Cognitive Function Trajectories and Factors among Chinese Older Adults with Subjective Memory Decline: CHARLS Longitudinal Study Results (2011–2018)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Cognitive Assessment
2.2.1. Subjective Memory Decline
2.2.2. Cognitive Function
2.3. Covariates
2.3.1. Demographic and Health-Related Variables
2.3.2. Instrumental Activities of Daily Living (IADL)
2.3.3. Depression
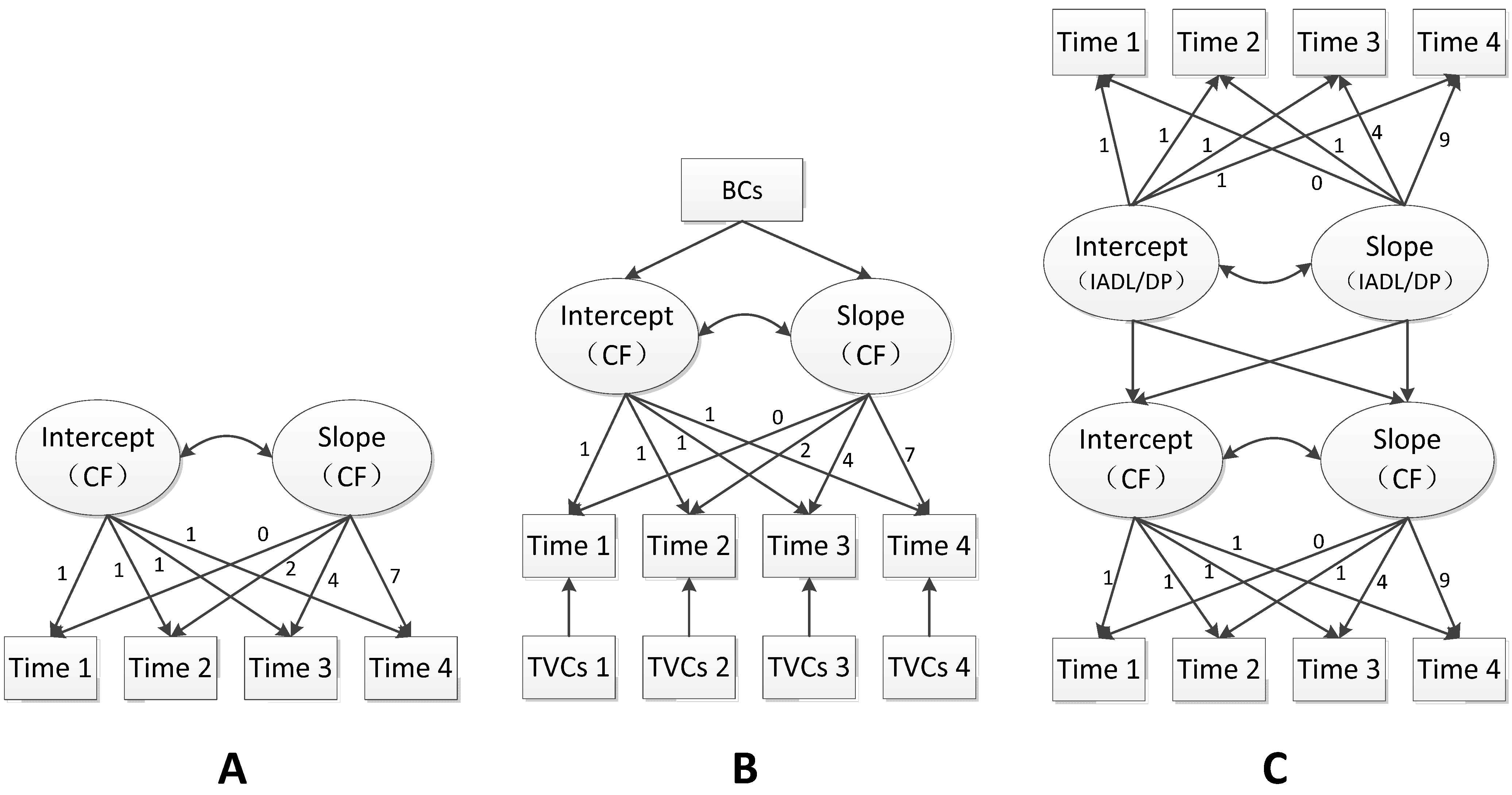
2.4. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Univariate LGCM of Cognitive Function
3.3. Conditional LGCM
3.4. Unconditional Parallel Process LGCM
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Gallistel, C.R. Finding numbers in the brain. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2017, 373, 20170119. [Google Scholar] [CrossRef] [PubMed]
- Delazer, M.; Kemmler, G.; Benke, T. Health numeracy and cognitive decline in advanced age. Neuropsychol. Dev. Cognition. Sect. B Aging Neuropsychol. Cogn. 2013, 20, 639–659. [Google Scholar] [CrossRef] [PubMed]
- Howieson, D.B.; Holm, L.A.; Kaye, J.A.; Oken, B.S.; Howieson, J. Neurologic function in the optimally healthy oldest old. Neuropsychological evaluation. Neurology 1993, 43, 1882–1886. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Li, X.; Zhang, J.; Yang, C.; Tao, W.; Zhang, S.; Zhang, Z.; Peng, D. White matter integrity disruption in the pre-dementia stages of Alzheimer’s disease: From subjective memory impairment to amnestic mild cognitive impairment. Eur. J. Neurol. 2019, 26, 800–807. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; van der Flier, W.M.; et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2014, 10, 844–852. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; Buckley, R.F.; van der Flier, W.M.; Han, Y.; Molinuevo, J.L.; Rabin, L.; Rentz, D.M.; Rodriguez-Gomez, O.; Saykin, A.J.; et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020, 19, 271–278. [Google Scholar] [CrossRef]
- Cherbuin, N.; Sargent-Cox, K.; Easteal, S.; Sachdev, P.; Anstey, K.J. Hippocampal atrophy is associated with subjective memory decline: The PATH Through Life study. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2015, 23, 446–455. [Google Scholar] [CrossRef]
- Jessen, F.; Wiese, B.; Bachmann, C.; Eifflaender-Gorfer, S.; Haller, F.; Kölsch, H.; Luck, T.; Mösch, E.; van den Bussche, H.; Wagner, M.; et al. Prediction of dementia by subjective memory impairment: Effects of severity and temporal association with cognitive impairment. Arch. Gen. Psychiatry 2010, 67, 414–422. [Google Scholar] [CrossRef]
- Jessen, F.; Wolfsgruber, S.; Wiese, B.; Bickel, H.; Mösch, E.; Kaduszkiewicz, H.; Pentzek, M.; Riedel-Heller, S.G.; Luck, T.; Fuchs, A.; et al. AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2014, 10, 76–83. [Google Scholar] [CrossRef]
- Langa, K.M.; Levine, D.A. The diagnosis and management of mild cognitive impairment: A clinical review. Jama 2014, 312, 2551–2561. [Google Scholar] [CrossRef]
- Small, G.W. What we need to know about age related memory loss. BMJ (Clin. Res. Ed.) 2002, 324, 1502–1505. [Google Scholar] [CrossRef]
- Amariglio, R.E.; Mormino, E.C.; Pietras, A.C.; Marshall, G.A.; Vannini, P.; Johnson, K.A.; Sperling, R.A.; Rentz, D.M. Subjective cognitive concerns, amyloid-β, and neurodegeneration in clinically normal elderly. Neurology 2015, 85, 56–62. [Google Scholar] [CrossRef]
