High Resistance to Antibiotics Recommended in Standard Treatment Guidelines in Ghana: A Cross-Sectional Study of Antimicrobial Resistance Patterns in Patients with Urinary Tract Infections between 2017–2021
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
2.2.1. General Setting
2.2.2. Specific Setting
2.2.3. Sample Collection and Processing
2.3. Study Population
2.4. Data Variables
2.5. Sources of Data, Data Collection and Validation
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics and Proportions of All Identified Bacterial Isolates in Urine Samples
3.2. Uropathogen Characteristics
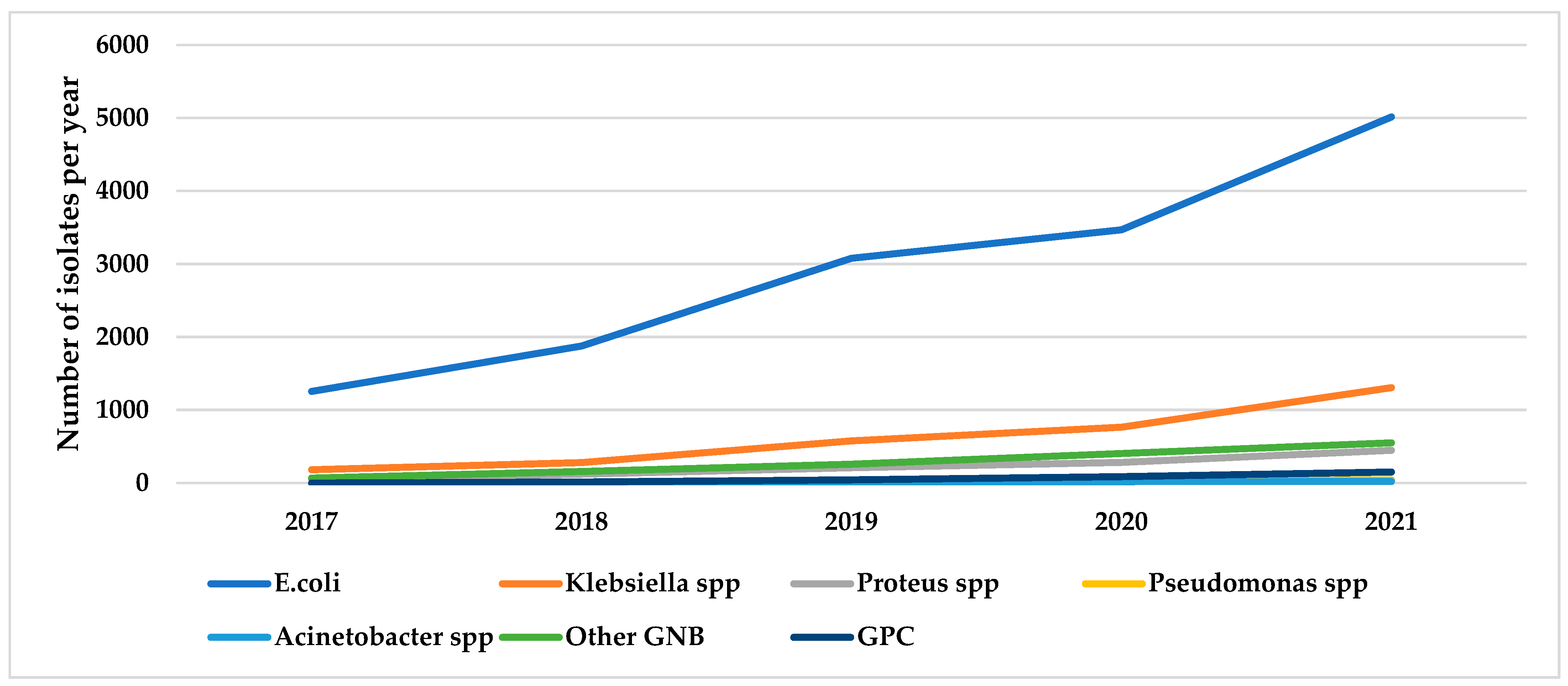
3.3. Trends in Urinary Bacterial Isolates over Five Years, 2017–2021
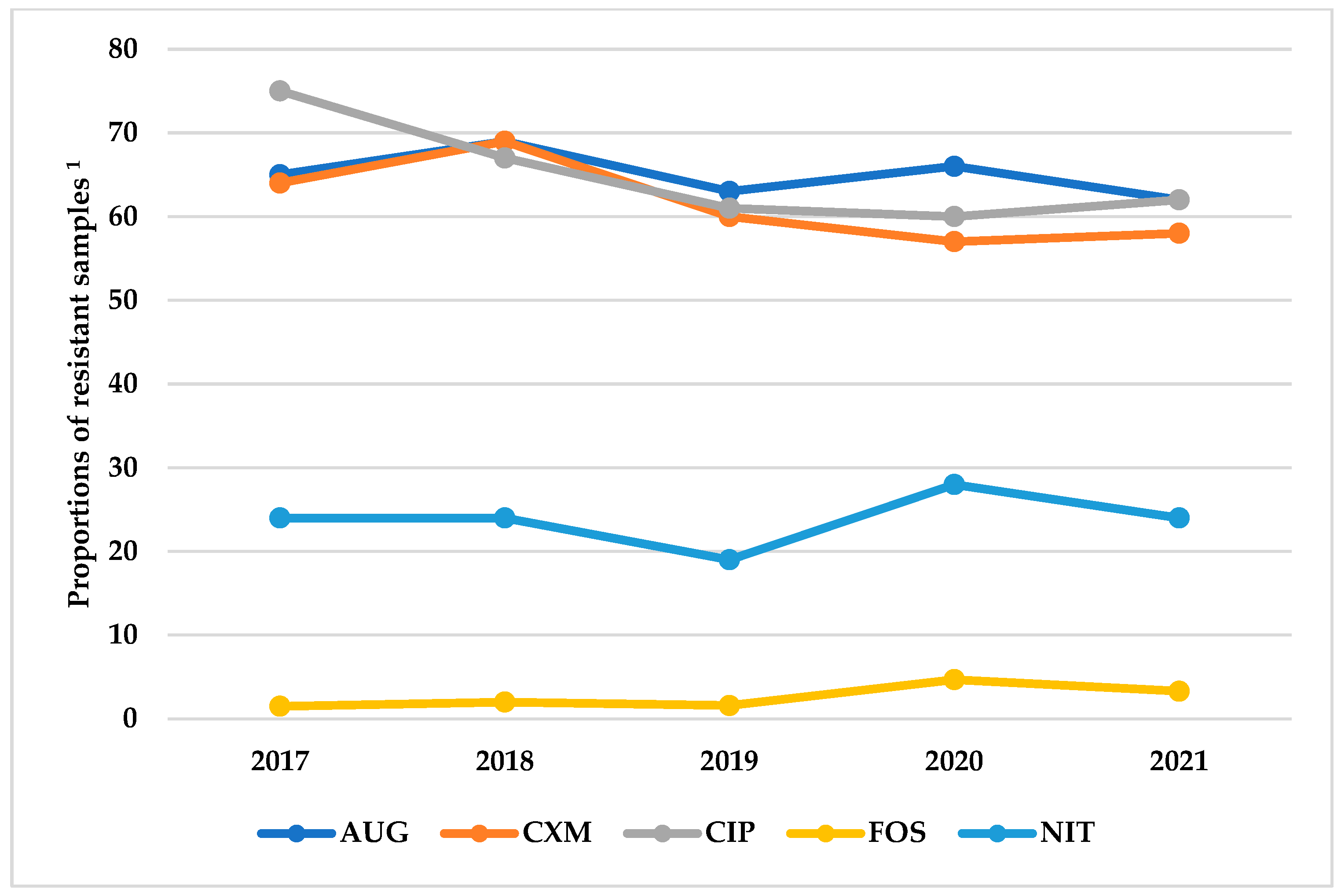
3.4. Resistance Patterns of Antibiotics Grouped by Route of Administration and the WHO AWaRe Classification
3.5. Multi-Drug Resistance and ESBL Positivity
3.6. Factors Associated with Antimicrobial Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Antimicrobial resistance. In Factsheet; World Health Organization (WHO): Geneva, Switzerland, 2020. [Google Scholar]
- Zalewska-pi, B. Phage Therapy as a Novel Strategy in the Treatment of Urinary Tract Infections Caused by E. coli. Antibiotics 2020, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Joshua, M.; Raymond, M. Virulence factors and antibiotic resistance patterns of uropathogenic Escherichia coli. Afr. J. Microbiol. Res. 2014, 8, 3678–3686. [Google Scholar] [CrossRef][Green Version]
- Stamm, W.E.; Norrby, S.R. Urinary Tract Infections: Disease Panorama and Challenges. J. Infect. Dis. 2001, 183, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, R.; Johansen, T.E.B. Eau Guidelines on Urological Infections; 2017; pp. 247–269. Available online: https://www.researchgate.net/publication/284761422_Guidelines_on_urological_infections. (accessed on 30 November 2022).
