood Press. 2015, 13, 58–61. [Google Scholar] [CrossRef][Green Version]
- Oh, R.C.; Arter, J.L.; Tiglao, S.M.; Larson, S.L. Exertional rhabdomyolysis: A case series of 30 hospitalized patients. Mil. Med. 2015, 180, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Stahl, K.; Rastelli, E.; Schoser, B. A systematic review on the definition of rhabdomyolysis. J. Neurol. 2020, 267, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Tietze, D.C.; Borchers, J. Exertional rhabdomyolysis in the athlete: A clinical review. Sports Health 2014, 6, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D.; Goodwin, N.; Rayner, B.L.; Branken, T.; Taylor, R.K. Water Intoxication: A possible complication during endurance exercise. Wilderness Environ. Med. 2005, 16, 221–227. [Google Scholar] [CrossRef]
- Hew-Butler, T.; Loi, V.; Pani, A.; Rosner, M.H. Exercise-associated hyponatremia: 2017 update. Front. Med. 2017, 4, 21. [Google Scholar] [CrossRef]
- Noakes, T. Hyponatremia in distance runners: Fluid and sodium balance during exercise. Curr. Sports Med. Rep. 2002, 1, 197–207. [Google Scholar] [CrossRef]
- Convertino, V.A.; Armstrong, L.E.; Coyle, E.F.; Mack, G.W.; Sawka, M.N.; Senay Jr, L.C.; Sherman, W.M. American College of Sports Medicine. Position Stand. Exercise and fluid replacement. Med. Sci. Sports Exerc. 1996, 28, i–ix. [Google Scholar] [CrossRef]
- Noakes, T.D.; Sharwood, K.; Collins, M.; Perkins, D.R. The dipsomania of great distance: Water intoxication in an Ironman triathlete. Br. J. Sports Med. 2004, 38, e16. [Google Scholar] [CrossRef]
- Sharwood, K.A.; Collins, M.; Goedecke, J.H.; Wilson, G.; Noakes, T.D. Weight changes, medical complications, and performance during an Ironman triathlon. Br. J. Sports Med. 2004, 38, 718–724. [Google Scholar] [CrossRef]
- Speedy, D.B.; Rogers, I.; Safih, S.; Foley, B. Hyponatremia and seizures in an ultradistance triathlete. J. Emerg. Med. 2000, 18, 41–44. [Google Scholar] [CrossRef]
- Hew-Butler, T.; Ayus, J.C.; Kipps, C.; Maughan, R.J.; Mettler, S.; Meeuwisse, W.H.; Page, A.J.; Reid, S.A.; Rehrer, N.J.; Roberts, W.O.; et al. Statement of the second international exercise-associated hyponatremia consensus development conference, New Zealand, 2007. Clin. J. Sport Med. 2008, 18, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M.; Kearns, A.K.; Rouzier, P.; Rubin, R.; Thompson, P.D. Serum creatine kinase levels and renal function measures in exertional muscle damage. Med. Sci. Sports Exerc. 2006, 38, 623. [Google Scholar] [CrossRef] [PubMed]
- Chorley, J.; Cianca, J.; Divine, J. Risk factors for exercise-associated hyponatremia in non-elite marathon runners. Clin. J. Sport Med. 2007, 17, 471–477. [Google Scholar] [CrossRef]
- Freund, B.J.; Shizuru, E.M.; Hashiro, G.M.; Claybaugh, J.R. Hormonal, electrolyte, and renal responses to exercise are intensity dependent. J. Appl. Physiol. 1991, 70, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Takamata, A.K.I.R.A.; Mack, G.W.; Stachenfeld, N.S.; Nadel, E.R. Body temperature modification of osmotically induced vasopressin secretion and thirst in humans. Am. J. Physiol. Integr. Comp. Physiol. 1995, 269, R874–R880. [Google Scholar] [CrossRef]