- Fu, R.; Liu, Y. Intergenerational Socioeconomic Mobility and Cognitive Impairment Among Chinese Older Adults: Gender Differences. J. Appl. Gerontol. Off. J. South. Gerontol. Soc. 2022, 41, 1733–1743. [Google Scholar] [CrossRef]
- Lee, J.; Sung, J.; Choi, M. The factors associated with subjective cognitive decline and cognitive function among older adults. J. Adv. Nurs. 2020, 76, 555–565. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.M. Factors affecting cognitive function according to gender in community-dwelling elderly individuals. Epidemiol. Health 2017, 39, e2017054. [Google Scholar] [CrossRef]
- Balash, Y.; Mordechovich, M.; Shabtai, H.; Giladi, N.; Gurevich, T.; Korczyn, A.D. Subjective memory complaints in elders: Depression, anxiety, or cognitive decline? Acta Neurol. Scand. 2013, 127, 344–350. [Google Scholar] [CrossRef]
- Cordier, R.; Chen, Y.W.; Clemson, L.; Byles, J.; Mahoney, N. Subjective memory complaints and difficulty performing activities of daily living among older women in Australia. Aust. Occup. Ther. J. 2019, 66, 227–238. [Google Scholar] [CrossRef]
- Leoutsakos, J.M.; Forrester, S.N.; Corcoran, C.D.; Norton, M.C.; Rabins, P.V.; Steinberg, M.I.; Tschanz, J.T.; Lyketsos, C.G. Latent classes of course in Alzheimer’s disease and predictors: The Cache County Dementia Progression Study. Int. J. Geriatr. Psychiatry 2015, 30, 824–832. [Google Scholar] [CrossRef]
- Tu, L.; Lv, X.; Yuan, C.; Zhang, M.; Fan, Z.; Xu, X.; Zeng, Y.; Yu, X.; Wang, H. Trajectories of cognitive function and their determinants in older people: 12 years of follow-up in the Chinese Longitudinal Healthy Longevity Survey. Int. Psychogeriatr. 2020, 32, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Min, J.W. A longitudinal study of cognitive trajectories and its factors for Koreans aged 60 and over: A latent growth mixture model. Int. J. Geriatr. Psychiatry 2018, 33, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Tein, J.Y.; Zhang, M.; Zhen, F.; Huang, F.; Huang, Y.; Yao, Y.; Mei, J. The need to belong: A parallel process latent growth curve model of late life negative affect and cognitive function. Arch. Gerontol. Geriatr. 2020, 89, 104049. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, Y.; Smith, J.P.; Strauss, J.; Yang, G. Cohort profile: The China Health and Retirement Longitudinal Study (CHARLS). Int. J. Epidemiol. 2014, 43, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Bai, A.; Huang, X.; Gao, Y.; Liu, L. Association Between Sleep and Motoric Cognitive Risk Syndrome Among Community-Dwelling Older Adults: Results from the China Health and Retirement Longitudinal Study. Front. Aging Neurosci. 2021, 13, 774167. [Google Scholar] [CrossRef]
- Xiang, Y.; Zare, H.; Guan, C.; Gaskin, D. The impact of rural-urban community settings on cognitive decline: Results from a nationally-representative sample of seniors in China. BMC Geriatr. 2018, 18, 323. [Google Scholar] [CrossRef]
- Qin, T.; Yan, M.; Fu, Z.; Song, Y.; Lu, W.; Fu, A.; Yin, P. Association between anemia and cognitive decline among Chinese middle-aged and elderly: Evidence from the China health and retirement longitudinal study. BMC Geriatr. 2019, 19, 305. [Google Scholar] [CrossRef]
