Endotoxin as a Marker for Water Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Sampling
2.2. Membrane Filtration Method
2.3. BacterisK Assay
2.4. Kinetic-QCL™ LAL Assay
2.5. Statistical Analysis
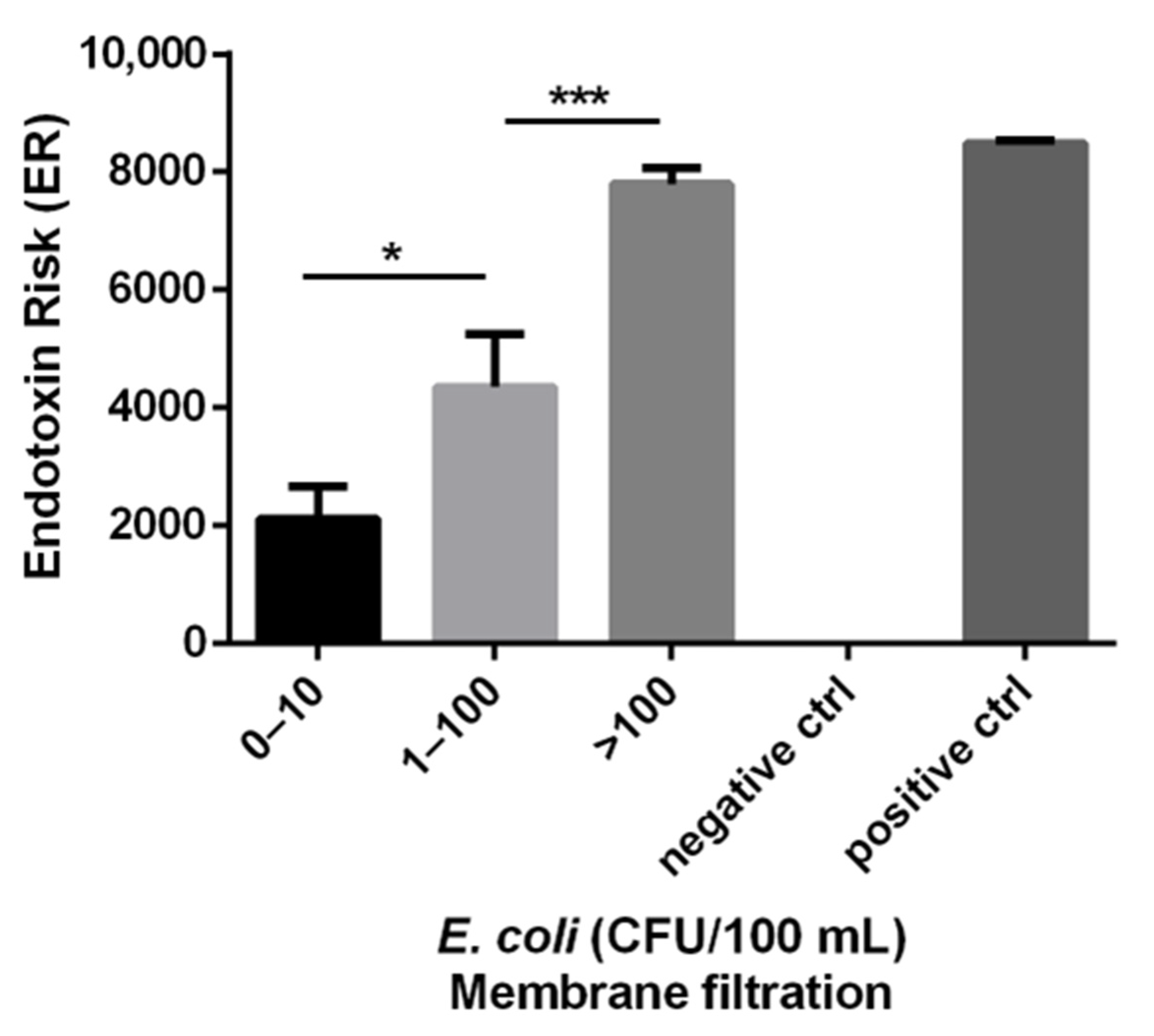
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fenwick, A. Waterborne infectious diseases--could they be consigned to history? Science 2006, 313, 1077–1081. [Google Scholar] [CrossRef]
- Borrego, J.J.; Arrabal, F.; de Vicente, A.; Gomez, L.F.; Romero, P. Study of Microbial Inactivation in the Marine Environment. J. Water Pollut. Control Fed. 1983, 55, 297–302. [Google Scholar]
- Anderson, W.B.; Slawson, R.M.; Mayfield, C.I. A review of drinking-water-associated endotoxin, including potential routes of human exposure. Can. J. Microbiol. 2002, 48, 567–587. [Google Scholar] [CrossRef]
- Field, K.G.; Samadpour, M. Fecal source tracking, the indicator paradigm, and managing water quality. Water Res. 2007, 41, 3517–3538. [Google Scholar] [CrossRef]
- Novak Babič, M.; Gunde-Cimerman, N.; Vargha, M.; Tischner, Z.; Magyar, D.; Veríssimo, C.; Sabino, R.; Viegas, C.; Meyer, W.; Brandão, J. Fungal Contaminants in Drinking Water Regulation? A Tale of Ecology, Exposure, Purification and Clinical Relevance. Int. J. Environ. Res. Public Health 2017, 14, 636. [Google Scholar] [CrossRef]
- ISO. Water Quality—Enumeration of Escherichia Coli and Coliform Bacteria—Part 1: Membrane Filtration Method for Waters with Low Bacterial Background Flora. 2014. Available online: https://www.iso.org/obp/ui/#iso:std:iso:9308:-1:ed-3:v1: (accessed on 18 October 2022).