- Cairns, R.S.; Hew-Butler, T. Incidence of exercise-associated hyponatremia and its association with nonosmotic stimuli of arginine vasopressin in the GNW100s ultra-endurance marathon. Clin. J. Sport Med. 2015, 25, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Hew-Butler, T.; Rosner, M.H.; Fowkes-Godek, S.; Dugas, J.P.; Hoffman, M.D.; Lewis, D.P.; Maughan, R.J.; Miller, K.C.; Montain, S.J.; Rehrer, N.J.; et al. Statement of the third international exercise-associated hyponatremia consensus development conference, Carlsbad, California, 2015. Clin. J. Sport Med. 2015, 25, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Buono, M.J.; Sjoholm, N.T. Effect of physical training on peripheral sweat production. J. Appl. Physiol. 1988, 65, 811–814. [Google Scholar] [CrossRef]
- Hew-Butler, T.; Jordaan, E.; Stuempfle, K.J.; Speedy, D.B.; Siegel, A.J.; Noakes, T.D.; Soldin, S.J.; Verbalis, J.G. Osmotic and nonosmotic regulation of arginine vasopressin during prolonged endurance exercise. J. Clin. Endocrinol. Metab. 2008, 93, 2072–2078. [Google Scholar] [CrossRef]
- Verbalis, J.G.; Goldsmith, S.R.; Greenberg, A.; Schrier, R.W.; Sterns, R.H. Hyponatremia treatment guidelines 2007: Expert panel recommendations. Am. J. Med. 2007, 120, S1–S21. [Google Scholar] [CrossRef] [PubMed]
- Chlíbková, D.; Rosemann, T.; Posch, L.; Matoušek, R.; Knechtle, B. Pre-and post-race hydration status in hyponatremic and non-hyponatremic ultra-endurance athletes. Chin. J. Physiol. 2016, 59, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.J.S.; Snipe, R.; Camões-Costa, V.; Scheer, V.; Murray, A. The impact of gastrointestinal symptoms and dermatological injuries on nutritional intake and hydration status during ultramarathon events. Sport Med. Open 2016, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E.; Vet-Joop, K.; Sturk, A.; Stegen, J.H.J.C.; Senden, J.; Saris, W.H.M.; Wagenmakers, A.J.M. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin. Sci. 2000, 98, 47–55. [Google Scholar] [CrossRef]
- Stuempfle, K.J.; Hoffman, M.D. Gastrointestinal distress is common during a 161-km ultramarathon. J. Sports Sci. 2015, 33, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Stuempfle, K.J.; Hoffman, M.D.; Hew-Butler, T. Association of gastrointestinal distress in ultramarathoners with race diet. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 103–109. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Nutrition for endurance sports: Marathon, triathlon, and road cycling. J. Sports Sci. 2011, 29, 91–99. [Google Scholar] [CrossRef]
- Stellingwerff, T.; Cox, G.R. Systematic review: Carbohydrate supplementation on exercise performance or capacity of varying durations. Appl. Physiol. Nutr. Metab. 2014, 39, 998–1011. [Google Scholar] [CrossRef]
- Fudge, B.W.; Westerterp, K.R.; Kiplamai, F.K.; Onywera, V.O.; Boit, M.K.; Kayser, B.; Pitsiladis, Y.P. Evidence of negative energy balance using doubly labelled water in elite Kenyan endurance runners prior to competition. Br. J. Nutr. 2006, 95, 59–66. [Google Scholar] [CrossRef]
- Onywera, V.O.; Kiplamai, F.K.; Tuitoek, P.J.; Boit, M.K.; Pitsiladis, Y.P. Food and macronutrient intake of elite Kenyan distance runners. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 709–719. [Google Scholar] [CrossRef]