- Plan, G.A.; Resistance, A.; Regulations, I.H.; Atb, T.; Atb, N. A Technical Guide to Implementing the World Health Organization’ s AWaRe Antibiotic Classification in MTaPS Program Countries; 2019; pp. 1–7. Available online: https://www.mtapsprogram.org/wp-content/uploads/2021/03/USAID-MTaPS_Implementing-WHO-AWaRe-Classification.pdf (accessed on 30 November 2022).
- Kaye, K.S.; Nguyen, H.H.; Rybak, M.J. Clinical review guidelines for management and care transitions in the emergency. J. Emerg. Med. 2015, 48, 508–519. [Google Scholar] [CrossRef]
- Tenney, J.; Hudson, N.; Alnifaidy, H.; Li, J.T.C.; Fung, K.H. Risk factors for aquiring multidrug-resistant organisms in urinary tract infections: A systematic literature review. Saudi Pharm. J. 2018, 26, 678–684. [Google Scholar] [CrossRef]
- Patients, O.; Donkor, E.S. Urinary Tract Infections among Bladder Outlet Obstruction Patients in Accra, Ghana: Aetiology, Antibiotic Resistance, and Risk Factors. Diseases 2018, 6, 65. [Google Scholar] [CrossRef]
- Sangeda, R.Z.; Paul, F.; Mtweve, D.M. Prevalence of urinary tract infections and antibiogram of uropathogens isolated from children under five attending Bagamoyo District Hospital in Tanzania: A cross-sectional study [version 1; peer review: Awaiting peer review]. F1000Res 2021, 10, 449. [Google Scholar] [CrossRef]
- Forson, A.O.; Tsidi, W.B.; Adjei, D.N.; Quarchie, M.N.; Nkrumah, N.O. Escherichia coli bacteriuria in pregnant women in Ghana: Antibiotic resistance patterns and virulence factors. BMC Res. Notes 2018, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gambrah, E.; Owusu-ofori, A.; Biney, E.; Oppong, C.; Cof, S.E. Diagnosis and treatment of urinary tract infections in hospitalized adults in Ghana: The role of the clinical microbiology laboratory in improving antimicrobial stewardship. Int. J. Infect. Dis. 2020, 102, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Horlortu, P.Z.; Dayie, N.T.K.D.; Obeng-nkrumah, N.; Labi, A. Community acquired urinary tract infections among adults in Accra, Ghana. Infect. Drug Resist. 2019, 12, 2059–2067. [Google Scholar]
- Yevutsey, S.K.; Buabeng, K.O.; Aikins, M.; Anto, B.P.; Biritwum, R.B.; Frimodt-Møller, N.; Gyansa-Lutterodt, M. Situational analysis of antibiotic use and resistance in Ghana: Policy and regulation. BMC Public Health 2017, 17, 896. [Google Scholar] [CrossRef] [PubMed]
- Global Action Plan on Antimicrobial Resistance. Available online: https://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1 (accessed on 20 August 2022).
- Agyekum, E.B.; Amjad, F.; Mohsin, M.; Ansah, M.N.S. A bird’s eye view of Ghana’s renewable energy sector environment: A Multi-Criteria Decision-Making approach. Util. Policy 2021, 70, 101219. [Google Scholar] [CrossRef]
- Ministry of Health|Ghana official website [internet]. Role and function. 2015. Available online: https://dhsprogram.com/pubs/pdf/spa6/02chapter02.pdf (accessed on 20 August 2021).
- Ministry of Health-Ghana National Drugs Programme (GNDP). Standard Treatment Guidelines; 2017 Ministry of Health: Accra, Ghana, 2017; pp. 401–403. ISBN 9789988257873.