- World Health Organisation. Developing Drinking-Water Quality Regulations and Standards: General Guidance with a Special Focus on Countries with Limited Resources; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organisation. WHO Guidelines Approved by the Guidelines Review Committee. In Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization Copyright © World Health Organization 2017: Geneva, Switzerland, 2017. [Google Scholar]
- Jorgensen, J.H.; Carvajal, H.F.; Chipps, B.E.; Smith, R.F. Rapid detection of gram-negative bacteriuria by use of the Limulus endotoxin assay. Appl. Microbiol. 1973, 26, 38–42. [Google Scholar] [CrossRef]
- Evans, T.M.; Schillinger, J.E.; Stuart, D.G. Rapid determination of bacteriological water quality by using limulus lysate. Appl. Environ. Microbiol. 1978, 35, 376–382. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Lee, J.C.; Alexander, G.A.; Wolf, H.W. Comparison of Limulus assay, standard plate count, and total coliform count for microbiological assessment of renovated wastewater. Appl. Environ. Microbiol. 1979, 37, 928–931. [Google Scholar] [CrossRef]
- Haas, C.N.; Meyer, M.A.; Paller, M.S.; Zapkin, M.A. The utility of endotoxins as a surrogate indicator in potable water microbiology. Water Res. 1983, 17, 803–807. [Google Scholar] [CrossRef]
- Watson, S.; Novitsky, T.; Quinby, H.; Valois, F. Determination of bacterial number and biomass in the marine environment. Appl. Environ. Microbiol. 1977, 33, 940–946. [Google Scholar] [CrossRef]
- Sattar, A.A.; Jackson, S.K.; Bradley, G. The potential of lipopolysaccharide as a real-time biomarker of bacterial contamination in marine bathing water. J. Water Health 2014, 12, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Sattar, A.A.; Abate, W.; Fejer, G.; Bradley, G.; Jackson, S.K. Evaluation of the proinflammatory effects of contaminated bathing water. J. Toxicol. Environ. Health A 2019, 82, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Choi, S. Gram-negative marine bacteria: Structural features of lipopolysaccharides and their relevance for economically important diseases. Mar. Drugs 2014, 12, 2485–2514. [Google Scholar] [CrossRef] [PubMed]
- Douwes, J.; Versloot, P.; Hollander, A.; Heederik, D.; Doekes, G. Influence of various dust sampling and extraction methods on the measurement of airborne endotoxin. Appl. Environ. Microbiol. 1995, 61, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Abate, W.; Sattar, A.A.; Liu, J.; Conway, M.E.; Jackson, S.K. Evaluation of recombinant factor C assay for the detection of divergent lipopolysaccharide structural species and comparison with Limulus amebocyte lysate-based assays and a human monocyte activity assay. J. Med. Microbiol. 2017, 66, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.; Snozzi, M.; Koster, W.; Bartram, J.; Ronchi, E.; Fewtrell, L.; World Health Organization; Water, S.; Health, T. Assessing Microbial Safety of Drinking Water: Improving Approaches and Methods; Dufour, A., Snozzi, M., Koster, W., Bartram, J., Ronchi, E., Fewtrell, L., Eds.; IWA Publishing: London, UK, 2003; pp. 237–292. [Google Scholar]
- Méndez, J.; Audicana, A.; Cancer, M.; Isern, A.; Llaneza, J.; Moreno, B.; Navarro, M.; Tarancón, M.L.; Valero, F.; Ribas, F.; et al. Assessment of drinking water quality using indicator bacteria and bacteriophages. J. Water Health 2004, 2, 201–214. [Google Scholar] [CrossRef]
- Johnson, C.N.; Bowers, J.C.; Griffitt, K.J.; Molina, V.; Clostio, R.W.; Pei, S.; Laws, E.; Paranjpye, R.N.; Strom, M.S.; Chen, A.; et al. Ecology of Vibrio parahaemolyticus and Vibrio vulnificus in the coastal and estuarine waters of Louisiana, Maryland, Mississippi, and Washington (United States). Appl. Environ. Microbiol. 2012, 78, 7249–7257. [Google Scholar] [CrossRef]