- Costa, R.J.; Miall, A.; Khoo, A.; Rauch, C.; Snipe, R.; Camões-Costa, V.; Gibson, P. Gut-training: The impact of two weeks repetitive gut-challenge during exercise on gastrointestinal status, glucose availability, fuel kinetics, and running performance. Appl. Physiol. Nutr. Metab. 2017, 42, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Poortmans, J.R. Exercise and renal function. Exerc. Sport Sci. Rev. 1977, 5, 255–294. [Google Scholar] [CrossRef] [PubMed]
- Clausen, J.P. Effect of physical training on cardiovascular adjustments to exercise in man. Physiol. Rev. 1977, 57, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yeh, W.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Banas, B.; Schlöndorff, D. Signaling danger: Toll-like receptors and their potential roles in kidney disease. J. Am. Soc. Nephrol. 2004, 15, 854–867. [Google Scholar] [CrossRef] [PubMed]
- Dauphinee, S.M.; Karsan, A. Lipopolysaccharide signaling in endothelial cells. Lab. Investig. 2006, 86, 9–22. [Google Scholar] [CrossRef]
- Lim, C.L.; Mackinnon, L.T. The roles of exercise-induced immune system disturbances in the pathology of heat stroke—The dual pathway model of heat stroke. Sport Med. 2006, 36, 39–64. [Google Scholar] [CrossRef]
- Selkirk, G.A.; Mclellan, T.M.; Wright, H.E.; Rhind, S.G. Mild endotoxemia, NF-κB translocation, and cytokine increase during exertional heat stress in trained and untrained individuals. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, 611–623. [Google Scholar] [CrossRef]
- Shaltout, H.A.; Eggebeen, J.; Marsh, A.P.; Brubaker, P.H.; Laurienti, P.J.; Burdette, J.H.; Basu, S.; Morgan, A.; Dos Santos, P.C.; Norris, J.L.; et al. Effects of supervised exercise and dietary nitrate in older adults with controlled hypertension and/or heart failure with preserved ejection fraction. Nitric. Oxide 2017, 69, 78–90. [Google Scholar] [CrossRef]
- Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Ekblom, B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. 2007, 191, 59–66. [Google Scholar] [CrossRef]
- Domínguez, R.; Cuenca, E.; Maté-Muñoz, J.L.; García-Fernández, P.; Serra-Paya, N.; Estevan, M.C.L.; Herreros, P.V.; Garnacho-Castaño, M.V. Effects of beetroot juice supplementation on cardiorespiratory endurance in athletes. A systematic review. Nutrients 2017, 9, 43. [Google Scholar] [CrossRef]
- Williams, J.K.; Smallwood, M.J.; Benjamin, N.; D’Souza, R.J.; Shore, A.C.; Winyard, P.G.; Gilchrist, M. Renal nitrate clearance in chronic kidney disease. Nitric. Oxide 2020, 97, 16–19. [Google Scholar] [CrossRef]
- McMahon, N.F.; Leveritt, M.D.; Pavey, T.G. The effect of dietary nitrate supplementation on endurance exercise performance in healthy adults: A systematic review and meta-analysis. Sport Med. 2017, 47, 735–756. [Google Scholar] [CrossRef]
- Ravindra, P.V.; Janhavi, P.; Divyashree, S.; Muthukumar, S.P. Nutritional interventions for improving the endurance performance in athletes. Arch. Physiol. Biochem. 2020, 128, 851–858. [Google Scholar] [CrossRef]
- Wagner, D.A.; Schultz, D.S.; Deen, W.M.; Young, V.R.; Tannenbaum, S.R. Metabolic fate of an oral dose of 15N-labeled nitrate in humans: Effect of diet supplementation with ascorbic acid. Cancer Res. 1983, 43, 1921–1925. [Google Scholar] [PubMed]
