Idiopathic Anaphylaxis? Analysis of Data from the Anaphylaxis Registry for West Pomerania Province, Poland
Abstract
1. Introduction
- An immediate onset reaction—anaphylactic shock during the first exposure to intravenous cetuximab;
- A delayed onset reaction—occurring 3–6 h after ingestion of mammalian meat, e.g., beef, pork or offal.
2. Objective
3. Materials and Methods
3.1. Study Design and Data Collection
3.2. The Basic Questionnaire Was a Simplified Version of the Network for Online-Registration of Anaphylaxis Survey (NORA) from Berlin [9]; More Details in Other Publication [39] and Appendix B
3.3. Basal Measurement of Tryptase and sIgE
3.4. Statistical Analysis
4. Results
4.1. Descriptive Statistics
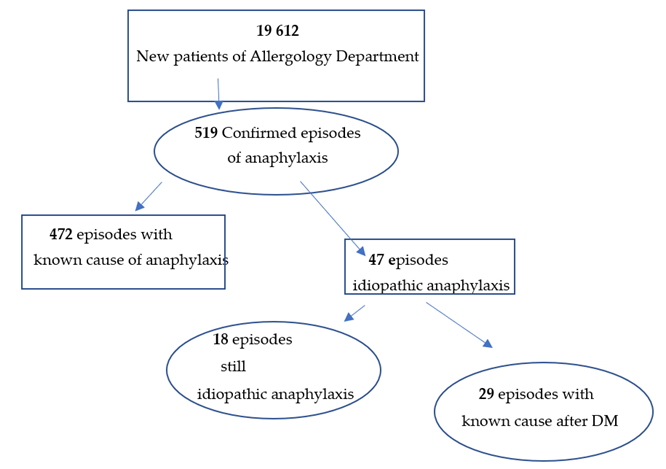
4.1.1. The Structure of the Research
- -
- In 15 cases, ingestion of wheat flour, and more specifically, of omega-5-gliadin;
- -
- In 8 cases, ingestion of fruit containing LTP;
- -
- In 6 cases, episodes of nocturnal anaphylaxis waking patients up were explained, as an α-Gal allergy to meat was found.
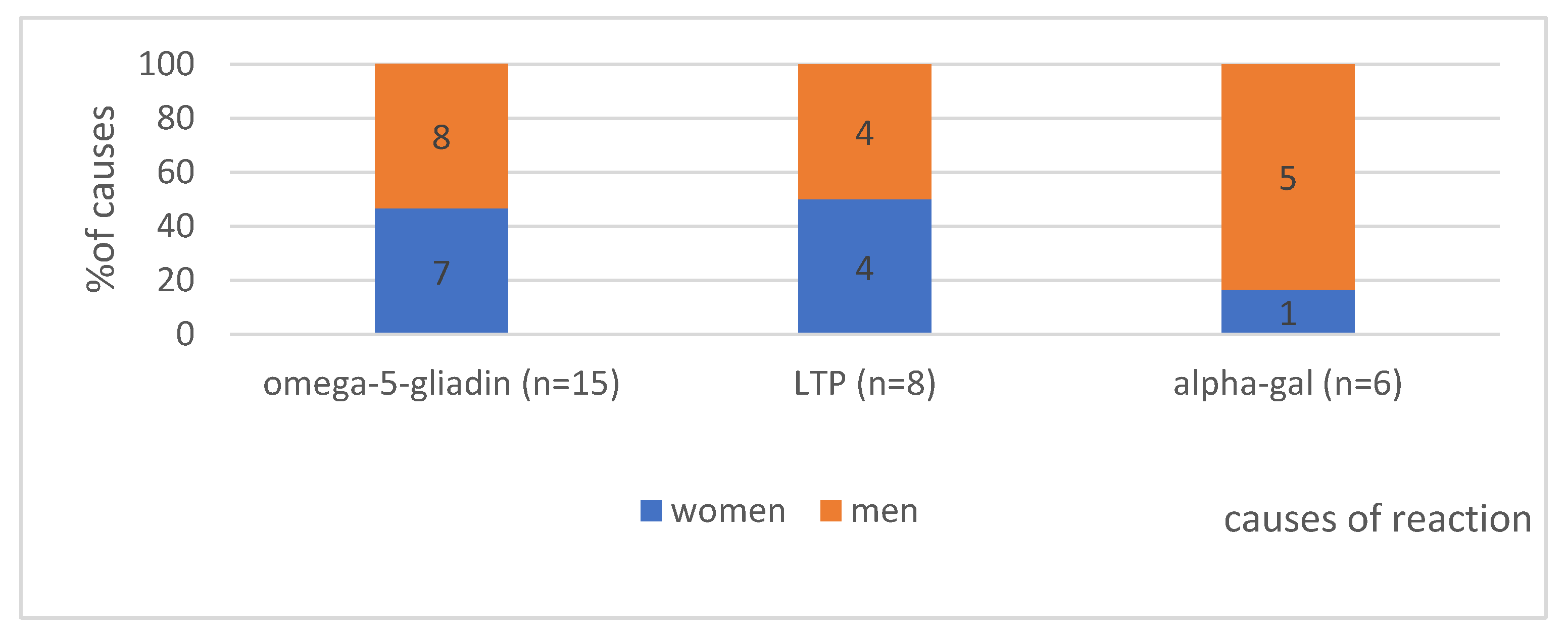
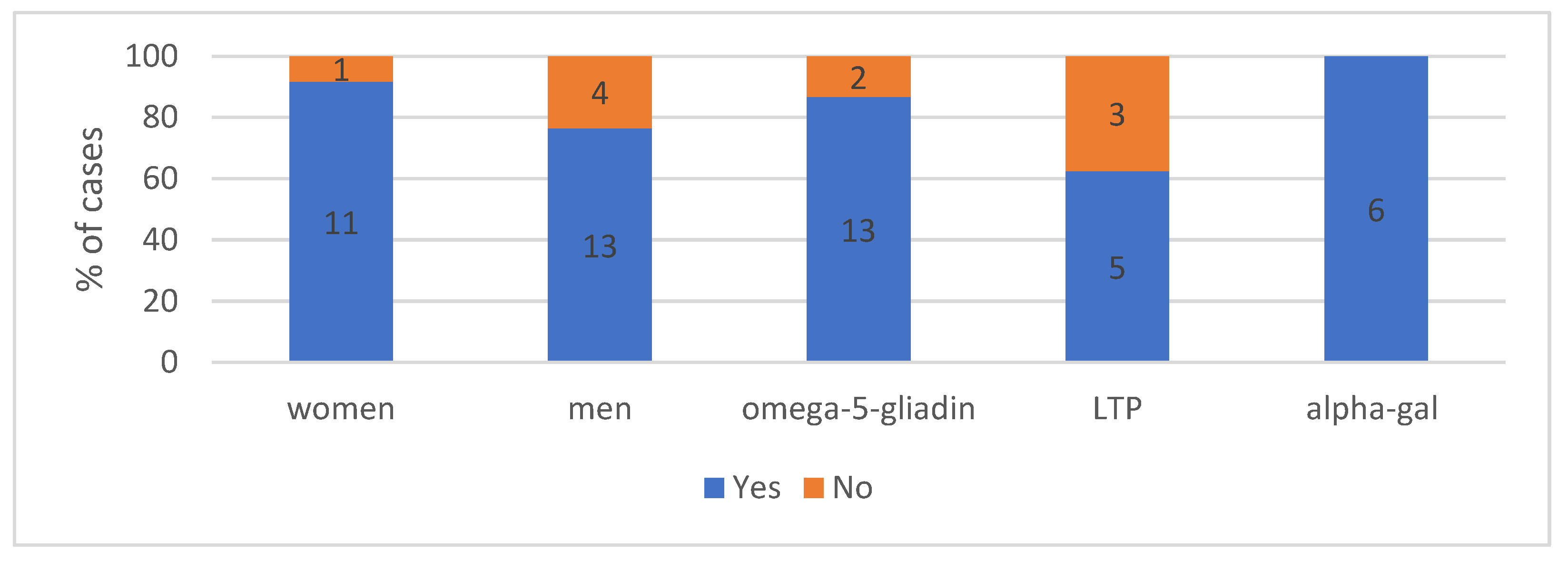
4.1.2. The Gender in Analysed Group According to the Cause of Anaphylaxis (n = 29)—Shows Figure 1
4.1.3. Age at First Occurrence of Reaction in the Analysed Group—Can Be Seen in Figure 2
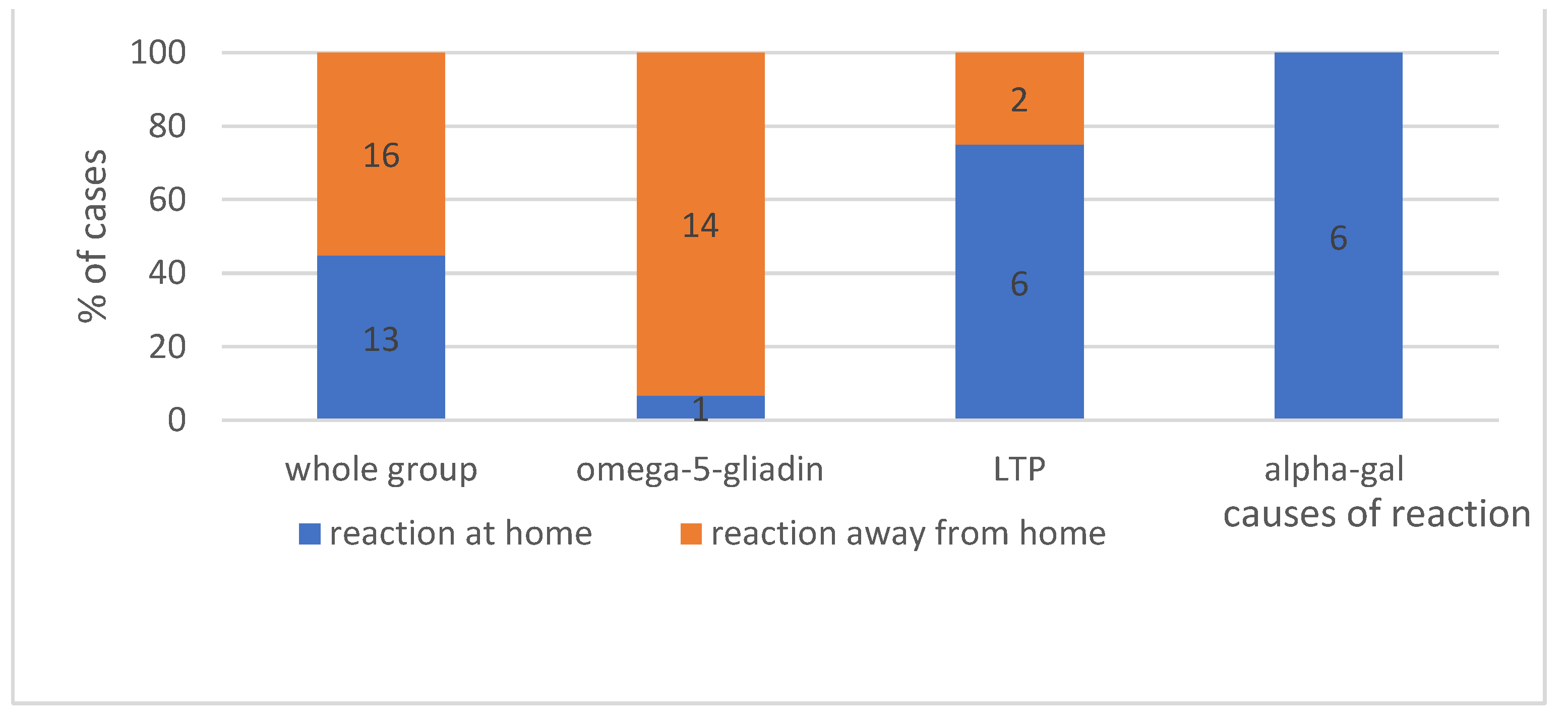
4.1.4. The Place Where Reaction Occurs—Shows Figure 3
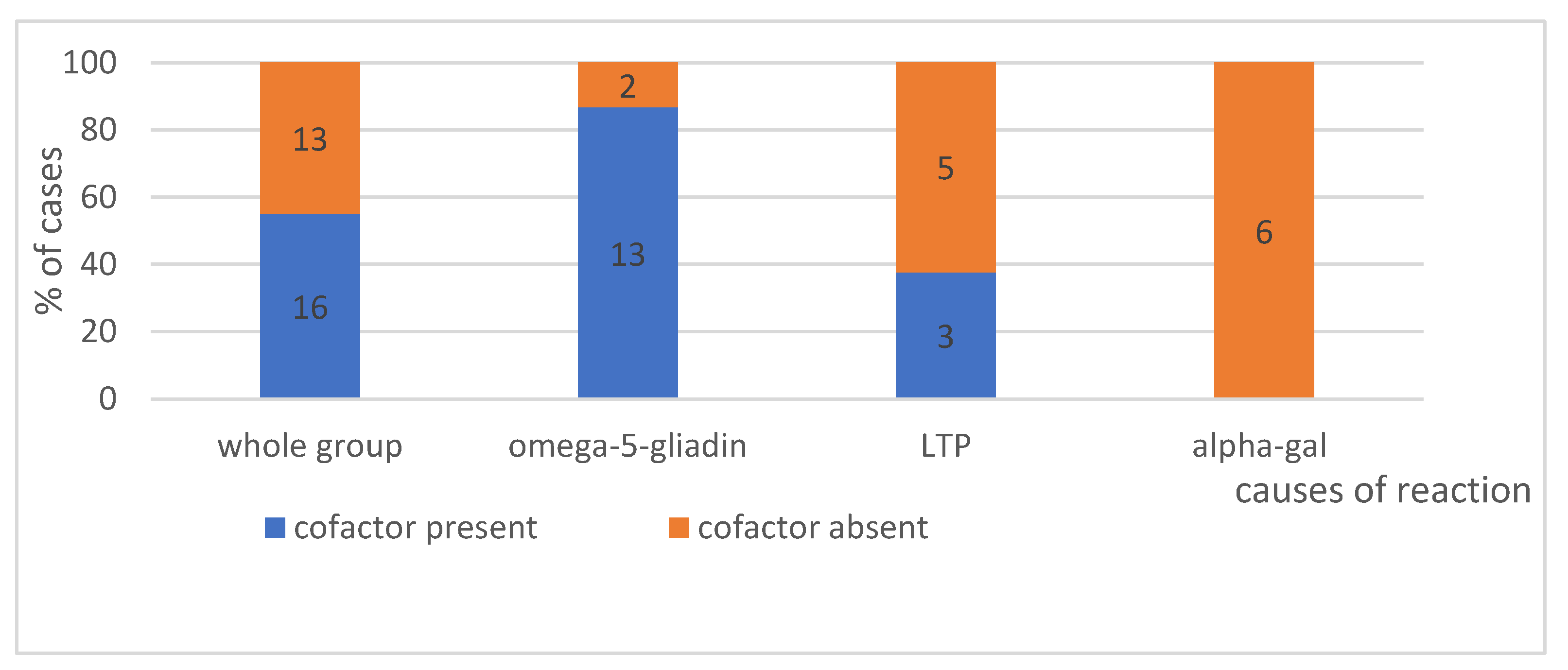
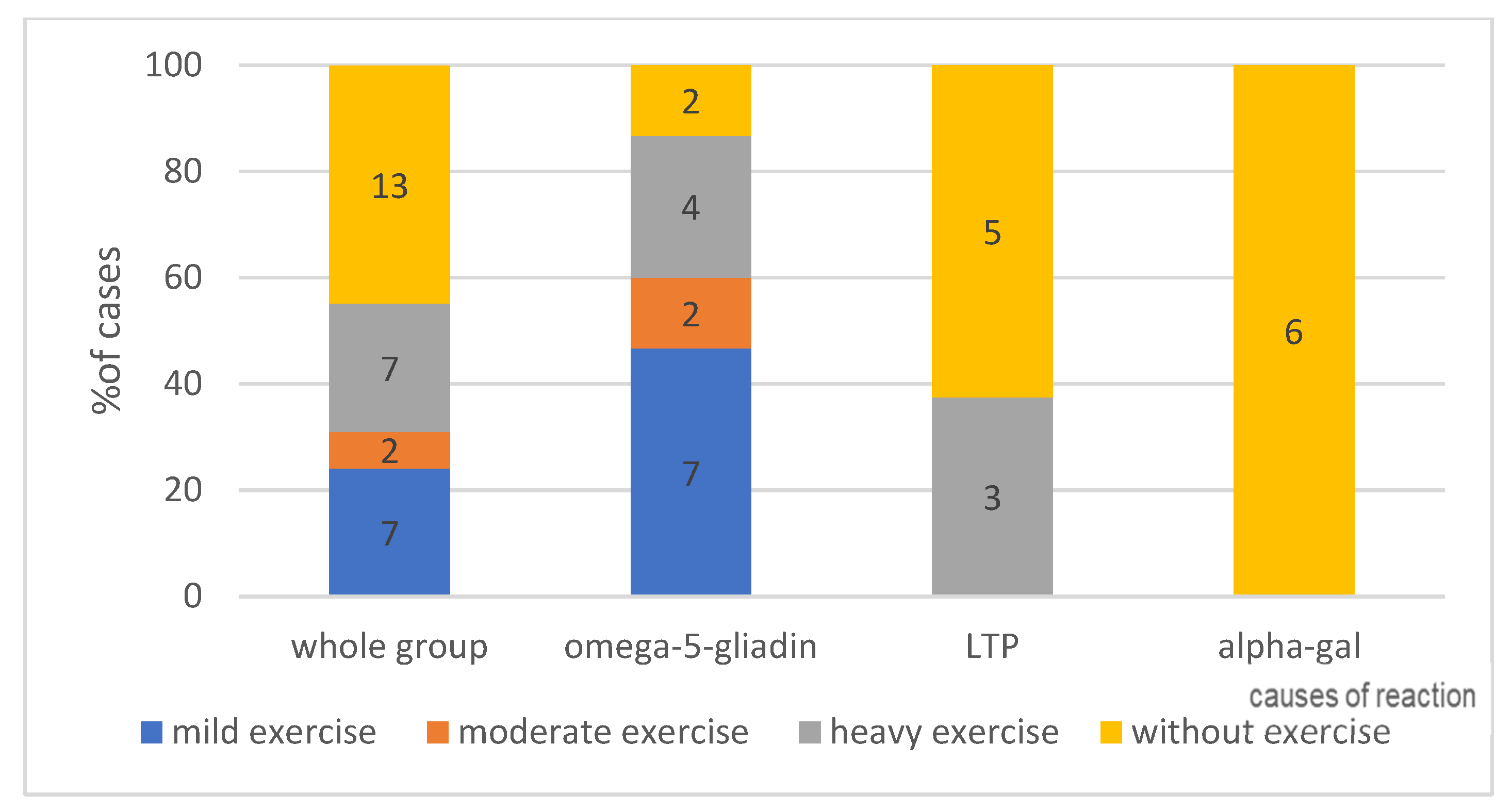
4.1.5. Effort as a Cofactor, According to Its Intensity—Can Be Seen in Figure 4—Present or No and in Figure 5—Intensity of Effort
4.2. Comparison of the Cases of Anaphylaxis Previously Classified as Idiopathic, Considering Their Causes (after the Use of MD); Early and Late Reactions, Medical Interventions (n = 29)
4.2.1. Time from Food Ingestion to Reaction Onset According to Triggering Factors—Shows Figure 6
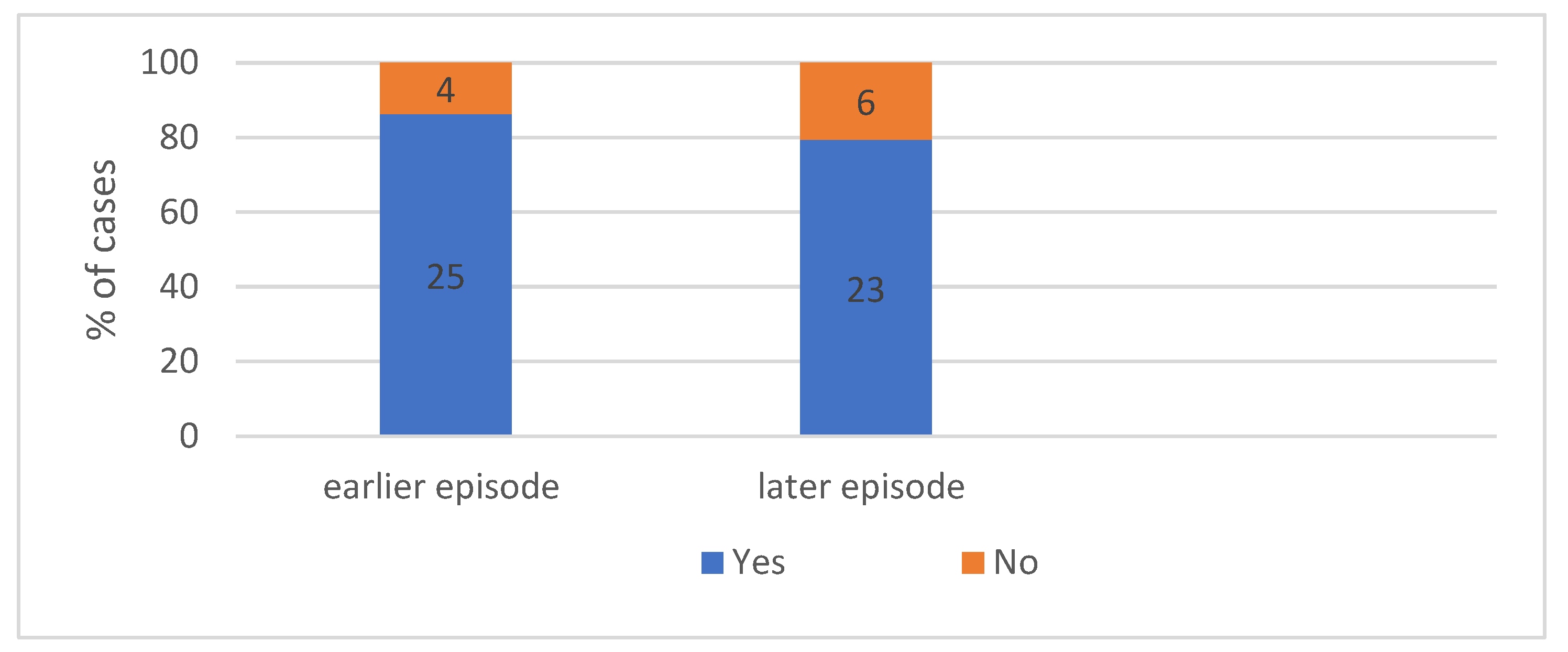
4.2.2. Patient Characteristics According to Previous Reactions. We Can Seen in Figure 7—Occurrence Both of Earlier and Later Episodes of Anaphylaxis
4.2.3. Patient Characteristics According to Later Reactions
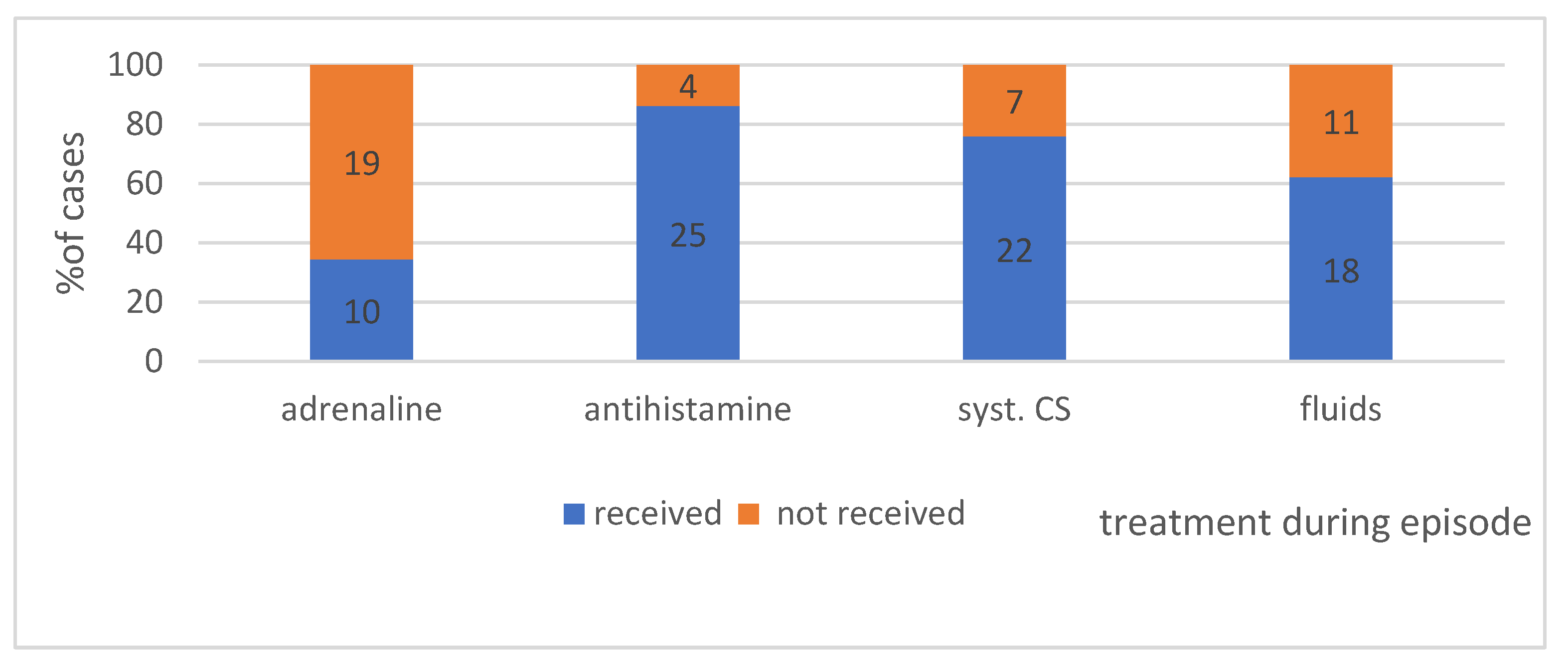
4.2.4. Treatment Applied during the Episodes in Individual Groups—Shows Figure 8
Was Intervention of Healthcare Professionals Necessary during the Episodes of Anaphylaxis in Individual Groups (n = 29)?
In How Many Cases Did Emergency Medical Service Have to Be Called to the Site Where the Reaction Occurred?
How Many Cases Required Hospital Treatment/Observation
4.3. What Medications Were Administered during the Episode of Anaphylaxis? We Can Seen in Figure 9
4.4. Providing the Patient with Emergency Kit after an Episode of Anaphylaxis—Shows Figure 10
4.5. Results of Additional Tests for Basal Tryptase and sIgE—Can Be Seen in Table 2
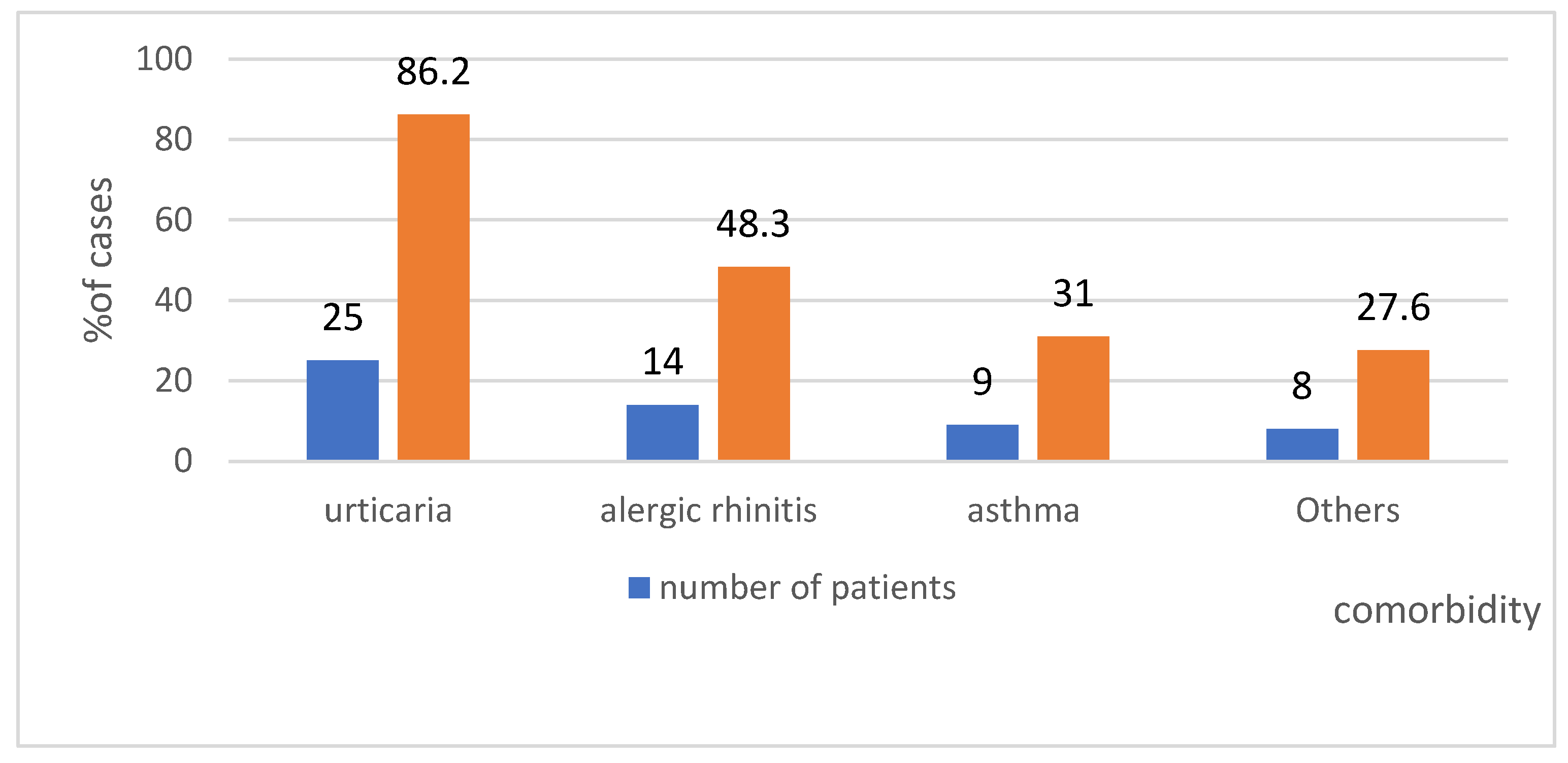
4.6. Comorbidity in the Study Group—Shows Figure 11
4.7. Time from Reaction Onset to Arrival at a Centre, Based on the Study Group
5. Discussion
6. Limitations
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
References