- Prashanth, B.V.S. Urine Culture Contamination: A One-Year Retrospective Study at the Tertiary Care Hospital. 29 December 2014. Available online: https://go.gale.com/ps/i.do?id=GALE%7CA469639434&sid=googleScholar&v=2.1&it=r&linkaccess=abs&issn=22784748&p=HRCA&sw=w&userGroupName=anon~70fd146c (accessed on 5 November 2021).
- Altheide, S.T. Biochemical and Culture-Based Approaches to Identification in the Diagnostic Microbiology Laboratory. Available online: http://clsjournal.ascls.org/content/early/2020/01/20/ascls.119.001875 (accessed on 5 November 2021).
- Antimicrobial Susceptibility Testing by the Kirby-Bauer Disc Diffusion Method. Available online: http://www.annclinlabsci.org/content/3/2/135.short (accessed on 5 November 2021).
- CLSI; Dolinsky, A.L.; Ohiro, R.K.; Fan, W.; Xiao, C.; Wu, F. National Committee for Clinical Laboratory Standards. 2000. Performance standard for antimicrobial susceptibility testing. Document M100–S10. J. Int. Med. Res. 2019, 46, 18. Available online: http://www.epa.gov/nerlcwww/1604sp02.pdf%0Ahttp://www.emeraldinsight.com/doi/10.1108/08876049410065598%0Ahttps://clsi.org/media/1469/m100s27_sample.pdf%0Ahttp://shop.clsi.org/site/Sample_pdf/M100S27_sample.pdf (accessed on 30 October 2022).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- McNutt, L.A.; Wu, C.; Xue, X.; Hafner, J.P. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am. J. Epidemiol. 2003, 157, 940–943. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, R.; Grosso, S.; Lorenzi, G.; Bruschetta, G.; Camporese, A. Evaluation of the new Sysmex UF-5000 fl uorescence fl ow cytometry analyser for ruling out bacterial urinary tract infection and for prediction of Gram negative bacteria in urine cultures. Clin. Chim. Acta 2018, 484, 171–178. [Google Scholar] [CrossRef]
- Wojno, K.J.; Baunoch, D.; Luke, N.; Opel, M.; Korman, H.; Kelly, C.; Jafri, S.M.A.; Keating, P.; Hazelton, D.; Hindu, S.; et al. Multiplex PCR Based Urinary Tract Infection (UTI) Analysis Compared to Traditional Urine Culture in Identifying Significant Pathogens in Symptomatic Patients. Urology 2019, 136, 119–126. [Google Scholar] [CrossRef]
- Jalil, M.B.; Al Atbee, M.Y.N. The prevalence of multiple drug resistance Escherichia coli and Klebsiella pneumoniae isolated from patients with urinary tract infections. J. Clin. Lab. Anal. 2022, 36, e24619. [Google Scholar] [CrossRef]
- Rizwan, M.; Akhtar, M.; Najmi, A.K.; Singh, K.; Res, D.; Delhi, N. Escherichia coli and Klebsiella pneumoniae Sensitivity/Resistance Pattern Towards Antimicrobial Agents in Primary and Simple Urinary Tract Infection Patients Visiting University Hospital of Jamia Hamdard New Delhi. Drug Res. 2018, 68, 415–420. [Google Scholar] [CrossRef]
- Maredia, N.N.; Fanning, M.J.; Christie, A.L.; Prokesch, B.C.; Zimmern, P.E. Adverse effects of chronic nitrofurantoin therapy in women with recurrent urinary tract infections in an outpatient setting. World J. Urol. 2020, 39, 2597–2603. [Google Scholar] [CrossRef]