- Sagnella, G.A.; Markandu, N.D.; Onipinla, A.K.; Chelliah, R.; Singer, D.R.J.; Macgregor, G.A. Plasma and urinary nitrate in essential hypertension. J. Hum. Hypertens. 1997, 11, 587–588. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dykhuizen, R.S.; Copland, M.; Smith, C.C.; Douglas, G.; Benjamin, N. Plasma nitrate concentration and urinary nitrate excretion in patients with gastroenteritis. J. Infect. 1995, 31, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Mian, A.I.; Du, Y.; Garg, H.K.; Caviness, A.C.; Goldstein, S.L.; Bryan, N.S. Urinary nitrate might be an early biomarker for pediatric acute kidney injury in the emergency department. Pediatr. Res. 2011, 70, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Goligorsky, M.S.; Brodsky, S.V.; Noiri, E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int. 2002, 61, 855–861. [Google Scholar] [CrossRef]
- Kwon, O.; Hong, S.; Ramesh, G. Diminished NO generation by injured endothelium and loss of macula densa nNOS may contribute to sustained acute kidney injury after ischemia-reperfusion. Am. J. Physiol. Physiol. 2009, 296, 25–33. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Gualano, B.; Carpentier, A. Nitrate supplementation and human exercise performance: Too much of a good thing? Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 599–604. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Nitrate in vegetables-Scientific Opinion of the Panel on Contaminants in the Food chain. EFSA J. 2008, 6, 689. [Google Scholar] [CrossRef]
- Calvert, J.W.; Lefer, D.J. Role of β-adrenergic receptors and nitric oxide signaling in exercise-mediated cardioprotection. Physiology 2013, 28, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.H.; Rusiecki, J.A.; Lynch, C.F.; Cantor, K.P. Nitrate in public water supplies and the risk of renal cell carcinoma. Cancer Causes Control 2007, 18, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Larsen, F.J.; Weitzberg, E. Supplementation with nitrate and nitrite salts in exercise: A word of caution. J. Appl. Physiol. 2011, 111, 616–617. [Google Scholar] [CrossRef]
- Carpentier, A.; Stragier, S.; Bréjeon, C.; Poortmans, J.R. Nitrate supplementation, exercise, and kidney function: Are there detrimental effects? Med. Sci. Sports Exerc. 2015, 47, 1519–1522. [Google Scholar] [CrossRef]
- Zouhal, H.; Groussard, C.; Minter, G.; Vincent, S.; Cretual, A.; Gratas-Delamarche, A.; Delamarche, P.; Noakes, T.D. Inverse relationship between percentage body weight change and finishing time in 643 forty-two-kilometre marathon runners. Br. J. Sports Med. 2011, 45, 1101–1105. [Google Scholar] [CrossRef]
- Chlíbková, D.; Knechtle, B.; Rosemann, T.; Žákovská, A.; Tomášková, I.; Shortall, M.; Tomášková, I. Changes in foot volume, body composition, and hydration status in male and female 24-hour ultra-mountain bikers. J. Int. Soc. Sports Nutr. 2014, 11, 18–21. [Google Scholar] [CrossRef]
- Glace, A.B.; Murphy, C. Severe hyponatremia develops in a runner following a half-marathon. J. Am. Acad PAs. 2008, 21, 27–29. [Google Scholar] [CrossRef]
- Atkins, W.C.; Butts, C.L.; Kelly, M.R.; Troyanos, C.; Laursen, R.M.; Duckett, A.; Emerson, D.M.; Rosa-Caldwell, M.E.; McDermott, B.P. Acute Kidney Injury Biomarkers and Hydration Outcomes at the Boston Marathon. Front. Physiol. 2022, 12, 2402. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.; Meyer, N.; et al. International Olympic Committee (IOC) consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Beals, K.A.; Meyer, N.L. Female athlete triad update. Clin. Sports Med. 2007, 26, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Fragala, M.S.; Watson, G.; Volek, J.S.; Rubin, M.R.; French, D.N.; Maresh, C.M.; Vingren, J.L.; Hatfield, D.L.; Spiering, B.A.; et al. Hormonal responses to a 160-km race across frozen Alaska. Br. J. Sports Med. 2008, 42, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Kupchak, B.R.; Volk, B.M.; Kunces, L.J.; Kraemer, W.J.; Hoffman, M.D.; Phinney, S.D.; Volek, J.S. Alterations in coagulatory and fibrinolytic systems following an ultra-marathon. Eur. J. Appl. Physiol. 2013, 113, 2705–2712. [Google Scholar] [CrossRef]
