Triptolide Attenuates Muscular Inflammation and Oxidative Stress in a Delayed-Onset Muscle Soreness Animal Model
Abstract
1. Introduction
2. Materials and Methods
2.1. DOMS Animal Model
2.2. In Vivo Study Design
2.3. Pain Behavior Test
2.4. Serum Biochemical Analysis
2.5. Western Blot
2.6. Measuring Lipid Peroxidation Level in Muscle
2.7. Measuring Muscular Glutathione (GSH) Level
2.8. Measuring Pro-Inflammatory Cytokines in Muscles
2.9. Measuring Nitrite Concentration
2.10. Histological Evaluation of Muscular Injury
2.11. Measuring Hepatic MPO Activity
2.12. Statistical Analysis
3. Results
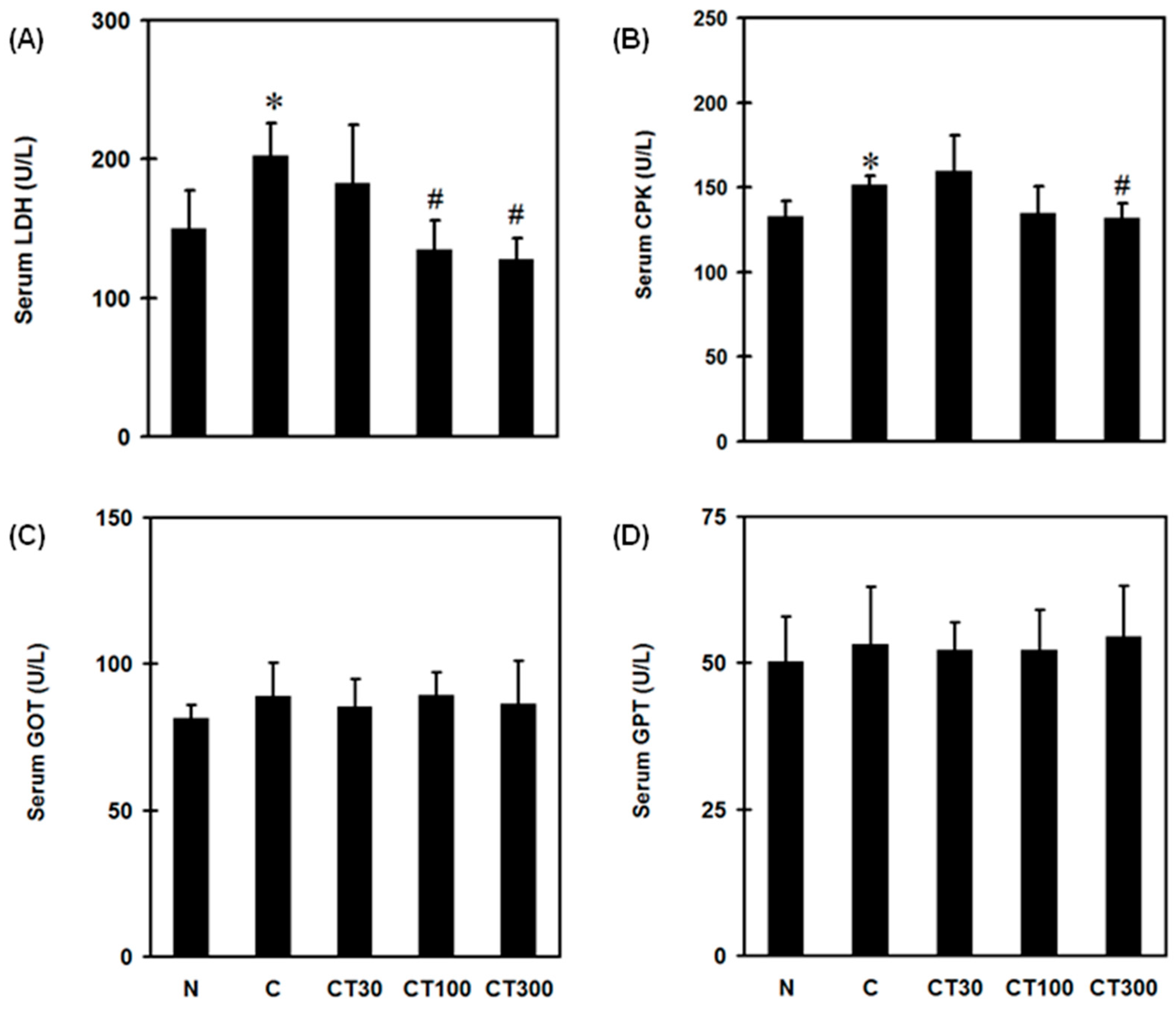
3.1. Triptolide Reduces Pain, Serum LDH and CPK Levels without Affecting Hepatic Function in the DOMS Rat Model
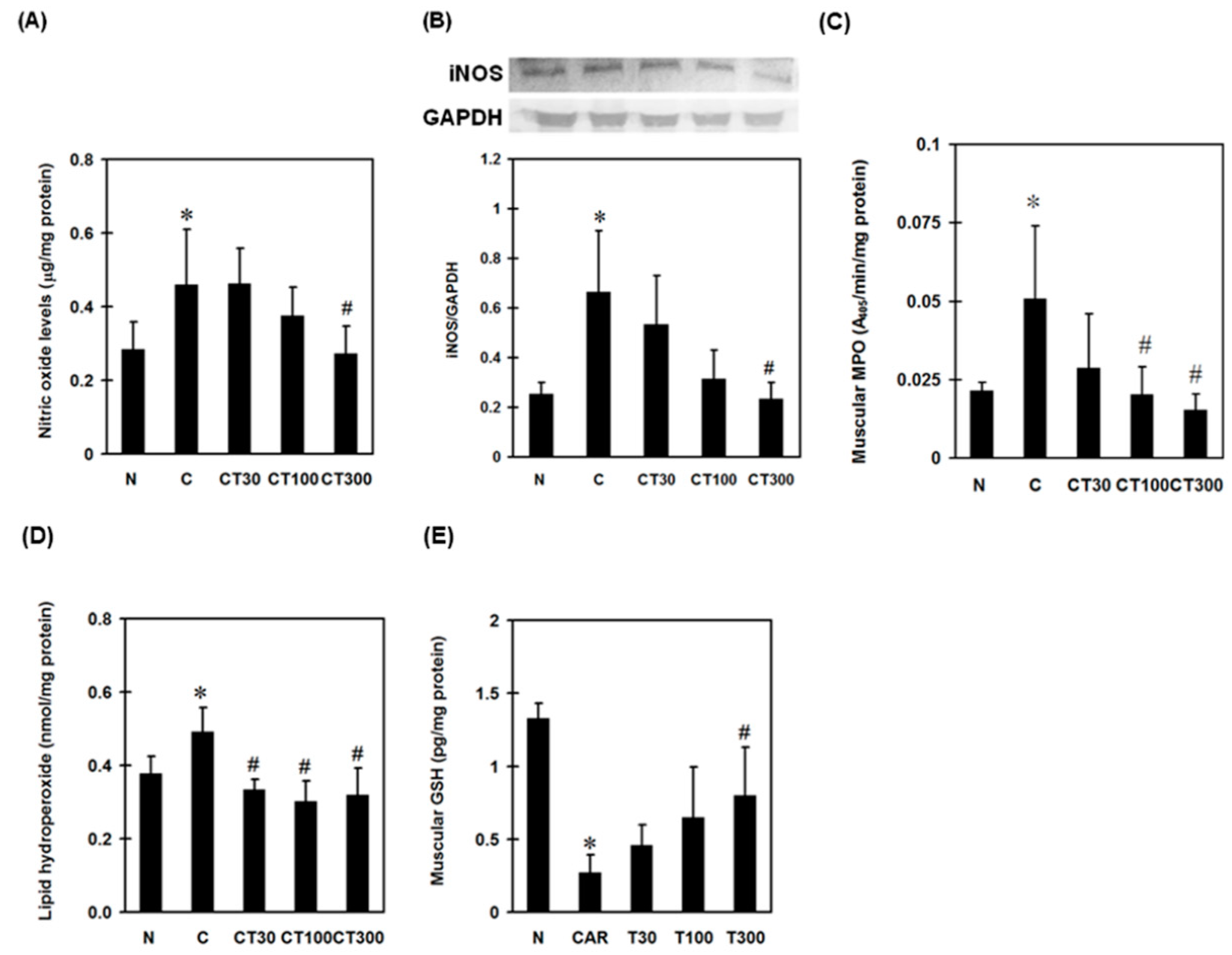
3.2. Triptolide Suppresses NO, iNOS, Reactive Nitrogen Species (RNS), and MPO Expression and Oxidative Stress in the DOMS Rat Model
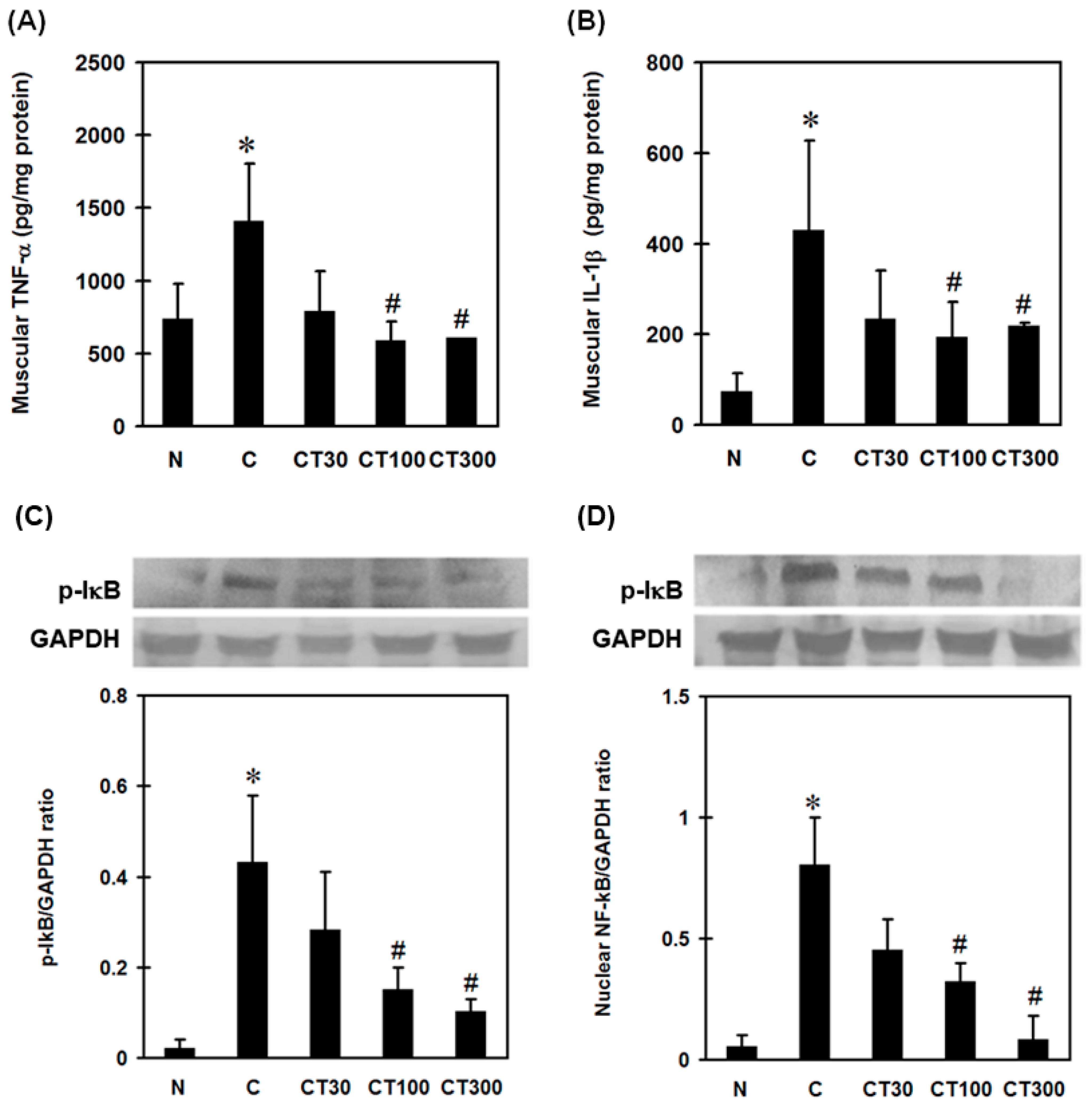
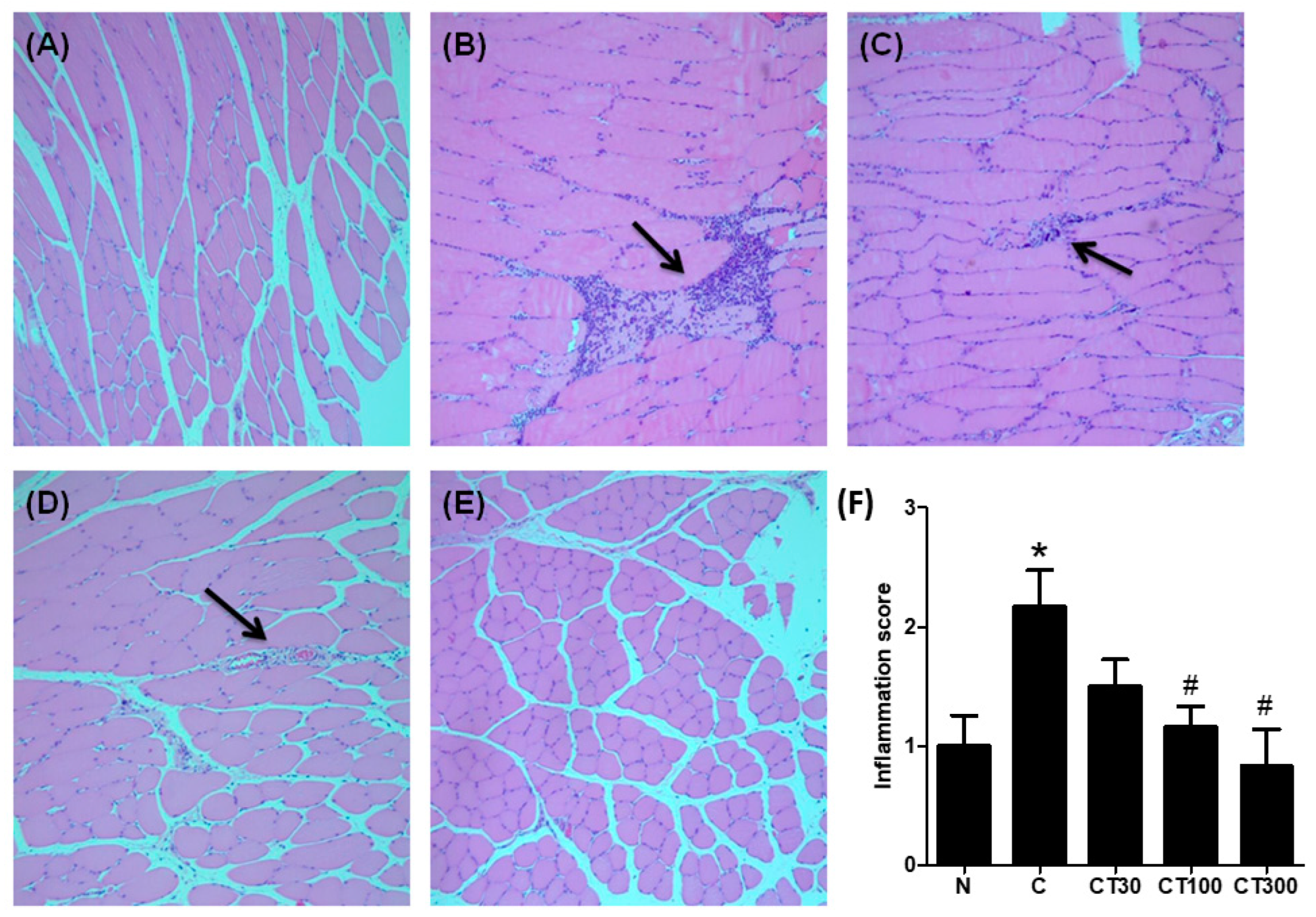
3.3. Triptolide Decreases Expression Levels of TNF-α, IL-1β, Nuclear NF-ĸB, and Leukocyte Infiltration in the DOMS Rat Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meamarbashi, A. Herbs and natural supplements in the prevention and treatment of delayed-onset muscle soreness. Avicenna J. Phytomedicine 2017, 7, 16. [Google Scholar]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Saborido, A.; Naudí, A.; Portero-Otín, M.; Pamplona, R.; Megías, A. Stanozolol treatment decreases the mitochondrial ROS generation and oxidative stress induced by acute exercise in rat skeletal muscle. J. Appl. Physiol. 