- Madani, Y.; Mann, B. Nitrofurantoin-induced lung disease and prophylaxis of urinary tract infections. Nat. Publ. Gr. 2012, 21, 337–341. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sugianli, A.K.; Ginting, F.; Parwati, I.; de Jong, M.D.; van Leth, F. Antimicrobial resistance among uropathogens in the Asia-Pacific region: A systematic review. JAC-Antimicrob. Resist. 2021, 3, dlab003. [Google Scholar] [CrossRef] [PubMed]
- Gekenidis, M.; Kläui, A.; Smalla, K.; Drissner, D. Transferable Extended-Spectrum β-Lactamase (ESBL) Plasmids in Enterobacteriaceae from Irrigation Water. Microorganisms 2020, 8, 978. [Google Scholar] [CrossRef] [PubMed]
- Simmering, J.E.; Tang, F.; Cavanaugh, J.E.; Polgreen, L.A.; Polgreen, P.M. The Increase in Hospitalizations for Urinary Tract Infections and the Associated Costs in the United States, 1998–2011. pen Forum Infect. Dis. 2017, 4, ofw281. [Google Scholar] [CrossRef]
- Pujades-rodriguez, M.; West, R.M.; Wilcox, M.H.; Sandoe, J. Lower Urinary Tract Infections: Management, Outcomes and Risk Factors for Antibiotic Re-prescription in Primary Care. EClinicalMedicine 2019, 14, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Health, H.F.; Suson, K.D.; Health, H.F. Henry Ford Health Scholarly Commons Re: Luke Harper, T. Blanc, M. Peycelon; et al. Circumcision and Risk of Febrile Urinary Tract Infection in Boys with Posterior Urethral Valves: Result of the CIRCUP Randomized Trial. Eur. Urol. Lett. Ed. 2021, 81, 64–72. [Google Scholar] [CrossRef]
- Klaus, R.; Lange-sperandio, B. Chronic Kidney Disease in Boys with Posterior Urethral Valves–Pathogenesis, Prognosis and Management. Biomedicines 2022, 10, 1894. [Google Scholar] [CrossRef]
- Nicolle, L.E. Catheter Associated Urinary Tract Infections; 2014; pp. 1–8. Available online: https://aricjournal.biomedcentral.com/articles/10.1186/2047-2994-3-23 (accessed on 20 August 2022).
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 1453–1457. [Google Scholar] [CrossRef]
- Sugianli, A.K.; Ginting, F.; Kusumawati, R.L.; Parwati, I.; De Jong, M.D.; Van Leth, F.; Schultsz, C. Laboratory-based versus population-based surveillance of antimicrobial resistance to inform empirical treatment for suspected urinary tract infection in Indonesia. PLoS ONE 2020, 15, e0230489. [Google Scholar] [CrossRef]
| Characteristics | n | (%) | |
|---|---|---|---|
| Total number of patients | 20,010 | (100) | |
| Age in years | |||
| <15 | 1474 | (7.4) | |
| 15–44 | 7771 | (38.8) | |
| 45–64 | 4539 | 22.7 | |
| ≥65 | 6040 | (30.2) | |
| Unknown | 186 | 0.9 | |
| Sex | |||
| Male | 5484 | (27.4) | |
| Female | 14,505 | (72.5) | |
| Unknown | 21 | (0.1) | |
| Geographic location of urine specimens | |||
| Accra | 12,702 | (63.5) | |
| Ashanti | 4.266 | (21.3) | |
| Others | 3042 | (15.2) | |
| Antimicrobial substance in urine 2 | Present | 1986 | (9.9) |
| Number of bacterial isolates identified | |||
| One | 19,217 | (96.0) | |
| Two | 786 | (3.9) | |
| Three | 7 | (<0.1) | |
| Antimicrobial resistance | |||
| Resistance to at least one antimicrobial | 13,079 | (65.3) | |
| MDR 3 in at least one isolate | 12,609 | (63.0) |
| Gram-Negative Bacteria (GNB) | Gram-Positive Bacteria (GPC) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | Klebsiella spp. | Proteus spp. | Pseudomonas spp. | Acinetobacter spp. | Other GNB | |||||||||
| n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | n (%) 2 | ||||||||