- Bai, A.; Tao, L.; Huang, J.; Tao, J.; Liu, J. Effects of physical activity on cognitive function among patients with diabetes in China: A nationally longitudinal study. BMC Public Health 2021, 21, 481. [Google Scholar] [CrossRef]
- Huang, Z.; Maurer, J. Validity of Self-Rated Memory Among Middle-Aged and Older Chinese Adults: Results From the China Health and Retirement Longitudinal Study (CHARLS). Assessment 2019, 26, 1582–1593. [Google Scholar] [CrossRef]
- Sha, T.; Cheng, W.; Yan, Y. Prospective associations between pulse pressure and cognitive performance in Chinese middle-aged and older population across a 5-year study period. Alzheimer’s Res. Ther. 2018, 10, 29. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, Y. Effects of education on cognition at older ages: Evidence from China’s Great Famine. Soc. Sci. Med. (1982) 2013, 98, 54–62. [Google Scholar] [CrossRef]
- Luo, L.; Wang, G.; Zhou, H.; Zhang, L.; Ma, C.X.; Little, J.P.; Yu, Z.; Teng, H.; Yin, J.Y.; Wan, Z. Sex-specific longitudinal association between baseline physical activity level and cognitive decline in Chinese over 45 years old: Evidence from the China health and retirement longitudinal study. Aging Ment. Health 2022, 26, 1721–1729. [Google Scholar] [CrossRef]
- Liu, M.; Du, X.; Sun, Y.; Zhou, A.; Sun, S.; Wu, Y. The mediating role of cognition in the relationship between sleep duration and instrumental activities of daily living disability among middle-aged and older Chinese. Arch. Gerontol. Geriatr. 2021, 94, 104369. [Google Scholar] [CrossRef]
- Baron, E.C.; Davies, T.; Lund, C. Validation of the 10-item Centre for Epidemiological Studies Depression Scale (CES-D-10) in Zulu, Xhosa and Afrikaans populations in South Africa. BMC Psychiatry 2017, 17, 6. [Google Scholar] [CrossRef]
- Brailean, A.; Aartsen, M.J.; Muniz-Terrera, G.; Prince, M.; Prina, A.M.; Comijs, H.C.; Huisman, M.; Beekman, A. Longitudinal associations between late-life depression dimensions and cognitive functioning: A cross-domain latent growth curve analysis. Psychol. Med. 2017, 47, 690–702. [Google Scholar] [CrossRef]
- Harring, J.R.; Strazzeri, M.M.; Blozis, S.A. Piecewise latent growth models: Beyond modeling linear-linear processes. Behav. Res. Methods 2021, 53, 593–608. [Google Scholar] [CrossRef]
- Chou, C.P.; Bentler, P.M.; Satorra, A. Scaled test statistics and robust standard errors for non-normal data in covariance structure analysis: A Monte Carlo study. Br. J. Math. Stat. Psychol. 1991, 44 Pt 2, 347–357. [Google Scholar] [CrossRef]
- Malhotra, N.; Lopes, E.L.; Veiga, R.T. Structural Equation Modeling with Lisrel: An Initial Vision. REMark Rev. Bras. De Mark. 2014, 13, 28–43. [Google Scholar] [CrossRef]
- Muthén, L.K.; Muthén, B. Mplus User’s Guide, 7th ed.; Autho: Los Angeles, CA, USA, 2012. [Google Scholar]
- Burr, J.A.; Han, S.H.; Peng, C. Childhood Friendship Experiences and Cognitive Functioning in Later Life: The Mediating Roles of Adult Social Disconnectedness and Adult Loneliness. Gerontologist 2020, 60, 1456–1465. [Google Scholar] [CrossRef]
- Numbers, K.; Crawford, J.D.; Kochan, N.A.; Draper, B.; Sachdev, P.S.; Brodaty, H. Participant and informant memory-specific cognitive complaints predict future decline and incident dementia: Findings from the Sydney Memory and Ageing Study. PLoS ONE 2020, 15, e0232961. [Google Scholar] [CrossRef]