2011, 110, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Scicchitano, M.; Bosco, F.; Musolino, V.; Carresi, C.; Scarano, F.; Maiuolo, J.; Nucera, S.; Maretta, A.; Paone, S.; et al. Modulation of nitric oxide synthases by oxidized LDLs: Role in vascular inflammation and atherosclerosis development. Int. J. Mol. Sci. 2019, 20, 3294. [Google Scholar] [CrossRef]
- Conti, A.; Miscusi, M.; Cardali, S.M.; Germanò, A.; Suzuki, H.; Cuzzocrea, S.; Tomasello, F. Nitric oxide in the injured spinal cord: Synthases cross-talk, oxidative stress and inflammation. Brain Res. Rev. 2007, 54, 205–218. [Google Scholar] [CrossRef]
- Clancy, R.; Gomez, P.; Abramson, S. Nitric oxide sustains nuclear factor kappaB activation in cytokine-stimulated chondrocytes. Osteoarthr. Cartil. 2004, 12, 552–558. [Google Scholar] [CrossRef]
- Yu, S.M.; Han, Y.; Kim, S.J. Simvastatin abolishes nitric oxide-and reactive oxygen species-induced cyclooxygenase-2 expression by blocking the nuclear factor κB pathway in rabbit articular chondrocytes. Cell Biol. Int. 2020, 44, 2153–2162. [Google Scholar] [CrossRef]
- Dong, G.K.; Zhang, X.T.; Ma, L.Q.; Li, N.; Ma, C.L.; Cong, B.; Gu, Z.Y. Nitric oxide mediated TNF-α, IL-1β gene expression in liver induced by crush injury of rat’s soft tissues. Fa Yi Xue Za Zhi 2014, 30, 250–252. [Google Scholar]
- Vuolteenaho, K.; Moilanen, T.; Knowles, R.; Moilanen, E. The role of nitric oxide in osteoarthritis. Scand. J. Rheumatol. 2007, 36, 247–258. [Google Scholar] [CrossRef]
- Klebanoff, S. Peroxidases in Chemistry and Biology; CRC Press: Boca Raton, FL, USA, 1991; pp. 1–35. [Google Scholar]
- Deby-Dupont, G.; Deby, C.; Lamy, M. Neutrophil myeloperoxidase revisited: It’s role in health and disease. Intensivmed. Und Notf. 1999, 36, 500–513. [Google Scholar] [CrossRef]