- Neubauer, O.; König, D.; Wagner, K.H. Recovery after an Ironman triathlon: Sustained inflammatory responses and muscular stress. Eur. J. Appl. Physiol. 2008, 104, 417–426. [Google Scholar] [CrossRef]
- Karkoulias, K.; Habeos, I.; Charokopos, N.; Tsiamita, M.; Mazarakis, A.; Pouli, A.; Spiropoulos, K. Hormonal responses to marathon running in non-elite athletes. Eur. J. Int. Med. 2008, 19, 598–601. [Google Scholar] [CrossRef]
- Semple, C.G.; Thomson, J.A.; Beastall, G.H. Endocrine responses to marathon running. Br. J. Sports Med. 1985, 19, 148–151. [Google Scholar] [CrossRef]
- Roupas, N.D.; Mamali, I.; Maragkos, S.; Leonidou, L.; Armeni, A.K.; Markantes, G.K.; Tsekouras, A.; Sakellaropoulos, G.C.; Markou, K.B.; Georgopoulos, N.A. The effect of prolonged aerobic exercise on serum adipokine levels during an ultra-marathon endurance race. Hormones 2013, 12, 275–282. [Google Scholar] [CrossRef]
- Hartley, L.H.; Mason, J.W.; Hogan, R.P.; Jones, L.G.; Kotchen, T.A.; Mougey, E.H.; Wherry, F.E.; Pennington, L.L.; Ricketts, P.T. Multiple hormonal responses to prolonged exercise in relation to physical training. J. Appl. Physiol. 1972, 33, 607–610. [Google Scholar] [CrossRef]
- Collins, K.J.; Weiner, J.S. Endocrinological aspects of exposure to high environmental temperatures. Physiol. Rev. 1968, 48, 785–839. [Google Scholar] [CrossRef]
- Friedl, K.E.; Moore, R.J.; Hoyt, R.W.; Marchitelli, L.J.; Martinez-lopez, L.E.; Askew, E.W. Endocrine markers of semistarvation in healthy lean men in a multistressor environment. J. Appl. Physiol. 2000, 88, 1820–1830. [Google Scholar] [CrossRef] [PubMed]
- Tauler, P.; Martinez, S.; Moreno, C.; Martínez, P.; Aguilo, A. Changes in salivary hormones, immunoglobulin A, and C-reactive protein in response to ultra-endurance exercises. Appl. Physiol. Nutr. Metab. 2014, 39, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Yousef, E.A.; El-kannishy, S.; Arfa, L.F.; Mahmoud, R. Plasma cortisol level and its relation to in-hospital mortality in acute kidney injury patients. J. Egypt Soc. Nephrol. Trans. 2021, 21, 91–97. [Google Scholar] [CrossRef]
- Rüst, C.A.; Knechtle, B.; Knechtle, P.; Rosemann, T. Similarities and differences in anthropometry and training between recreational male 100-km ultra-marathoners and marathoners. J. Sports Sci. 2012, 30, 1249–1257. [Google Scholar] [CrossRef]
- Reid, S.A.; Speedy, D.B.; Thompson, J.M.; Noakes, T.D.; Mulligan, G.; Page, T.; Campbell, R.G.; Milne, C. Study of hematological and biochemical parameters in runners completing a standard marathon. Clin. J. Sport Med. 2004, 14, 344–353. [Google Scholar] [CrossRef]
- Sinert, R.; Kohl, L.; Rainone, T.; Scalea, T. Exercise-induced rhabdomyolysis. Ann. Emerg. Med. 1994, 23, 1301–1306. [Google Scholar] [CrossRef]
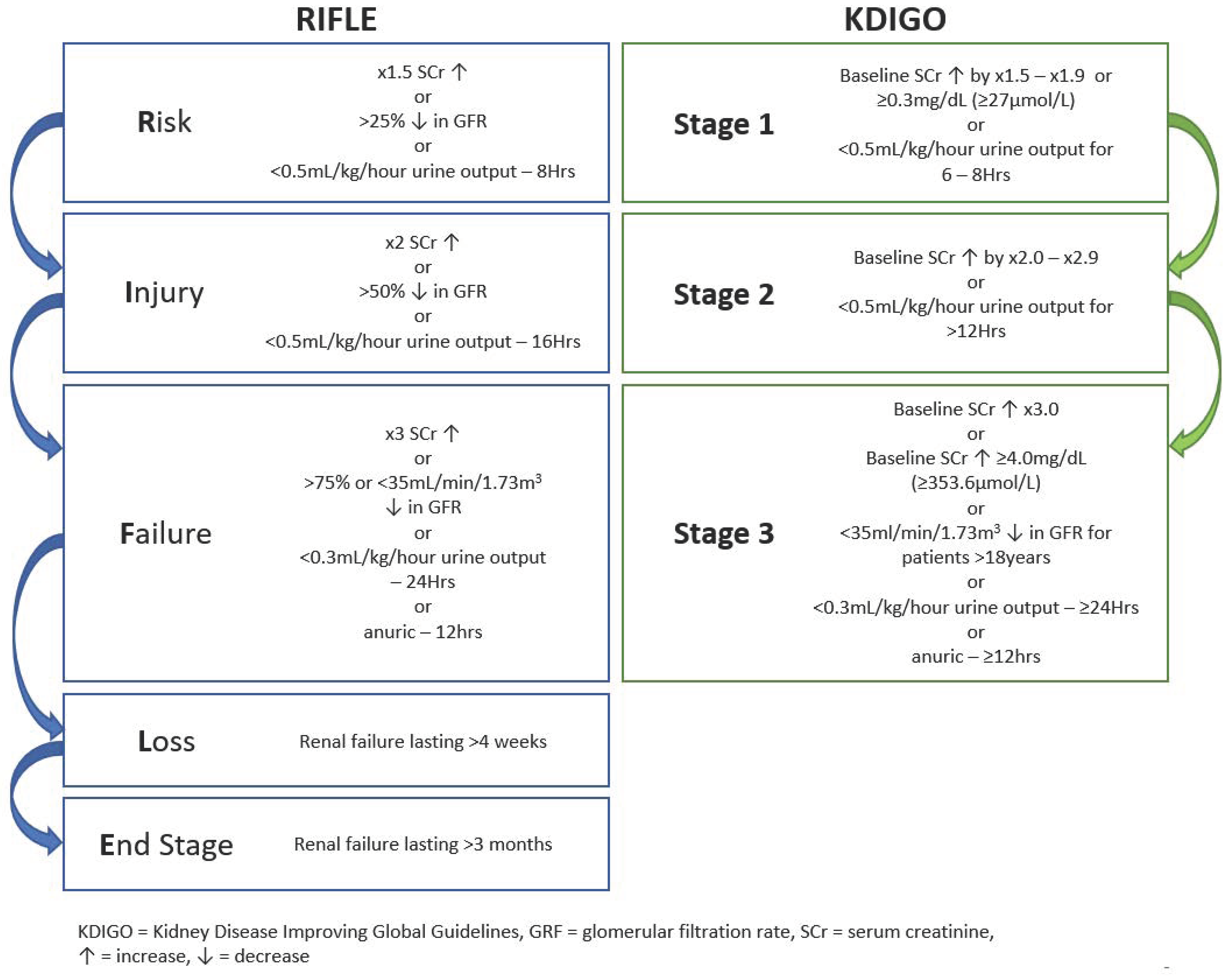
 = increase,
= increase,  = decrease.