| Age groups in years | ||||||||||||||
| <15 | 997 | (6.8) | 272 | (8.7) | 104 | (9.3) | 5 | (5.0) | 9 | (18.8) | 143 | (9.9) | 26 | (8.8) |
| 15–44 | 5887 | (40.1) | 1141 | (36.7) | 374 | (33.4) | 15 | (15.0) | 17 | (35.4) | 501 | (34.8) | 137 | (46.4) |
| 45–64 | 3329 | (22.7) | 735 | (23.6) | 237 | (21.2) | 21 | (21.0) | 4 | (8.3) | 309 | (21.5) | 64 | (21.7) |
| ≥65 | 4341 | (29.5) | 942 | (30.3) | 395 | (35.3) | 59 | (59.0) | 18 | (37.5) | 472 | (32.8) | 65 | (22.0) |
| Regions | ||||||||||||||
| Accra | 9437 | (64.2) | 1921 | (61.7) | 723 | (64.6) | 65 | (65.0) | 27 | (56.3) | 824 | (57.2) | 186 | (63.1) |
| Ashanti | 3080 | (21.0) | 659 | (21.2) | 280 | (25.0) | 18 | (18.0) | 6 | (12.5) | 348 | (24.2) | 77 | (26.1) |
| Others | 2178 | (14.8) | 532 | (17.1) | 117 | (10.4) | 17 | (17.0) | 15 | (31.3) | 268 | (18.6) | 32 | (10.8) |
| Total | 14695 | (100) | 3112 | (100) | 1120 | (100) | 100 | (100) | 48 | (100) | 1440 | (100) | 295 | (100) |
| Ampicillin | Amoxicillin Clavulanate | Nitrofurantoin | Ciprofloxacin | Cefuroxime | Fosfomycin | |
|---|---|---|---|---|---|---|
| n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | |
| E. coli | N/A | 9411 (64.0) | 3575 (24.3) | 9161 (62.3) | 8851 (60.2) | 435 (2.9) |
| Klebsiella spp. | N/A | 2083 (66.9) | 1639 (52.7) | 1659 (53.3) | 1986 (63.8) | 308 (9.9) |
| Proteus spp. | N/A | 105 (9.4) | N/A | 151 (13.5) | 91 (8.13) | 6 (14.0) |
| Acinetobacter spp. | N/A | N/A | N/A | 9 (18.8) | N/A | N/A |
| Pseudomonas spp. | N/A | N/A | N/A | 41 (41) | N/A | N/A |
| Enterococcus faecalis | 9 (3.3) | N/A | 4 (1.5) | N/A | N/A | 5 (1.9) |
| Organism | Ak | Caz | Cro | Mem | Piptaz | Tige |
|---|---|---|---|---|---|---|
| n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | |
| E. coli | 461 (3.1) | 7147 (48.62) | 7362 (50.1) | 42 (0.3) | 7148 (48.6) | 252 (1.7) |
| Klebsiella spp. | 116 (3.7) | 1802 (57.9) | 1834 (58.9) | 56 (1.8) | 1803 (57.9) | 173 (5.6) |
| Proteus spp. | 3 (0.3) | 46 (4.1) | 46 (4.1) | 3 (0.3) | 17 (39.5) | N/A |
| Acinetobacter spp. | 2 (4.2) | 17 (35.4) | N/A | 1 (2.1) | 14 (29.2) | N/A |
| Pseudomonas spp. | 21 (21) | 30 (30) | N/A | 19 (19) | 27 (27.0) | N/A |
| Organism | MDR | ESBL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | 2021 | Overall | 2017 | 2018 | 2019 | 2020 | 2021 | Overall | |
| n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | n (%) 2 | n (%) 2 | n (%) 2 | n (%) 2 | n (%) 2 | n (%) 2 | |
| Gram negatives | ||||||||||||
| E. coli | 871 (69.4) | 1309 (69.7) | 1958 (63.6) | 2182 (62.9) | 3087 (61.6) | 9407 (64.0) | 600 (47.8) | 975 (51.9) | 1585 (51.5) | 1692 (48.8) | 2492 (49.7) | 7344 (50.0) |
| Klebsiella spp. | 137 (74.5) | 216 (77.4) | 376 (65.2) | 522 (68.3) | 885 (67.7) | 2136 (68.6) | 106 (57.6) | 181 (64.9) | 329 (57.0) | 441 (57.7) | 765 (58.5) | 1822 (58.6) |
| Proteus spp. | 4(6.8) | 14 (11.6) | 33 (15.8) | 32 (11.4) | 59 (13.1) | 142 (12.68) | 4 (6.8) | 6 (5.0) | 16 (7.7) | 11 (3.9) | 22 (4.9) | 59 (5.3) |
| Pseudomonas spp. | 0 (0.0) | 1 (16.7) | 9 (40.9) | 14 (51.8) | 16 (35.6) | 40 (40) | N/A | N/A | N/A | N/A | N/A | N/A |