- Heinecke, J.W. Mechanisms of oxidative damage by myeloperoxidase in atherosclerosis and other inflammatory disorders. J. Lab. Clin. Med. 1999, 133, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Galijasevic, S.; Saed, G.M.; Diamond, M.P.; Abu-Soud, H.M. Myeloperoxidase up-regulates the catalytic activity of inducible nitric oxide synthase by preventing nitric oxide feedback inhibition. Proc. Natl. Acad. Sci. USA 2003, 100, 14766–14771. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.W.; Zhang, W.; Shi, Q.; Zheng, W.J.; Li, X.; Chen, H.; Wu, Q.-J.; Jiang, W.-L.; Li, H.B.; Gong, L.; et al. Comparison of Tripterygium wilfordii Hook F with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): A randomised, controlled clinical trial. Ann. Rheum. Dis. 2015, 74, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Goldbach-Mansky, R.; Wilson, M.; Fleischmann, R.; Olsen, N.; Silverfield, J.; Kempf, P.; Kivitz, A.; Sherrer, Y.; Pucino, F.; Csako, G.; et al. Comparison of Tripterygium wilfordii Hook F versus sulfasalazine in the treatment of rheumatoid arthritis: A randomized trial. Ann. Intern. Med. 2009, 151, 229–240. [Google Scholar] [CrossRef]
- Tao, X.; Davis, L.S.; Lipsky, P.E. Effect of an extract of the Chinese herbal remedy Tripterygium wilfordii Hook F on human immune responsiveness. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1991, 34, 1274–1281. [Google Scholar] [CrossRef]
- Yuan, K.; Li, X.; Lu, Q.; Zhu, Q.; Jiang, H.; Wang, T.; Huang, G.; Xu, A. Application and mechanisms of triptolide in the treatment of inflammatory diseases—A review. Front. Pharmacol. 2019, 10, 1469. [Google Scholar] [CrossRef]