- Nelson, M.E.; Andel, R.; Nedelska, Z.; Martinkova, J.; Cechova, K.; Markova, H.; Matuskova, V.; Nikolai, T.; Lerch, O.; Parizkova, M.; et al. The Association Between Homocysteine and Memory in Older Adults. J. Alzheimer’s Dis. JAD 2021, 81, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Rönnlund, M.; Nyberg, L.; Bäckman, L.; Nilsson, L.G. Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychol. Aging 2005, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Grysman, A. Gender and gender typicality in autobiographical memory: A replication and extension. Memory 2018, 26, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Jack, F.; MacDonald, S.; Reese, E.; Hayne, H. Maternal reminiscing style during early childhood predicts the age of adolescents’ earliest memories. Child Dev. 2009, 80, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Zaninotto, P.; Batty, G.D.; Allerhand, M.; Deary, I.J. Cognitive function trajectories and their determinants in older people: 8 years of follow-up in the English Longitudinal Study of Ageing. J. Epidemiol. Community Health 2018, 72, 685–694. [Google Scholar] [CrossRef]
- Wu, C. The mediating and moderating effects of depressive symptoms on the prospective association between cognitive function and activities of daily living disability in older adults. Arch. Gerontol. Geriatr. 2021, 96, 104480. [Google Scholar] [CrossRef]
- Hesseberg, K.; Bentzen, H.; Ranhoff, A.H.; Engedal, K.; Bergland, A. Disability in instrumental activities of daily living in elderly patients with mild cognitive impairment and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2013, 36, 146–153. [Google Scholar] [CrossRef]
- Jekel, K.; Damian, M.; Wattmo, C.; Hausner, L.; Bullock, R.; Connelly, P.J.; Dubois, B.; Eriksdotter, M.; Ewers, M.; Graessel, E.; et al. Mild cognitive impairment and deficits in instrumental activities of daily living: A systematic review. Alzheimer’s Res. Ther. 2015, 7, 17. [Google Scholar] [CrossRef]
- Mao, H.F.; Chang, L.H.; Tsai, A.Y.; Huang, W.W.; Tang, L.Y.; Lee, H.J.; Sun, Y.; Chen, T.F.; Lin, K.N.; Wang, P.N.; et al. Diagnostic accuracy of Instrumental Activities of Daily Living for dementia in community-dwelling older adults. Age Ageing 2018, 47, 551–557. [Google Scholar] [CrossRef]
- Spano, G.; Caffò, A.O.; Lanciano, T.; Curci, A.; Bosco, A. Visuospatial/executive abilities and mood affect the reliability of a subjective memory complaints measure. Aging Clin. Exp. Res. 2020, 32, 1317–1326. [Google Scholar] [CrossRef]
- Singh-Manoux, A.; Dugravot, A.; Fournier, A.; Abell, J.; Ebmeier, K.; Kivimäki, M.; Sabia, S. Trajectories of Depressive Symptoms Before Diagnosis of Dementia: A 28-Year Follow-up Study. JAMA Psychiatry 2017, 74, 712–718. [Google Scholar] [CrossRef]
- Zhang, B.; Lin, Y.; Hu, M.; Sun, Y.; Xu, M.; Hao, J.; Zhu, C. Associations between trajectories of depressive symptoms and rate of cognitive decline among Chinese middle-aged and older adults: An 8-year longitudinal study. J. Psychosom. Res. 2022, 160, 110986. [Google Scholar] [CrossRef]
- Ma, Y.; Liang, L.; Zheng, F.; Shi, L.; Zhong, B.; Xie, W. Association Between Sleep Duration and Cognitive Decline. JAMA Netw. Open 2020, 3, e2013573. [Google Scholar] [CrossRef]
- Scarpelli, S.; Bartolacci, C.; D’Atri, A.; Gorgoni, M.; De Gennaro, L. Mental Sleep Activity and Disturbing Dreams in the Lifespan. Int. J. Environ. Res. Public Health 2019, 16, 3658. [Google Scholar] [CrossRef]