- Hepner, D.L.; Castells, M.C. Anaphylaxis During the Perioperative Period. Anesth. Analg. 2003, 97, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Worm, M.; Ansotegui, I.J.; El-Gamal, Y.; Rivas, M.F.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Tanno, L.K.; et al. Time to revisit the definition and clinical criteria for anaphylaxis? World Allergy Organ. J. 2019, 12, 100066. [Google Scholar] [CrossRef] [PubMed]
- Panesar, S.S.; Javad, S.; De Silva, D.; Nwaru, B.I.; Hickstein, L.; Muraro, A.; Roberts, G.; Worm, M.; Bilò, M.B.; Cardona, V.; et al. The epidemiology of anaphylaxis in Europe: A systematic review. Allergy 2013, 68, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Tanno, L.K.; Bierrenbach, A.L.; Simons, F.; Cardona, V.; Thong, B.Y.H.; Molinari, N.; Calderon, M.A.; Worm, M.; Chang, Y.S. Critical view of anaphylaxis epidemiology: Open questions and new perspectives. Allergy Asthma Clin. Immunol. 2018, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Rodriguez, T.W.; Garcia-Neuer, M.; Alenazy, L.A.; Castells, M. Anaphylaxis in the 21-st century: Phenotypes, endotypes, and biomarkers. J. Asthma Allergy 2018, 11, 121–142. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Muñoz-Furlong, A.; Campbell, R.L.; Adkinson, N.F., Jr.; Bock, S.A.; Branum, A.; Brown, S.G.; Camargo, C.A., Jr.; Cydulka, R.; Galli, S.J.; et al. Second symposium on the definition and management of anaphylaxis: Summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium. J. Allergy Clin. Immunol. 2006, 117, 391–397. [Google Scholar] [CrossRef]
- Simons, F.E.R.; Ardusso, L.R.; Bilò, M.B.; Cardona, V.; Ebisawa, M.; El-Gamal, Y.M.; Lieberman, P.; Lockey, R.F.; Muraro, A.; Roberts, G.; et al. International consensus on (ICON) anaphylaxis. World Allergy Organ. J. 2014, 30, 9. [Google Scholar] [CrossRef]
- Muraro, A.; Roberts, G.; Worm, M.; Bilò, M.B.; Brockow, K.; Fernández Rivas, M.; Santos, A.F.; Zolkipli, Z.Q.; Bellou, A.; Beyer, K.; et al. Anaphylaxis: Guidelines from the European Academy of Allergy and Clinical Immunology. Allergy 2014, 69, 1026–1045. [Google Scholar] [CrossRef]
- Worm, M.; Moneret-Vautrin, A.; Scherer, K.; Lang, R.; Fernandez-Rivas, M.; Cardona, V.; Kowalski, M.L.; Jutel, M.; Poziomkowska-Gesicka, I.; Papadopoulos, N.G.; et al. First European data from the network of severe allergic reactions (NORA). Allergy 2014, 67, 1397–1404. [Google Scholar] [CrossRef]
- Bielen, C.; Bielen, L.; Likić, R. Incidence, etiology, predictors and outcomes of suspected drug hypersensitivity reactions in a tertiary care university hospital’s emergency department: A retrospective study. Wien. Klin. Wochenschr. 2019, 131, 329–336. [Google Scholar] [CrossRef]
- Simons, F.E.R.; Ebisawa, M.; Sanchez-Borges, M.; Thong, B.Y.; Worm, M.; Tanno, L.K.; Lockey, R.F.; El-Gamal, Y.M.; Brown, S.G.; Park, H.S.; et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ. J. 2015, 8, 32. [Google Scholar] [CrossRef]
- Clark, S.; Wei, W.; Rudders, S.A.; Camargo, C.A., Jr. Risk factors for severe anaphylaxis in patients receiving anaphylaxis treatment in US emergency departments and hospitals. J. Allergy Clin. Immunol. 2014, 134, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.M.; Kim, M.K.; Kang, H.R.; Kim, T.B.; Sohn, S.W.; Koh, Y.I.; Park, H.K.; Jang, G.C.; Kim, C.W.; Jee, Y.K.; et al. Predictors of the severity and serious outcomes of anaphylaxis in Korean adults: A multicenter retrospective case study. Allergy Asthma Immunol. Res. 2015, 7, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Ortiz, M.; Alvaro, M.; Piquer, M.; Giner, M.T.; Dominguez, O.; Lozano, J.; Jiménez-Feijoo, R.; Cambra, F.J.; Plaza, A.M. Life-threatening anaphylaxis to egg and milk oral immunotherapy in asthmatic teenagers. Ann. Allergy Asthma Immunol. 2014, 113, 482–484. [Google Scholar] [CrossRef]