- Chong, L.W.; Hsu, Y.C.; Chiu, Y.T.; Yang, K.C.; Huang, Y.T. Antifibrotic Effects of Triptolide on Hepatic Stellate Cells and Dimethylnitrosamine-intoxicated Rats. Phytother. Res. 2011, 25, 990–999. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Duan, W.; Liu, B.; Gong, P.; Ding, Y.; Wu, X. Anti-inflammatory effects of triptolide by inhibiting the NF-κB signalling pathway in LPS-induced acute lung injury in a murine model. Mol. Med. Rep. 2014, 10, 447–452. [Google Scholar] [CrossRef]
- Wang, B.; Ma, L.; Tao, X.; Lipsky, P. Triptolide, an active component of the Chinese herbal remedy Tripterygium wilfordii Hook F, inhibits production of nitric oxide by decreasing inducible nitric oxide synthase gene transcription. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2004, 50, 2995–3003. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, J.; Bao, X.; Chan, S.; Young, D.O.; Liu, D.; Shen, P. Triptolide attenuates oxidative stress, NF-κB activation and multiple cytokine gene expression in murine peritoneal macrophage. Int. J. Mol. Med. 2006, 17, 141–150. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Qin, W.-S.; Zeng, C.-H.; Zheng, C.-X.; Hong, Y.-M.; Lu, Y.-Z.; Li, L.-S.; Liu, Z.-H. Triptolide reduces proteinuria in experimental membranous nephropathy and protects against C5b-9-induced podocyte injury in vitro. Kidney Int. 2010, 77, 974–988. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Moore, S.A.; Sluka, K.A. Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain 2003, 104, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Philpott, H.T.; O’Brien, M.; McDougall, J.J. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain 2017, 158, 2442. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.