- Cox, S.R.; Ritchie, S.J.; Allerhand, M.; Hagenaars, S.P.; Radakovic, R.; Breen, D.P.; Davies, G.; Riha, R.L.; Harris, S.E.; Starr, J.M.; et al. Sleep and cognitive aging in the eighth decade of life. Sleep 2019, 42, zsz019. [Google Scholar] [CrossRef]
- Youn, J.C.; Kim, K.W.; Lee, D.Y.; Jhoo, J.H.; Lee, S.B.; Park, J.H.; Choi, E.A.; Choe, J.Y.; Jeong, J.W.; Choo, I.H.; et al. Development of the Subjective Memory Complaints Questionnaire. Dement. Geriatr. Cogn. Disord. 2009, 27, 310–317. [Google Scholar] [CrossRef]
- Troyer, A.K.; Rich, J.B. Psychometric properties of a new metamemory questionnaire for older adults. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2002, 57, P19–P27. [Google Scholar] [CrossRef]

| Baseline (n = 1465) | n/mean | %/SD | n/mean | %/SD | ||||
| Age | 65.47 | 4.56 | Male | 884 | 60.34% | |||
| Education | Female | 581 | 39.66% | |||||
| Illiterate | 165 | 11.26% | Smoking (Yes) | 706 | 48.19% | |||
| Primary school or below | 863 | 58.91% | Smoking (No) | 759 | 51.81% | |||
| Middle school | 289 | 19.73% | Drinking (Yes) | 432 | 37.75% | |||
| High school or above | 148 | 10.10% | Drinking (No) | 912 | 62.25% | |||
| Four Waves | 2011 | 2013 | 2015 | 2018 | ||||
| n/mean | %/SD | n/mean | %/SD | n/mean | %/SD | n/mean | %/SD | |
| Married with spouse present | 1243 | 84.85% | 1224 | 83.55% | 1184 | 80.82% | 1109 | 75.70% |
| Chronic diseases | ||||||||
| Hypertension | 459 | 31.46% | 521 | 35.59% | 587 | 40.65% | 747 | 50.99% |
| Dyslipidemia | 200 | 13.91% | 253 | 17.29% | 267 | 18.53% | 400 | 27.30% |
| Diabetes | 116 | 7.98% | 153 | 10.45% | 166 | 11.40 | 245 | 16.72% |
| Heart disease | 250 | 17.16% | 282 | 19.26% | 329 | 22.55% | 430 | 29.35% |
| Stroke | 34 | 2.32% | 44 | 3.00% | 45 | 3.09% | 151 | 10.31% |
| Memory-related disease | 27 | 1.85% | 28 | 1.91% | 44 | 3.01% | 93 | 6.35% |
| Sleeping time | ||||||||
| Night sleeping time (hours) | 6.17 | 1.79 | 6.04 | 1.72 | 6.18 | 1.90 | 6.08 | 2.05 |
| Napping time (minutes) | 36.25 | 43.17 | 43.29 | 46.59 | 41.82 | 44.76 | 47.35 | 54.86 |
| Cognitive functions | ||||||||
| Global cognition | 15.59 | 4.22 | 15.56 | 4.53 | 14.66 | 4.60 | 13.97 | 5.64 |
| Orientation | 4.09 | 1.09 | 4.09 | 1.17 | 4.04 | 1.16 | 3.84 | 1.16 |
| Episodic memory | 7.24 | 2.95 | 7.23 | 3.10 | 6.65 | 3.18 | 6.65 | 4.01 |
| Calculation | 3.50 | 1.74 | 3.50 | 1.72 | 3.26 | 1.80 | 2.87 | 1.89 |
| Constructability | 0.76 | 0.43 | 0.75 | 0.43 | 0.71 | 0.45 | 0.61 | 0.49 |
| IADL | 5.55 | 1.51 | 5.53 | 1.57 | 5.68 | 1.83 | 6.01 | 2.28 |
| Depression | 8.52 | 6.13 | 7.69 | 5.42 | 7.74 | 6.21 | 8.45 | 6.32 |
| Depression symptoms | 424 | 28.94% | 307 | 20.96% | 352 | 24.03% | 418 | 28.59% |
| A. Univariate LGCM | |||||||||||
| Mean | Variance | Fit Indexes | |||||||||
| n = 1465 | Intercept | Slope | Intercept | Slope | Correlation | χ2 | df | CFI | TLI | RMSEA | SRMR |
| Global cognition | 15.759 *** | −0.251 *** | 7.778 *** | 0.094 ** | 0.413 *** | 26.412 *** | 5 | 0.988 | 0.985 | 0.054 | 0.025 |
| Orientation | 4.134 *** | −0.038 *** | 0.507 *** | 0.008 ** | −0.011 | 25.549 *** | 5 | 0.980 | 0.976 | 0.053 | 0.030 |
| Episodic memory | 7.264 *** | −0.104 *** | 2.714 *** | 0.038 * | 0.161 ** | 20.759 *** | 5 | 0.984 | 0.980 | 0.046 | 0.022 |