- Triggiani, M.; Montagni, M.; Parente, R.; Ridolo, E. Anaphylaxis and cardiovascular diseases: A dangerous liaison. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Rueff, F.; Vos, B.; Oude Elberink, J.; Bender, A.; Chatelain, R.; Dugas-Breit, S.; Horny, H.P.; Küchenhoff, H.; Linhardt, A.; Mastnik, S.; et al. Predictors of clinical effectiveness of Hymenoptera venom immunotherapy. Clin. Exp. Allergy 2014, 44, 736–746. [Google Scholar] [CrossRef]
- Valent, P. Risk factors and management of severe life-threatening anaphylaxis in patients with clonal mast cell disorders. Clin. Exp. Allergy 2014, 44, 914–920. [Google Scholar] [CrossRef]
- Gülen, T.; Hägglund, H.; Sander, B.; Dahlén, B.; Nilsson, G. The presence of mast cell clonality in patients with unexplained anaphylaxis. Clin. Exp. Allergy 2014, 44, 1179–1187. [Google Scholar] [CrossRef]
- Álvarez-Twose, I.; Zanotti, R.; González-de-Olano, D.; Bonadonna, P.; Vega, A.; Matito, A.; Sánchez-Muñoz, L.; Morgado, J.M.; Perbellini, O.; García-Montero, A.; et al. Nonaggressive systemic mastocytosis (SM) without skin lesions associated with insect-induced anaphylaxis shows unique features versus other indolent SM. J. Allergy Clin. Immunol. 2014, 133, 520–528. [Google Scholar] [CrossRef]
- Krishna, M.T.; Worm, M.; Bilo, M.B. Editorial: Anaphylaxis—A Distinct Immunological Syndrome, but How Much Do We Really Understand? Front. Immunol. 2019, 10, 2943. [Google Scholar] [CrossRef]
- Vinagre-Aragón, A.; Zis, P.; Grunewald, R.A.; Hadjivassiliou, M. Movement Disorders Related to Gluten Sensitivity: A Systematic Review. Nutrients 2018, 10, 1034. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.B.; Uhde, M.; Green, P.H.; Alaedini, A. Autoantibodies in the Extraintestinal Manifestations of Celiac Disease. Nutrients 2018, 10, 1123. [Google Scholar] [CrossRef] [PubMed]
- Sievers, S.; Rawel, H.M.; Ringel, K.P.; Niggemann, B.; Beyer, K. Wheat protein recognition pattern in tolerant and allergic children. Pediatr. Allergy Immunol. 2016, 27, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Andreozzi, L.; Cipriani, F.; Giannetti, A.; Gallucci, M.; Caffarelli, C. Wheat Allergy in Children: A Comprehensive Update. Medicina 2019, 55, 400. [Google Scholar] [CrossRef] [PubMed]
- Czaja-Bulsa, G.; Bulsa, M. What Do We Know Now about IgE-Mediated Wheat Allergy in Children? Nutrients 2017, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Scherf, K.A.; Brockow, K.; Biedermann, T.; Koehler, P.; Wieser, H. Wheat-dependent exercise-induced anaphylaxis. Clin. Exp. Allergy 2016, 46, 10–20. [Google Scholar] [CrossRef]
- Matsuo, H.; Dahlström, J.; Tanaka, A.; Kohno, K.; Takahashi, H.; Furumura, M.; Morita, E. Sensitivity and specificity of recombinant omega-5 gliadin-specific IgE measurement for the diagnosis of wheat-dependent exercise-induced anaphylaxis. Allergy 2008, 63, 233–236. [Google Scholar] [CrossRef]