-Y.; Chien, S.-P.; Hsu, D.-Z.; Liu, M.-Y. Protective effect of sesamol on the pulmonary inflammatory response and lung injury in endotoxemic rats. Food Chem. Toxicol. 2010, 48, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.Z.; Chien, S.P.; Li, Y.H.; Chuang, Y.C.; Chang, Y.C.; Liu, M.Y. Sesame oil attenuates hepatic lipid peroxidation by inhibiting nitric oxide and superoxide anion generation in septic rats. J. Parenter. Enter. Nutr. 2008, 32, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Hsu, D.-Z.; Fu, Y.-H.; Liu, M.-Y. Sleep deprivation-induced multi-organ injury: Role of oxidative stress and inflammation. Excli J. 2015, 14, 672. [Google Scholar]
- Huang, S.-C.; Wu, J.-F.; Saovieng, S.; Chien, W.-H.; Hsu, M.-F.; Li, X.-F.; Lee, S.-D.; Huang, C.-Y.; Kuo, C.-H. Doxorubicin inhibits muscle inflammation after eccentric exercise. J. Cachexia Sarcopenia Muscle 2017, 8, 277–284. [Google Scholar] [CrossRef]
- Ng, S.W.; Zhang, H.; Hegde, A.; Bhatia, M. Role of preprotachykinin-A gene products on multiple organ injury in LPS-induced endotoxemia. J. Leukoc. Biol. 2008, 83, 288–295. [Google Scholar] [CrossRef]
- Kehl, L.J.; Fairbanks, C.A. Experimental animal models of muscle pain and analgesia. Exerc. Sport Sci. Rev. 2003, 31, 188–194. [Google Scholar] [CrossRef][Green Version]
- Kim, M.; Chun, J.; Jung, H.A.; Choi, J.S.; Kim, Y.S. Capillarisin attenuates exercise-induced muscle damage through MAPK and NF-κB signaling. Phytomedicine 2017, 32, 30–36. [Google Scholar] [CrossRef]