| Calculation | 3.591 *** | −0.095 *** | 0.839 *** | 0.007 | 0.007 | 20.226 ** | 5 | 0.975 | 0.971 | 0.046 | 0.030 |
| Constructability | 0.779 *** | −0.022 *** | 0.048 *** | <0.001 | <0.001 | 15.365 ** | 5 | 0.977 | 0.972 | 0.038 | 0.021 |
| B. Conditional LGCM | |||||||||||
| Global Cognition a | Orientation a | Episodic Memory a | Calculation a | Constructability a | |||||||
| n = 1426 | β | SE | β | SE | β | SE | β | SE | β | SE | |
| Intercept | 5.824 *** | 0.267 | 5.835 *** | 0.287 | 4.500 *** | 0.295 | 3.829 *** | 0.281 | 3.394 *** | 0.285 | |
| Intercept variance | 0.677 *** | 0.037 | 0.706 *** | 0.041 | 0.779 *** | 0.043 | 0.774 *** | 0.120 | 0.513 *** | 0.099 | |
| Slope | −1.142 ** | 0.260 | −0.265 | 0.207 | −0.972 ** | 0.291 | −1.215 ** | 0.462 | −0.389 | 3.382 | |
| Slope variance | 0.766 *** | 0.084 | 0.955 *** | 0.037 | 0.659 *** | 0.133 | 0.836 *** | 0.120 | 0.895 ** | 0.282 | |
| Correlation | 0.255 | 0.183 | −0.257 *** | 0.093 | 0.198 | 0.256 | −0.063 | 0.224 | 0.075 | 0.378 | |
| Intercept on Baseline | |||||||||||
| Age | −0.157 *** | 0.034 | 0.008 | 0.035 | −0.219 *** | 0.041 | −0.080 | 0.042 | −0.048 | 0.042 | |
| Gender | 0.010 | 0.045 | −0.024 | 0.046 | 0.123 * | 0.055 | −0.122 * | 0.056 | −0.097 | 0.056 | |
| ≤Primary school | −0.105 *** | 0.035 | −0.036 | 0.037 | −0.133 ** | 0.044 | −0.037 | 0.045 | 0.008 | 0.045 | |
| Middle school | 0.248 *** | 0.037 | 0.239 *** | 0.038 | 0.148 ** | 0.045 | 0.189 *** | 0.047 | 0.349 *** | 0.048 | |
| ≥High school | 0.368 *** | 0.039 | 0.315 *** | 0.041 | 0.308 *** | 0.049 | 0.229 *** | 0.050 | 0.328 *** | 0.052 | |
| Smoking status | 0.028 | 0.041 | 0.007 | 0.043 | 0.008 | 0.051 | 0.065 | 0.052 | 0.040 | 0.052 | |
| Drinking status | −0.038 | 0.036 | −0.027 | 0.038 | −0.053 | 0.045 | 0.010 | 0.046 | 0.032 | 0.046 | |
| Marriage | −0.030 | 0.680 | 0.010 | 0.072 | −0.058 | 0.089 | 0.047 | 0.089 | 0.026 | 0.095 | |
| Night sleeping time | 0.054 | 0.053 | −0.016 | 0.055 | 0.017 | 0.068 | 0.097 | 0.067 | 0.154 * | 0.103 | |
| Napping time | −0.052 | 0.051 | −0.032 | 0.053 | −0.106 | 0.066 | 0.023 | 0.066 | 0.020 | 0.071 | |
| CCVD | −0.002 | 0.075 | 0.113 | 0.077 | −0.131 | 0.097 | 0.051 | 0.098 | 0.154 | 0.103 | |
| IADL | −0.118 * | 0.051 | −0.128 * | 0.054 | −0.122 | 0.067 | −0.018 | 0.067 | −0.047 | 0.072 | |
| Depression | −0.108 | 0.056 | −0.129 * | 0.058 | −0.038 | 0.074 | −0.058 | 0.073 | −0.045 | 0.079 | |
| Slope on Baseline | |||||||||||
| Age | −0.158 ** | 0.061 | −0.073 | 0.057 | −0.130 | 0.070 | −0.155 | 0.094 | −0.085 | 0.102 | |
| Gender | 0.073 | 0.078 | −0.050 | 0.075 | 0.143 | 0.092 | −0.114 | 0.118 | 0.015 | 0.130 | |
| ≤Primary school | −0.023 | 0.062 | 0.011 | 0.059 | −0.046 | 0.071 | −0.004 | 0.090 | −0.062 | 0.105 | |
| Middle school | 0.226 ** | 0.068 | 0.061 | 0.061 | 0.243 ** | 0.080 | 0.159 | 0.102 | −0.123 | 0.113 | |
| ≥High school | 0.189 ** | 0.071 | −0.012 | 0.066 | 0.271 ** | 0.087 | −0.011 | 0.111 | 0.101 | 0.119 | |
| Smoking status | −0.092 | 0.072 | −0.118 | 0.069 | 0.055 | 0.082 | −0.136 | 0.108 | 0.051 | 0.120 | |
| Drinking status | 0.001 | 0.063 | 0.003 | 0.060 | 0.026 | 0.072 | −0.048 | 0.093 | −0.023 | 0.105 | |
| Marriage | 0.083 | 0.105 | −0.033 | 0.082 | 0.105 | 0.122 | 0.059 | 0.158 | −0.054 | 0.179 | |
| Night sleeping time | −0.103 | 0.087 | −0.033 | 0.081 | −0.003 | 0.101 | −0.261 | 0.139 | −0.211 | 0.159 | |