- Hofmann, S.C.; Fischer, J.; Eriksson, C.; Bengtsson Gref, O.; Biedermann, T.; Jakob, T. IgE detection to α/β/γ-gliadin and its clinical relevance in wheat-dependent exercise-induced anaphylaxis. Allergy 2012, 67, 1457–1460. [Google Scholar] [CrossRef]
- Pascal, M.; Muñoz-Cano, R.; Reina, Z.; Palacín, A.; Vilella, R.; Picado, C.; Juan, M.; Sánchez-López, J.; Rueda, M.; Salcedo, G.; et al. Lipid transfer protein syndrome: Clinical pattern, cofactor effect and profile of molecular sensitization to plant-foods and pollens. Clin. Exp. Allergy 2012, 42, 1529–1539. [Google Scholar] [CrossRef]
- Sancho, A.I.; Foxall, R.; Rigby, N.M.; Browne, T.; Zuidmeer, L.; van Ree, R.; Waldron, K.W.; Mills, E.C. Maturity and storage influence on the apple (Malus domestica) allergen Mal d 3, a nonspecific lipid transfer protein. J. Agric. Food Chem. 2006, 54, 5098–5104. [Google Scholar] [CrossRef]
- Pastorello, E.A.; Robino, A.M. Clinical role of lipid transfer proteins in food allergy. Mol. Nutr. Food Res. 2004, 48, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Ukleja-Sokołowska, N.; Bartuzi, Z. Alergia pokarmowa—Sytuacja społeczna i prawna. Alerg. Astma Immunol. 2015, 20, 88–93. [Google Scholar]
- Asero, R.; Mistrello, G.; Roncarolo, D.; Amato, S. Detection of some safe plant-derived foods for LTP-allergic patients. Int. Arch. Allergy Immunol. 2007, 144, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Platts-Mills, T.A.; Li, R.C.; Keshavarz, B.; Smith, A.R.; Wilson, J.M. Diagnosis and management of patients with the α-gal syndrome. J. Allergy Clin. Immunol. Pract. 2020, 8, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Lied, G.A.; Lund, K.B.; Storaas, T. Intraoperative ana-phylaxis to gelatin-based hemostatic agents: A case report. J. Asthma Allergy 2019, 12, 163–167. [Google Scholar] [CrossRef]
- Hilger, C.; Fischer, J.; Wölbing, F.; Biedermann, T. Role and mechanism of galactose-alpha-1,3-galactose in the elicitation of delayed anaphylactic reactions to red meat. Curr. Allergy Asthma Rep. 2019, 19, 3. [Google Scholar] [CrossRef]
- Cardona, V.; Ansotegui, I.J. Component-resolved diagnosis in anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 244–249. [Google Scholar] [CrossRef]
- Ring, J.; Messmer, K. Incidence and severity of anaphylactoid reactions to colloid volume substituted. Lancet 1977, 1, 466–469. [Google Scholar] [CrossRef]
- Poziomkowska-Gęsicka, I.; Kurek, M. Clinical Manifestations and Causes of Anaphylaxis. Analysis of 382 Cases from the Anaphylaxis Registry in West Pomerania Province in Poland. Int. J. Environ. Res. Public Health 2020, 17, 2787. [Google Scholar] [CrossRef]
- Kraft, M.; Dölle-Bierke, S.; Renaudin, J.M.; Ruëff, F.; Hofmeier, K.S.; Treudler, R.; Pföhler, C.; Hawranek, T.; Poziomkowska-Gęsicka, I.; Jappe, U.; et al. Anaphylaxis in Adults Differs from Reactions to Other Types of Food. J. Allergy Clin. Immunol. Pract. 2021, 9, 2844–2852.e5. [Google Scholar] [CrossRef]