- Withee, E.D.; Tippens, K.M.; Dehen, R.; Tibbitts, D.; Hanes, D.; Zwickey, H. Effects of Methylsulfonylmethane (MSM) on exercise-induced oxidative stress, muscle damage, and pain following a half-marathon: A double-blind, randomized, placebo-controlled trial. J. Int. Soc. Sport. Nutr. 2017, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Diehl, B.; Hoheisel, U.; Mense, S. Histological and neurophysiological changes induced by carrageenan in skeletal muscle of cat and rat. Agents Actions 1988, 25, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Guarner-Lans, V.; Rubio-Ruiz, M.E. Reductive stress in inflammation-associated diseases and the pro-oxidant effect of antioxidant agents. Int. J. Mol. Sci. 2017, 18, 2098. [Google Scholar] [CrossRef] [PubMed]
- Bloodsworth, A.; O’Donnell, V.B.; Freeman, B.A. Nitric oxide regulation of free radical–and enzyme-mediated lipid and lipoprotein oxidation. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1707–1715. [Google Scholar] [CrossRef]
- Halliwell, B.; Zhao, K.; Whiteman, M. Nitric oxide and peroxynitrite. The ugly, the uglier and the not so good: A personal view of recent controversies. Free Radic. Res. 1999, 31, 651–669. [Google Scholar] [CrossRef]
- Guzik, T.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation. J. Physiol. Pharm. 2003, 54, 469–487. [Google Scholar]
- DiDonato, J.A.; Mercurio, F.; Karin, M. NF-κB and the link between inflammation and cancer. Immunol. Rev. 2012, 246, 379–400. [Google Scholar] [CrossRef]
- Kang, J.L.; Lee, K.; Castranova, V. Nitric oxide up-regulates DNA-binding activity of nuclear factor-κB in macrophages stimulated with silica and inflammatory stimulants. Mol. Cell. Biochem. 2000, 215, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Rousseau, D.L.; Abu-Soud, H.M.; Stuehr, D.J. Heme coordination of NO in NO synthase. Proc. Natl. Acad. Sci. USA 1994, 91, 10512–10516. [Google Scholar] [CrossRef]