| Napping time | 0.058 | 0.086 | 0.006 | 0.081 | 0.137 | 0.101 | −0.055 | 0.127 | −0.017 | 0.145 | |
| CCVD | 0.157 | 0.117 | 0.111 | 0.113 | 0.226 | 0.135 | −0.049 | 0.175 | −0.072 | 0.200 | |
| IADL | 0.136 | 0.087 | 0.068 | 0.083 | 0.130 | 0.102 | 0.062 | 0.129 | 0.196 | 0.159 | |
| Depression | −0.013 | 0.096 | 0.053 | 0.091 | −0.116 | 0.111 | 0.089 | 0.142 | −0.138 | 0.166 | |
| TVCs → Cognitive Functions | |||||||||||
| T1(Marriage) → T1 | 0.021 | 0.042 | 0.005 | 0.043 | 0.027 | 0.047 | −0.025 | 0.046 | 0.018 | 0.047 | |
| T2(Marriage) → T2 | 0.073 * | 0.028 | 0.031 | 0.030 | 0.063 * | 0.032 | 0.028 | 0.034 | 0.027 | 0.033 | |
| T3(Marriage) → T3 | 0.054 * | 0.021 | 0.044 | 0.023 | 0.030 | 0.024 | 0.035 | 0.024 | 0.015 | 0.024 | |
| T4(Marriage) → T4 | 0.078 ** | 0.025 | 0.011 | 0.028 | 0.088 ** | 0.027 | 0.042 | 0.030 | −0.058 | 0.030 | |
| T1(Night sleeping time) → T1 | −0.033 | 0.037 | 0.009 | 0.038 | −0.031 | 0.041 | −0.017 | 0.046 | −0.077 | 0.042 | |
| T2(Night sleeping time) → T2 | −0.038 | 0.023 | −0.042 | 0.025 | −0.026 | 0.026 | −0.019 | 0.027 | −0.030 | 0.027 | |
| T3(Night sleeping time) → T3 | 0.006 | 0.020 | 0.020 | 0.022 | 0.001 | 0.023 | −0.008 | 0.025 | −0.007 | 0.024 | |
| T4(Night sleeping time) → T4 | 0.005 | 0.020 | −0.028 | 0.022 | 0.010 | 0.022 | 0.034 | 0.024 | −0.017 | 0.024 | |
| T1(Napping time) → T1 | 0.036 | 0.035 | 0.0019 | 0.037 | 0.063 | 0.040 | −0.012 | 0.040 | −0.021 | 0.041 | |
| T2(Napping time) → T2 | −0.022 | 0.022 | −0.012 | 0.024 | −0.035 | 0.025 | 0.010 | 0.026 | 0.018 | 0.025 | |
| T3(Napping time) → T3 | −0.016 | 0.020 | −0.032 | 0.022 | 0.003 | 0.023 | −0.023 | 0.025 | −0.018 | 0.025 | |
| T4(Napping time) → T4 | 0.011 | 0.020 | 0.017 | 0.024 | −0.008 | 0.023 | <0.001 | 0.025 | 0.001 | 0.025 | |
| T1(CCVD) → T1 | 0.072 | 0.050 | −0.006 | 0.052 | 0.143 * | 0.056 | −0.028 | 0.056 | −0.059 | 0.056 | |
| T2(CCVD) → T2 | 0.059 | 0.032 | 0.006 | 0.052 | 0.066 | 0.036 | −0.046 | 0.038 | 0.111 | 0.037 | |
| T3(CCVD) → T3 | 0.006 | 0.026 | −0.030 | 0.028 | 0.041 | 0.029 | <0.001 | 0.030 | −0.006 | 0.030 | |
| T4(CCVD) → T4 | 0.025 | 0.026 | −0.032 | 0.029 | 0.051 | 0.029 | 0.014 | 0.031 | −0.037 | 0.031 | |
| T1(IADL) → T1 | −0.022 | 0.036 | −0.044 | 0.037 | 0.010 | 0.041 | −0.017 | 0.041 | −0.090 * | 0.042 | |
| T2(IADL) → T2 | −0.053 * | 0.002 | −0.053 * | 0.024 | −0.034 | 0.025 | −0.024 | 0.027 | −0.052 * | 0.026 | |
| T3(IADL) → T3 | −0.040 | 0.021 | −0.055 * | 0.023 | −0.007 | 0.024 | −0.048 * | 0.026 | −0.091 *** | 0.025 | |
| T4(IADL) → T4 | −0.071 ** | 0.021 | −0.071 ** | 0.024 | −0.030 | 0.024 | −0.079 ** | 0.026 | −0.094 *** | 0.026 | |
| T1(Depression) → T1 | −0.110 ** | 0.039 | −0.006 | 0.041 | −0.160 *** | 0.044 | 0.011 | 0.044 | −0.064 | 0.045 | |
| T2(Depression) → T2 | −0.134 *** | 0.024 | −0.123 *** | 0.026 | −0.124 *** | 0.027 | −0.062 * | 0.029 | −0.078 ** | 0.028 | |
| T3(Depression) → T3 | −0.100 *** | 0.022 | −0.110 *** | 0.024 | −0.099 *** | 0.025 | −0.004 | 0.027 | −0.048 | 0.026 | |
| T4(Depression) → T4 | −0.157 *** | 0.022 | −0.099 *** | 0.025 | −0.158 *** | 0.025 | −0.070 ** | 0.027 | −0.026 | 0.028 | |
| Fit Indexes | |||||||||||
| χ2 | 98.558 | 126.072 ** | 106.172 * | 75.770 | 75.080 | ||||||
| df | 79 | 79 | 79 | 79 | 79 | ||||||
| CFI | 0.992 | 0.970 | 0.981 | 1.000 | 1.000 | ||||||