= decrease.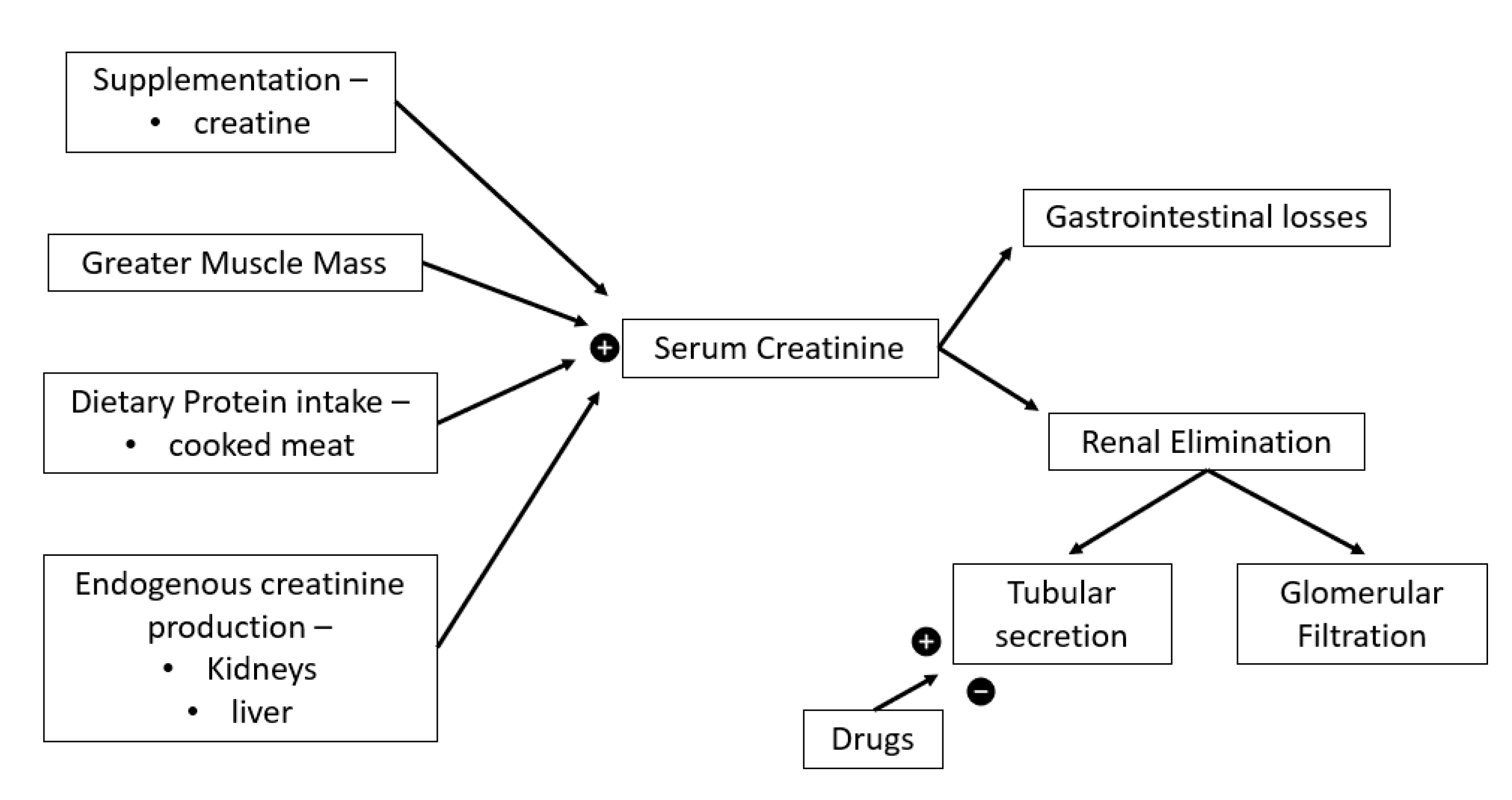
 = increase,
= increase,  = decrease.
= decrease.
 = increase,
= increase,  = decrease.