- Hurshman, A.R.; Marletta, M.A. Nitric oxide complexes of inducible nitric oxide synthase: Spectral characterization and effect on catalytic activity. Biochemistry 1995, 34, 5627–5634. [Google Scholar] [CrossRef]
- Evans, T.; Buttery, L.; Carpenter, A.; Springall, D.; Polak, J.; Cohen, J. Cytokine-treated human neutrophils contain inducible nitric oxide synthase that produces nitration of ingested bacteria. Proc. Natl. Acad. Sci. USA 1996, 93, 9553–9558. [Google Scholar] [CrossRef] [PubMed]
- Abu-Soud, H.M.; Wang, J.; Rousseau, D.L.; Fukuto, J.M.; Ignarro, L.J.; Stuehr, D.J. Neuronal Nitric Oxide Synthase Self-inactivates by Forming a Ferrous-Nitrosyl Complex during Aerobic Catalysis (∗). J. Biol. Chem. 1995, 270, 22997–23006. [Google Scholar] [CrossRef] [PubMed]
- Abu-Soud, H.M.; Ichimori, K.; Presta, A.; Stuehr, D.J. Electron transfer, oxygen binding, and nitric oxide feedback inhibition in endothelial nitric-oxide synthase. J. Biol. Chem. 2000, 275, 17349–17357. [Google Scholar] [CrossRef] [PubMed]
- Abu-Soud, H.M.; Ichimori, K.; Nakazawa, H.; Stuehr, D.J. Regulation of inducible nitric oxide synthase by self-generated NO. Biochemistry 2001, 40, 6876–6881. [Google Scholar] [CrossRef]
- Brown, S.J.; Child, R.B.; Day, S.H.; Donnelly, A.E. Exercise-induced skeletal muscle damage and adaptation following repeated bouts of eccentric muscle contractions. J. Sport. Sci. 1997, 15, 215–222. [Google Scholar] [CrossRef]
- Willoughby, D.; McFarlin, B.; Bois, C. Interleukin-6 expression after repeated bouts of eccentric exercise. Int. J. Sport. Med. 2003, 24, 15–21. [Google Scholar] [CrossRef]