- Cho, H.; Kim, D.; Choo, Y.; Park, J.; Choi, J.; Jang, D.; Kim, T.; Jeong, J.W.; Kwon, J.W. Common causes of emergency department visits for anaphylaxis in Korean community hospitals: A cross-sectional study. Medicine 2019, 98, e14114. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Estrada, A.; Silvers, S.K.; Klein, A.; Zell, K.; Wang, X.F.; Lang, D.M. Epidemiology of anaphylaxis at a tertiary care center: A report of 730 cases. Ann. Allergy Asthma Immunol. 2017, 118, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Heaps, A.; Carter, S.; Selwood, C.; Moody, M.; Unsworth, J.; Deacock, S.; Sumar, N.; Bansal, A.; Hayman, G.; El-Shanawany, T.; et al. The utility of the ISAC allergen array in the investigation of idiopathic anaphylaxis. Clin. Exp. Immunol. 2014, 177, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Zurbano-Azqueta, L.; Antón-Casas, E.; Duque-Gómez, S.; Jiménez-Gómez, I.; Fernández-Pellón, L.; López-Gutiérrez, J. Alpha-gal syndrome. Allergy to red meat and gelatin. Rev. Clin. Esp. 2022, 222, 401–405. [Google Scholar] [CrossRef]
- Commins, S.P.; Platts-Mills, T.A. Delayed anaphylaxis to red meat in patients with IgE specific for galactose alpha-1,3-galactose (alpha-gal). Curr. Allergy Asthma Rep. 2013, 13, 72–77. [Google Scholar] [CrossRef]
- Pattanaik, D.; Lieberman, P.; Lieberman, J.; Pongdee, T.; Keene, A.T. The changing face of anaphylaxis in adults and adolescents. Ann. Allergy Asthma Immunol. 2018, 121, 594–597. [Google Scholar] [CrossRef]
- Khan, N.U.; Shakeel, N.; Makda, A.; Mallick, A.S.; Ali Memon, M.; Hashmi, S.H.; Khan, U.R.; Razzak, J.A. Anaphylaxis: Incidence, presentation, causes and outcome in patients in a tertiary-care hospital in Karachi, Pakistan. QJM Int. J. Med. 2013, 106, 1095–1101. [Google Scholar] [CrossRef]
- Nabavi, M.; Lavavpour, M.; Arshi, S.; Bemanian, M.H.; Esmaeilzadeh, H.; Molatefi, R.; Rekabi, M.; Ahmadian, J.; Eslami, N.; Shokri, S.; et al. Characteristics, etiology and treatment of pediatric and adult anaphylaxis in Iran. Iran. J. Allergy Asthma Immunol. 2017, 16, 480–487. [Google Scholar]
- Venturini, M.; Lobera, T.; Sebastián, A.; Portillo, A.; Oteo, J.A. IgE to alpha-Gal in foresters and forest workers from La Rioja, north of Spain. J. Investig. Allergol. Clin. Immunol. 2018, 28, 106–112. [Google Scholar] [CrossRef]
- Tripathi, A.; Commins, S.P.; Heymann, P.W.; Platts-Mills, T.A. Delayed anaphylaxis to red meat masquerading as idiopathic anaphylaxis. J. Allergy Clin. Immunol. Pract. 2014, 2, 259–265. [Google Scholar] [CrossRef]
- Caponetto, P.; Fischer, J.; Biedermann, T. Gelatin-containing sweets can elicit anaphylaxis in a patient with sensitization to galactose-a-1,3-galactose. J. Allergy Clin. Immunol. Pract. 2013, 1, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Commins, S.P.; Satinover, S.M.; Hosen, J.; Mozena, J.; Borish, L.; Lewis, B.D.; Woodfolk, J.A.; Platts-Mills, T.A. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J. Allergy Clin. Immunol. 2009, 123, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Pisazka, V.; Duscher, G.; Hodžić, A.; Reider, N.; Allerberger, F. Alpha-gal allergy after a tick bite in Austria. Wien. Klin. Wochenschr. 2019, 131, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Hebsaker, J.; Caponetto, P.; Platts-Mills, T.A.; Biedermann, T. Galactose-alpha-1,3-galactose sensitization is a prerequisite for pork-kidney allergy and cofactor-related mammalian meat anaphylaxis. J. Allergy Clin. Immunol. 2014, 134, 755–759. [Google Scholar] [CrossRef]
- Christensen, M.J.; Eller, E.; Mortz, C.G.; Brockow, K.; Bindslev-Jensen, C. Exercise Lowers Threshold and Increases Severity, but Wheat-Dependent, Exercise-Induced Anaphylaxis Can Be Elicited at Rest. J. Allergy Clin. Immunol. Pract. 2018, 6, 514–520. [Google Scholar] [CrossRef]