= decrease.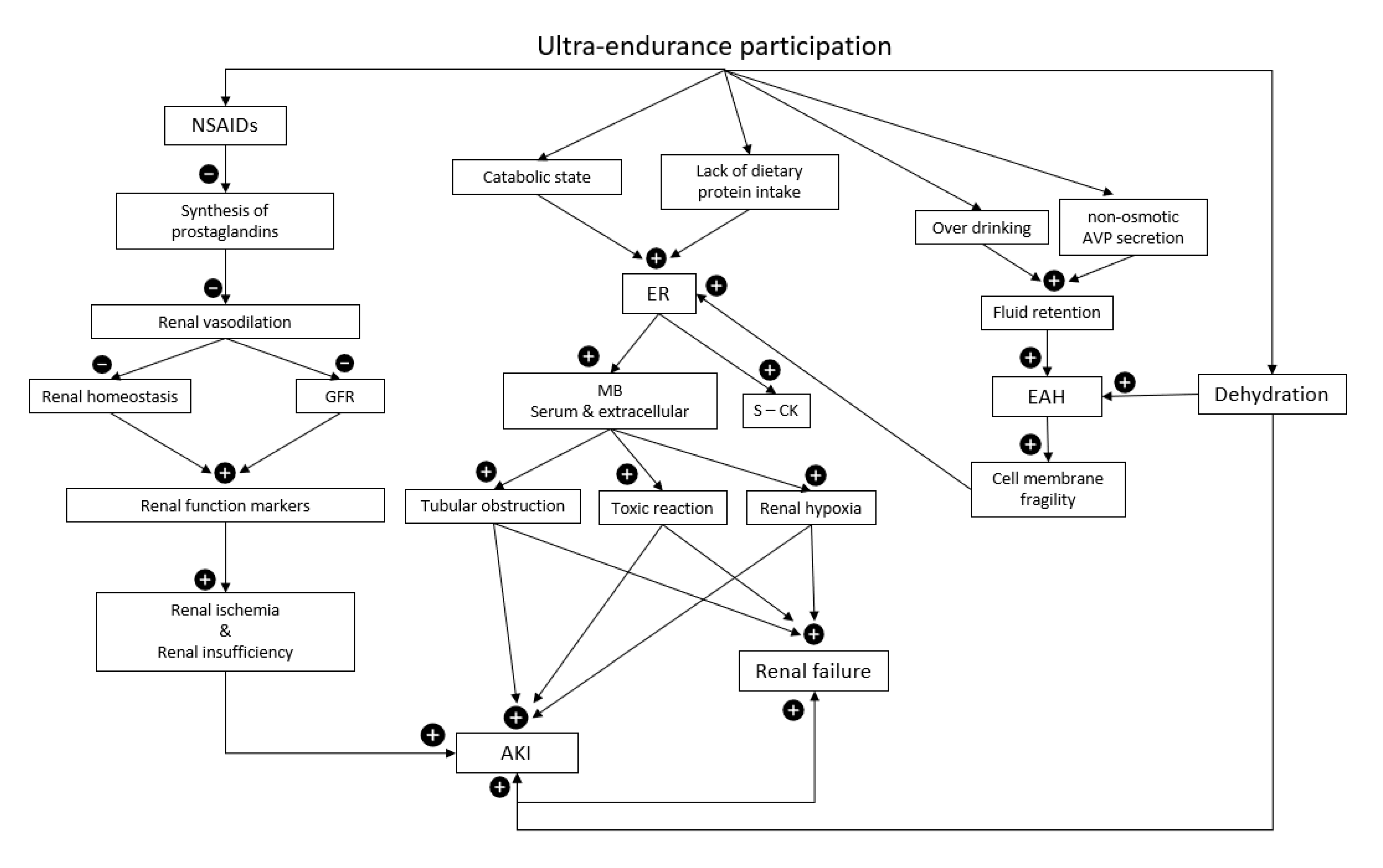
| Study | Cases | Event | Pre–Race | Diagnosis | Follow-up/ Recovery | NSAIDs |
|---|---|---|---|---|---|---|
| 35 | n = 4 | Ultramarathon (89.3 km) | NS | 4/4 = AKI 4/4 = ER 3/4 = EAH | Hospitalization and acute renal dialysis (2–21 days) | 3/4 = YES |
| 34 | n = 5 | Ultramarathon (161 km) | NS | 3/5 = AKI 5/5 = ER 5/5 = EAH | Intravenous fluids Hospitalization | 4/5 = Yes |
| 23 | n = 26 | Ultramarathon (100 km) | Yes | 22/26 = AKI 18/22 = Stage I AKI 4/22 = Stage II AKI 26/26 = ER | NS | Positive history |
| 57 | n = 627 | Ultramarathon (161 km) | Yes | 227/627 = AKI risk 31/627 = AKI | NS | NS |
| 39 | n = 30 | Multi-stage Ultramarathon (177 km) | Yes | 55–85% = RIFLE criteria Total over 3 check points 31 = AKI risk 6 = AKI | Normalised prior to start of each new stage—24 h | NS |
| 26 | n = 26 | Ultramarathon (100 km) | Yes | 22/26 = AKI 18/26 = stage I AKI 4/26 = stage II AKI | Hospitalisation—1 day | NS |
| 46 | n = 6 | Ultramarathon (217 km) | Yes | RIFLE risk only met by reduction to GFR (>25%) from 84 km no change in SCr | No medical intervention needed | Negative use |
| 40 | n = 50 | Marathon & Ultramarathon (100 km & 308 km) | YES | 100 km (n = 17) = greatest increase in SCr, all above upper reference limit | NS | NS |
| 58 | n = 1 | Ultramarathon (90 km) | NS | Acute tubular necrosis (ATN), ARF, & ER | Hospitalisation—10 days | Yes |
| 47 | n = 47 | Ultramarathon (80 km) | Yes | Total over 3 check points 8 = Stage I AKI 1 = Stage II AKI | Day 9 = normalised | NS |
| 25 | n = 128 | Multi-stage Ultramarathons | Yes | 80–102/128 = AKI per check point Total over 3 check points 179 = AKI Risk 101 = AKI | NS | NS |
| 43 | n = 37 | Ultramarathon (100 km) | Yes | 18/37 = AKI | NS | Yes |
| 48 | n = 16 | Ultramarathon (60 km) | Yes | 6/16 = AKI | NS | NS |
| 49 | n = 13 | Ultramarathon (149 km) | Yes | SCr = increased ~1/3 | NS | NS |
| 50 | n = 2 | 2-man- relay 24 h ultramarathon | Yes | SCr = increased Cr clearance = reduced | SCr = normal after 24 h | NS |