- Foschini, D.; Prestes, J. Acute hormonal and immune responses after a bi-set strength training. Fit. Perform. J. (Online Ed.) 2007, 6, 38–44. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Z.; Ji, J.; Wang, X.; Wang, T.; Zhang, Y.; Tai, T.; Chen, M.; Sun, L.; Li, X.; et al. Gene expression profiling and pathway analysis of hepatotoxicity induced by triptolide in Wistar rats. Food Chem. Toxicol. 2013, 58, 495–505. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-C.; Tsai, C.-C.; Ko, P.-Y.; Kwan, T.-H.; Liu, M.-Y.; Wu, P.-T.; Jou, I.-M. Triptolide Attenuates Muscular Inflammation and Oxidative Stress in a Delayed-Onset Muscle Soreness Animal Model. Int. J. Environ. Res. Public Health 2022, 19, 16685. https://doi.org/10.3390/ijerph192416685
Hsu C-C, Tsai C-C, Ko P-Y, Kwan T-H, Liu M-Y, Wu P-T, Jou I-M. Triptolide Attenuates Muscular Inflammation and Oxidative Stress in a Delayed-Onset Muscle Soreness Animal Model. International Journal of Environmental Research and Public Health. 2022; 19(24):16685. https://doi.org/10.3390/ijerph192416685
Chicago/Turabian StyleHsu, Che-Chia, Chin-Chuan Tsai, Po-Yen Ko, Ting-Hsien Kwan, Ming-Yie Liu, Po-Ting Wu, and I-Ming Jou. 2022. "Triptolide Attenuates Muscular Inflammation and Oxidative Stress in a Delayed-Onset Muscle Soreness Animal Model" International Journal of Environmental Research and Public Health 19, no. 24: 16685. https://doi.org/10.3390/ijerph192416685
APA StyleHsu, C.-C., Tsai, C.-C., Ko, P.-Y., Kwan, T.-H., Liu, M.-Y., Wu, P.-T., & Jou, I.-M. (2022). Triptolide Attenuates Muscular Inflammation and Oxidative Stress in a Delayed-Onset Muscle Soreness Animal Model. International Journal of Environmental Research and Public Health, 19(24), 16685. https://doi.org/10.3390/ijerph192416685