- Christensen, M.J.; Eller, E.; Mortz, C.G.; Brockow, K.; Bindslev-Jensen, C. Wheat-Dependent Cofactor-Augmented Anaphylaxis: A Prospective Study of Exercise, Aspirin, and Alcohol Efficacy as Cofactors. J. Allergy Clin. Immunol. Pract. 2019, 7, 114–121. [Google Scholar] [CrossRef]
- Brockow, K.; Kneissl, D.; Valentini, L.; Zelger, O.; Grosber, M.; Kugler, C.; Werich, M.; Darsow, U.; Matsuo, H.; Morita, E.; et al. Using a gluten oral food challenge protocol to improve diagnosis of wheat-dependent exercise-induced anaphylaxis. J. Allergy Clin. Immunol. 2015, 135, 977–984. [Google Scholar] [CrossRef]
- Scherf, K.A.; Lindenau, A.C.; Valentini, L.; Collado, M.C.; García-Mantrana, I.; Christensen, M.; Tomsitz, D.; Kugler, C.; Biedermann, T.; Brockow, K. Cofactors of wheat-dependent exercise-induced anaphylaxis do not increase highly individual gliadin absorption in healthy volunteers. Clin. Transl. Allergy 2019, 9, 19. [Google Scholar] [CrossRef]
- Hompes, S.; Dölle, S.; Grünhagen, J.; Grabenhenrich, L.; Worm, M. Elicitors and co-factors in food-induced anaphylaxis in adults. Clin. Transl. Allergy 2013, 3, 38. [Google Scholar] [CrossRef]
- Aihara, M.; Miyazawa, M.; Osuna, H.; Tsubaki, K.; Ikebe, T.; Aihara, Y.; Ikezawa, Z. Food-dependent exercise-induced anaphylaxis: Influence of concurrent aspirin administration on skin testing and provocation. Br. J. Dermatol. 2002, 146, 466–472. [Google Scholar] [CrossRef]
- Poziomkowska-Gęsicka, I.; Kostrzewska, M.; Kurek, M. Comorbidities and Cofactors of Anaphylaxis in Patients with Moderate to Severe Anaphylaxis. Analysis of Data from the Anaphylaxis Registry for West Pomerania Province, Poland. Int. J. Environ. Res. Public Health 2021, 18, 333. [Google Scholar] [CrossRef] [PubMed]
- Múgica-García, M.V.; Tejedor-Alonso, M.A.; Moro-Moro, M.; Esteban-Hernández, J.; Rojas-Perez-Ezquerra, P.E.; Vila-Albelda, C.; Rosado-Ingelmo, A. Self-Management of Anaphylaxis Is Not Optimal. J. Investig. Allergol. Clin. Immunol. 2015, 25, 408–415. [Google Scholar] [PubMed]
- Mostmans, Y.; Grosber, M.; Blykers, M.; Mols, P.; Naeije, N.; Gutermuth, J. Adrenalin in anaphylaxis treatment and self-administration: Experiences from an inner city emergency department. Allergy 2017, 72, 492–497. [Google Scholar] [CrossRef]
- Ring, J.; Klimek, L.; Worm, M. Adrenaline in the Acute Treatment of Anaphylaxis. Dtsch. Arztebl. Int. 2018, 115, 528–534. [Google Scholar] [CrossRef] [PubMed]
- McLean-Tooke, A.P.; Bethune, C.A.; Fay, A.C.; Spickett, G.P. Adrenaline in the treatment of anaphylaxis: What is the evidence? BMJ 2003, 327, 1332–1335. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Ten Broek, V.; Brown, S.G.A.; Simons, F.E.R. H1-antihistamines for the treatment of anaphylaxis: Cochrane systematic review. Allergy 2007, 62, 830–837. [Google Scholar] [CrossRef]
- Nurmatov, U.B.; Rhatigan, E.; Simons, F.E.; Sheikh, A. H2-antihistamines for the treatment of anaphylaxis with and without shock: A systematic review. Ann. Allergy Asthma Immunol. 2014, 112, 126–131. [Google Scholar] [CrossRef]
- Bilò, M.B.; Cichocka-Jarosz, E.; Pumphrey, R.; Oude-Elberink, J.N.; Lange, J.; Jakob, T.; Bonadonna, P.; Fernandez, J.; Kosnik, M.; Helbling, A.; et al. Self-medication of anaphylactic reactions due to Hymenoptera stings-an EAACI Task Force Consensus Statement. Allergy 2016, 71, 931–943. [Google Scholar] [CrossRef]