- Collier, S.A.; Deng, L.; Adam, E.A.; Benedict, K.M.; Beshearse, E.M.; Blackstock, A.J.; Bruce, B.B.; Derado, G.; Edens, C.; Fullerton, K.E.; et al. Estimate of Burden and Direct Healthcare Cost of Infectious Waterborne Disease in the United States. Emerg. Infect. Dis. 2021, 27, 140–149. [Google Scholar] [CrossRef]
- Aiken, G.R. Fluorescence and dissolved organic matter: A chemist’s perspective: Chapter 2. Aquat. Org. Matter Fluoresc. 2014, 35, 35. [Google Scholar]
- Sorensen, J.P.R.; Diaw, M.T.; Pouye, A.; Roffo, R.; Diongue, D.M.L.; Faye, S.C.; Gaye, C.B.; Fox, B.G.; Goodall, T.; Lapworth, D.J.; et al. In-situ fluorescence spectroscopy indicates total bacterial abundance and dissolved organic carbon. Sci. Total Environ. 2020, 738, 139419. [Google Scholar] [CrossRef]
- Carstea, E.M.; Popa, C.L.; Baker, A.; Bridgeman, J. In situ fluorescence measurements of dissolved organic matter: A review. Sci. Total Environ. 2020, 699, 134361. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.P.R.; Baker, A.; Cumberland, S.A.; Lapworth, D.J.; MacDonald, A.M.; Pedley, S.; Taylor, R.G.; Ward, J.S.T. Real-time detection of faecally contaminated drinking water with tryptophan-like fluorescence: Defining threshold values. Sci. Total Environ. 2018, 622–623, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Bain, R.; Cronk, R.; Wright, J.; Yang, H.; Slaymaker, T.; Bartram, J. Fecal contamination of drinking-water in low- and middle-income countries: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001644. [Google Scholar] [CrossRef] [PubMed]

| E. coli CFU/100 mL of Water | ||||
|---|---|---|---|---|
| 0–10 (Low Risk) | 11–100 (Intermediate Risk) | >100 (High Risk) | Total | |
| <500 ER | 9 | 0 | 0 | 9 |
| 500–7000 ER | 12 | 9 | 3 | 24 |
| >7000 ER | 2 | 5 | 29 | 36 |
| Total | 23 | 14 | 32 | 69 |
| Sample ID | First Result | Second Result | Mean | Standard Deviation | Variation Coefficient |
|---|---|---|---|---|---|
| 31 | 1770 | 2085 | 1928 | 223 | 12% |
| 32 | 1080 | 1120 | 1100 | 28 | 3% |
| 33 | 2650 | 2470 | 2560 | 127 | 5% |
| 34 | 6155 | 7075 | 6615 | 651 | 10% |
| 35 | 4840 | 4830 | 4835 | 7 | 0% |
| 36 | 3930 | 4610 | 4270 | 481 | 11% |
| 37 | 8485 | 8505 | 8495 | 14 | 0% |
| 38 | 7365 | 7530 | 7448 | 117 | 2% |
| 39 | 7755 | 7795 | 7775 | 28 | 0% |
| 40 | 8320 | 8320 | 8320 | 0 | 0% |
| 41 | 8280 | 8385 | 8333 | 74 | 1% |
| 42 | 8495 | 8495 | 8495 | 0 | 0% |
| 44 | 8395 | 8365 | 8380 | 21 | 0% |
| 45 | 8445 | 8455 | 8450 | 7 | 0% |
| 47 | 8415 | 8415 | 8415 | 0 | 0% |
| Mean: 3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sattar, A.A.; Good, C.R.; Saletes, M.; Brandão, J.; Jackson, S.K. Endotoxin as a Marker for Water Quality. Int. J. Environ. Res. Public Health 2022, 19, 16528. https://doi.org/10.3390/ijerph192416528
Sattar AA, Good CR, Saletes M, Brandão J, Jackson SK. Endotoxin as a Marker for Water Quality. International Journal of Environmental Research and Public Health. 2022; 19(24):16528. https://doi.org/10.3390/ijerph192416528
Chicago/Turabian StyleSattar, Anas A., Christian R. Good, Margaux Saletes, João Brandão, and Simon K. Jackson. 2022. "Endotoxin as a Marker for Water Quality" International Journal of Environmental Research and Public Health 19, no. 24: 16528. https://doi.org/10.3390/ijerph192416528
APA StyleSattar, A. A., Good, C. R., Saletes, M., Brandão, J., & Jackson, S. K. (2022). Endotoxin as a Marker for Water Quality. International Journal of Environmental Research and Public Health, 19(24), 16528. https://doi.org/10.3390/ijerph192416528