| Acinetobacter spp. | 0 (0.0) | 0 (0) | 0 (0.0) | 6 (31.6) | 4 (18.2) | 10 (20.8) | N/A | N/A | N/A | N/A | N/A | N/A |
| Other Gram-negative rods | 65 (94.3) | 140 (89.1) | 220 (85.3) | 348 (86.1) | 425 (77.0) | 1198 (83.2) | 44 (63.8) | 90 (57.3) | 131 (50.8) | 126 (31.2) | 185 (33.5) | 576 (40) |
| Factors | Total n 1 | MDR n (%) 2 | PR (95% CI) | aPR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Age in years | |||||
| <15 | 1474 | 854 (57.9) | Ref | Ref | |
| 15–44 | 7771 | 4595 (59.1) | 1.01 (0.97–1.06) | 1.05 (0.99–1.09) | 0.06 |
| 45–64 | 4539 | 3020 (66.5) | 1.14 (1.09–1.20) | 1.14 (1.08–1.19) | <0.001 |
| ≥5 | 6040 | 4207 (69.6) | 1.20 (1.14–1.25) | 1.16 (1.11–1.22) | <0.001 |
| Sex | |||||
| Male | 5484 | 3989 (72.7) | 1.20 (1.17–1.22) | 1.13 (1.11–1.16) | <0.001 |
| Female | 14,505 | 8783 (60.5) | Ref | Ref | |
| Specimen location 3 | |||||
| Accra | 12,702 | 7931 (62.4) | Ref | Ref | |
| Ashanti | 4266 | 2722 (63.8) | 1.02 (0.99–1.05) | 1.04 (1.01–1.07) | 0.002 |
| Others | 3042 | 2131 (63.8) | 1.12 (1.09–1.15) | 1.09 (1.07–1.13) | <0.001 |
| Antimicrobial substance in urine | |||||
| Present | 1986 | 1742 (87.1) | 1.43 (1.40–1.46) | 1.40 (1.37–1.43) | <0.001 |
| Absent | 18,007 | 11,030 (61.2) | Ref | Ref |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asamoah, B.; Labi, A.-K.; Gupte, H.A.; Davtyan, H.; Peprah, G.M.; Adu-Gyan, F.; Nair, D.; Muradyan, K.; Jessani, N.S.; Sekyere-Nyantakyi, P. High Resistance to Antibiotics Recommended in Standard Treatment Guidelines in Ghana: A Cross-Sectional Study of Antimicrobial Resistance Patterns in Patients with Urinary Tract Infections between 2017–2021. Int. J. Environ. Res. Public Health 2022, 19, 16556. https://doi.org/10.3390/ijerph192416556
Asamoah B, Labi A-K, Gupte HA, Davtyan H, Peprah GM, Adu-Gyan F, Nair D, Muradyan K, Jessani NS, Sekyere-Nyantakyi P. High Resistance to Antibiotics Recommended in Standard Treatment Guidelines in Ghana: A Cross-Sectional Study of Antimicrobial Resistance Patterns in Patients with Urinary Tract Infections between 2017–2021. International Journal of Environmental Research and Public Health. 2022; 19(24):16556. https://doi.org/10.3390/ijerph192416556
Chicago/Turabian StyleAsamoah, Benjamin, Appiah-Korang Labi, Himanshu A. Gupte, Hayk Davtyan, Georgette Marfo Peprah, Forster Adu-Gyan, Divya Nair, Karlos Muradyan, Nasreen S. Jessani, and Paul Sekyere-Nyantakyi. 2022. "High Resistance to Antibiotics Recommended in Standard Treatment Guidelines in Ghana: A Cross-Sectional Study of Antimicrobial Resistance Patterns in Patients with Urinary Tract Infections between 2017–2021" International Journal of Environmental Research and Public Health 19, no. 24: 16556. https://doi.org/10.3390/ijerph192416556
APA StyleAsamoah, B., Labi, A.-K., Gupte, H. A., Davtyan, H., Peprah, G. M., Adu-Gyan, F., Nair, D., Muradyan, K., Jessani, N. S., & Sekyere-Nyantakyi, P. (2022). High Resistance to Antibiotics Recommended in Standard Treatment Guidelines in Ghana: A Cross-Sectional Study of Antimicrobial Resistance Patterns in Patients with Urinary Tract Infections between 2017–2021. International Journal of Environmental Research and Public Health, 19(24), 16556. https://doi.org/10.3390/ijerph192416556