| TLI | 0.987 | 0.950 | 0.969 | 1.006 | 1.009 | ||||||
| RMSEA | 0.013 | 0.020 | 0.016 | <0.001 | <0.001 | ||||||
| SRMR | 0.010 | 0.010 | 0.010 | 0.008 | 0.007 | ||||||
| C. Unconditional PP-LGCM | |||||
|---|---|---|---|---|---|
| N = 1462 | β | SE | β | SE | |
| Global cognition on IADL a | Orientation on IADL a | ||||
| I(IADL) → I(GC) | −0.347 *** | 0.064 | I(IADL) → I(OR) | −0.368 *** | 0.069 |
| S(IADL) → I(GC) | 0.067 | 0.083 | S(IADL) → I(OR) | 0.106 | 0.088 |
| I(IADL) → S(GC) | 0.364 * | 0.151 | I(IADL) → S(OR) | 0.252 * | 0.120 |
| S(IADL) → S(GC) | −0.637 ** | 0.184 | S(IADL) →S(OR) | −0.417 ** | 0.147 |
| Episodic memory on IADL a | Calculation on IADL a | ||||
| I(IADL) → I(EM) | −0.260 ** | 0.070 | I(IADL) → I(CA) | −0.223 ** | 0.075 |
| S(IADL) → I(EM) | 0.001 | 0.088 | S(IADL) → I(CA) | 0.067 | 0.094 |
| I(IADL) → S(EM) | 0.244 | 0.155 | I(IADL) → S(CA) | 0.316 | 0.188 |
| S(IADL) → S(EM) | −0.437 * | 0.196 | S(IADL) → S(CA) | −0.592 * | 0.249 |
| Constructability on IADL a | Global cognition on Depression a | ||||
| I(IADL) → I(CO) | −0.427 *** | 0.090 | I(DP) → I(GC) | −0.408 *** | 0.043 |
| S(IADL) → I(CO) | 0.243 * | 0.113 | S(DP) → I(GC) | 0.178 | 0.117 |
| I(IADL) → S(CO) | 0.625 | 0.326 | I(DP) → S(GC) | −0.054 | 0.115 |
| S(IADL) → S(CO) | −0.891 * | 0.428 | S(DP) → S(GC) | −0.868 ** | 0.286 |
| Orientation on Depression a | Episodic memory on Depression a | ||||
| I(DE) → I(OR) | −0.349 *** | 0.047 | I(DE) → I(EM) | −0.383 *** | 0.050 |
| S(DE) → I(OR) | 0.172 | 0.126 | S(DE) → I(EM) | 0.188 | 0.133 |
| I(DE) → S(OR) | 0.037 | 0.093 | I(DE) → S(EM) | −0.102 | 0.129 |
| S(DE) → S(OR) | −0.559 * | 0.239 | S(DE) → S(EM) | −0.880 * | 0.339 |
| Calculation on Depression a | Constructability on Depression a | ||||
| I(DE) → I(CA) | −0.223 *** | 0.049 | I(DE) → I(CO) | −0.358 *** | 0.051 |
| S(DE) → I(CA) | 0.074 | 0.123 | S(DE) → I(CO) | 0.088 | 0.130 |
| I(DE) → S(CA) | 0.005 | 0.106 | I(DE) → S(CO) | 0.122 | 0.136 |
| S(DE) → S(CA) | −0.455 | 0.278 | S(DE) → S(CO) | −0.412 | 0.348 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Li, M.; Wu, C. Cognitive Function Trajectories and Factors among Chinese Older Adults with Subjective Memory Decline: CHARLS Longitudinal Study Results (2011–2018). Int. J. Environ. Res. Public Health 2022, 19, 16707. https://doi.org/10.3390/ijerph192416707
Ma C, Li M, Wu C. Cognitive Function Trajectories and Factors among Chinese Older Adults with Subjective Memory Decline: CHARLS Longitudinal Study Results (2011–2018). International Journal of Environmental Research and Public Health. 2022; 19(24):16707. https://doi.org/10.3390/ijerph192416707
Chicago/Turabian StyleMa, Chifen, Mengyuan Li, and Chao Wu. 2022. "Cognitive Function Trajectories and Factors among Chinese Older Adults with Subjective Memory Decline: CHARLS Longitudinal Study Results (2011–2018)" International Journal of Environmental Research and Public Health 19, no. 24: 16707. https://doi.org/10.3390/ijerph192416707
APA StyleMa, C., Li, M., & Wu, C. (2022). Cognitive Function Trajectories and Factors among Chinese Older Adults with Subjective Memory Decline: CHARLS Longitudinal Study Results (2011–2018). International Journal of Environmental Research and Public Health, 19(24), 16707. https://doi.org/10.3390/ijerph192416707