- Ring, J.; Beyer, K.; Biedermann, T.; Bircher, A.; Duda, D.; Fischer, J.; Friedrichs, F.; Fuchs, T.; Gieler, U.; Jakob, T.; et al. Guideline for acute therapy and management of anaphylaxis: S2 Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Association of German Allergologists (AeDA), the Society of Pediatric Allergy and Environmental Medicine (GPA), the German Academy of Allergology and Environmental Medicine (DAAU), the German Professional Association of Pediatricians (BVKJ), the Austrian Society for Allergology and Immunology (ÖGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Anaesthesiology and Intensive Care Medicine (DGAI), the German Society of Pharmacology (DGP), the German Society for Psychosomatic Medicine (DGPM), the German Working Group of Anaphylaxis Training and Education (AGATE) and the patient organization German Allergy and Asthma Association (DAAB). Allergo J. Int. 2014, 23, 96–112. [Google Scholar]
- Choo, K.J.L.; Simons, F.E.R.; Sheikh, A. Glucocorticoids for the treatment of anaphylaxis: Cochrane systematic review. Allergy 2010, 65, 1205–1211. [Google Scholar] [CrossRef]
- Choo, K.J.; Simons, F.E.; Sheikh, A. Glucocorticoids for the treatment of anaphylaxis. Evid. Based Child Health 2013, 8, 1276–1294. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, P.; Nicklas, R.A.; Oppenheimer, J.; Kemp, S.F.; Lang, D.M.; Bernstein, D.I.; Bernstein, J.A.; Burks, A.W.; Feldweg, A.M.; Fink, J.N.; et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J. Allergy Clin. Immunol. 2010, 126, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Halken, S.; Muraro, A.; De Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R. European Academy of Allergy and Clinical Immunology, Food Allergy, Anaphylaxis Guidelines Group. EAACI guidelines: Anaphylaxis (2021 update). Allergy 2022, 77, 357–377. [Google Scholar]
- Gold, M.S.; Sainsbury, R. First aid anaphylaxis management inchildren who were prescribed an epinephrine autoinjectordevice (EpiPen). J. Allergy Clin. Immunol. 2000, 106 Pt 1, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Mullins, R.J. Anaphylaxis: Risk factors for recurrence. Clin. Exp. Allergy 2003, 33, 1033–1040. [Google Scholar] [CrossRef]
- Cianferoni, A.; Novembre, E.; Pucci, N.; Lombardi, E.; Bernardini, R.; Vierucci, A. Anaphylaxis: A 7-year follow-up survey of 46 children. Ann. Allergy Asthma Immunol. 2004, 92, 464–468. [Google Scholar] [CrossRef]
- Asero, R.; Antonicelli, L.; Arena, A.; Bommarito, L.; Caruso, B.; Colombo, G.; Crivellaro, M.; De Carli, M.; Della Torre, E.; Della Torre, F.; et al. Epinephrine autoinjector prescription in food-allergic adults:symptom-based only or allergen-based also? An Italian multi-centre study. Eur. Ann. Allergy Clin. Immunol. 2010, 42, 25–31. [Google Scholar]
- Sicherer, S.H.; Munoz-Furlong, A.; Sampson, H.A. Prevalence of seafood allergy in the United States determined by a random telephone survey. J. Allergy Clin. Immunol. 2004, 114, 159–165. [Google Scholar] [CrossRef]
- Commins, S.P.; Jerath, M.R.; Cox, K.; Erickson, L.D.; Platts-Mills, T. Delayed anaphylaxis to alpha-gal, an oligosaccharide in mammalian meat. Allergol. Int. 2016, 65, 16–20. [Google Scholar] [CrossRef]