| 51 | n = 5 | Ultramarathon (90 km) | Yes | 1 = female = collapsed with transient oliguria with renal tubular dysfunction | Renal dysfunction persisted = 14 days Full recovery = 1 year | NS |
| 52 | n = 8 | Ultramarathon | Yes | SCr = significantly increased | 24 h = returned to baseline | NS |
| 53 | n = 28 | Ultra-cycle | Yes | SCr = significantly increased | SCr elevated at 24 h | NS |
| 54 | n = 16 | Ultra-cycle | Yes | SCr = significantly increased | SCr normalised at 24 h | NS |
| 55 | n = 10 | Ultramarathon | Yes | SCr post-race = no significant increase SCr increase by 25% at 6 h post-race | SCr normalised at 48 h | NS |
| 56 | n = 113 | Ultramarathon & ultra-mountain bike (24 h & 100 km) | Yes | 2/113 = increased SCr = AKI 13/113 = EAH 6/113 = ER 2/113 = EAH & ER = runners | No medical intervention | NS |
| 3 | n = 1 | Multi-stage ultra-trail marathon (786 km) | Yes | ER No AKI | 9 days | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tidmas, V.; Brazier, J.; Bottoms, L.; Muniz, D.; Desai, T.; Hawkins, J.; Sridharan, S.; Farrington, K. Ultra-Endurance Participation and Acute Kidney Injury: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 16887. https://doi.org/10.3390/ijerph192416887
Tidmas V, Brazier J, Bottoms L, Muniz D, Desai T, Hawkins J, Sridharan S, Farrington K. Ultra-Endurance Participation and Acute Kidney Injury: A Narrative Review. International Journal of Environmental Research and Public Health. 2022; 19(24):16887. https://doi.org/10.3390/ijerph192416887
Chicago/Turabian StyleTidmas, Victoria, Jon Brazier, Lindsay Bottoms, Daniel Muniz, Terun Desai, Janine Hawkins, Sivakumar Sridharan, and Ken Farrington. 2022. "Ultra-Endurance Participation and Acute Kidney Injury: A Narrative Review" International Journal of Environmental Research and Public Health 19, no. 24: 16887. https://doi.org/10.3390/ijerph192416887
APA StyleTidmas, V., Brazier, J., Bottoms, L., Muniz, D., Desai, T., Hawkins, J., Sridharan, S., & Farrington, K. (2022). Ultra-Endurance Participation and Acute Kidney Injury: A Narrative Review. International Journal of Environmental Research and Public Health, 19(24), 16887. https://doi.org/10.3390/ijerph192416887