- Wen, L.; Zhou, J.; Yin, J.; Sun, J.L.; Sun, Y.; Wu, K.; Katial, R. Delayed Anaphylaxis to Red Meat Associated With Specific IgE Antibodies to Galactose. Allergy Asthma Immunol. Res. 2015, 7, 92–94. [Google Scholar] [CrossRef]
- Brzozowska, M.; Mokrzycka, N.; Porębski, G. Alpha-gal syndrome: The first report in Poland. Cent. Eur. J. Immunol. 2021, 46, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Stone, C.A., Jr.; Hemler, J.A.; Commins, S.P.; Schuyler, A.J.; Phillips, E.J.; Peebles, R.S.; Fahrenholz, J.M. Anaphylaxis after zoster vaccine: Implicating alpha-gal allergy as a possible mechanism. J. Allergy Clin. Immunol. 2017, 139, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Kutluǧ, Ş.; Hancıoǧlu, G.; Kökçü, Ş.İ.K.; Sancak, R.; Öztürk, F. Evaluation of red meat allergy patients and review of the literature. Turk. J. Pediatr. 2021, 63, 832–845. [Google Scholar] [CrossRef] [PubMed]



| WAO (Simons et al., 2011) | A serious life-threatening generalized or systemic hypersensitivity reaction. A serious allergic reaction that is rapid in onset and might cause death |
| EAACI (Panesar et al., 2013) | A severe life-threatening generalized or systemic hypersensitivity reaction. An acute, potentially fatal, multi-organ system allergic reaction. |
| AAAAI/ACAAI (Lieberman et al., 2010) | An acute life-threatening systemic reaction with varied mechanisms, clinical presentations, and severity that results from the sudden release of mediators from mast cells and basophils. |
| ASCIA (Brown et al., 2006) | Anaphylaxis is a serious, rapid-onset allergic reaction that may cause death. Severe anaphylaxis is characterized by life-threatening upper airway obstruction, bronchospasm and/or hypotension. |
| n | Average | SD | Min | Max | |
|---|---|---|---|---|---|
| Tryptase | 27 | 4.3 | 1.3 | 1.7 | 6.9 |
| 11 | 3.97 | 1.2 | 1.95 | 5.9 |
| 16 | 4.6 | 1.3 | 1.7 | 6.9 |
| Omega-5-gliadin | 15 | 9.5 | 10.7 | 0.5 | 38.8 |
| 7 | 9.7 | 9.1 | 0.5 | 26.9 |
| 8 | 9.3 | 12.6 | 0.5 | 38.8 |
| LTP | 8 | 19.8 | 33.8 | 0.4 | 100 |
| 4 | 25.4 | 49.7 | 0.4 | 100 |
| 4 | 14.3 | 10.6 | 3.93 | 23.9 |
| Alpha-gal | 6 | 54.9 | 43.4 | 1.46 | 100 |
| 1 | 1.46 | 1.46 | 1.46 | |
| 5 | 65.6 | 38.8 | 8.1 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poziomkowska-Gęsicka, I. Idiopathic Anaphylaxis? Analysis of Data from the Anaphylaxis Registry for West Pomerania Province, Poland. Int. J. Environ. Res. Public Health 2022, 19, 16716. https://doi.org/10.3390/ijerph192416716
Poziomkowska-Gęsicka I. Idiopathic Anaphylaxis? Analysis of Data from the Anaphylaxis Registry for West Pomerania Province, Poland. International Journal of Environmental Research and Public Health. 2022; 19(24):16716. https://doi.org/10.3390/ijerph192416716
Chicago/Turabian StylePoziomkowska-Gęsicka, Iwona. 2022. "Idiopathic Anaphylaxis? Analysis of Data from the Anaphylaxis Registry for West Pomerania Province, Poland" International Journal of Environmental Research and Public Health 19, no. 24: 16716. https://doi.org/10.3390/ijerph192416716
APA StylePoziomkowska-Gęsicka, I. (2022). Idiopathic Anaphylaxis? Analysis of Data from the Anaphylaxis Registry for West Pomerania Province, Poland. International Journal of Environmental Research and Public Health, 19(24), 16716. https://doi.org/10.3390/ijerph192416716






