First-Trimester Biochemical Serum Markers in Female Kidney Transplant Recipients—The Impact of Graft Function
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, J.E.; Reid, D.E.; Harrison, J.H.; Merrill, J.P. Successful pregnancies after human renal transplantation. N. Engl. J. Med. 1963, 269, 341–343. [Google Scholar] [CrossRef]
- Transplant Pregnancy Registry International-Gift of Life Institute. 2018 Annual Report. Available online: https://www.transplantpregnancyregistry.org/ (accessed on 11 July 2022).
- Durst, J.K.; Rampersad, R.M. Pregnancy in Women with Solid-Organ Transplants: A Review. Obstet. Gynecol. Surv. 2015, 70, 408–418. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Cabiddu, G.; Attini, R.; Gerbino, M.; Todeschini, P.; Perrino, M.L.; Manzione, A.M.; Piredda, G.B.; Gnappi, E.; Caputo, F.; et al. Outcomes of Pregnancies After Kidney Transplantation: Lessons Learned From CKD. A Comparison of Transplanted, Nontransplanted Chronic Kidney Disease Patients and Low-Risk Pregnancies: A Multicenter Nationwide Analysis. Transplantation 2017, 101, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Pandya, P.P.; Snijders, R.J.; Johnson, S.P.; De Lourdes Brizot, M.; Nicolaides, K.H. Screening for fetal trisomies by maternal age and fetal nuchal translucency thickness at 10 to 14 weeks of gestation. Br. J. Obstet. Gynaecol. 1995, 102, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, K.H. Screening for fetal aneuploidies at 11 to 13 weeks. Prenat. Diagn. 2011, 31, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.; Silva, M.; Papadopoulos, S.; Wright, A.; Nicolaides, K.H. Serum pregnancy-associated plasma protein-A in the three trimesters of pregnancy: Effects of maternal characteristics and medical history. Ultrasound Obstet. Gynecol. 2015, 46, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Amiri, F.S. Serum tumor markers in chronic kidney disease: As clinical tool in diagnosis, treatment and prognosis of cancers. Ren. Fail. 2016, 38, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Menon, M.C.; Bhaskaran, M.; Jhaveri, K.D.; Molmenti, E.; Muoio, V. Elevated human chorionic gonadotropin levels in patients with chronic kidney disease: Case series and review of literature. Indian J. Nephrol. 2013, 23, 424–427. [Google Scholar] [PubMed]
- Coskun, A.; Duran, S.; Apaydin, S.; Bulut, I.; Sariyar, M. Pregnancy-associated plasma protein-A: Evaluation of a new biomarker in renal transplant patients. Transplant. Proc. 2007, 39, 3072–3076. [Google Scholar] [CrossRef] [PubMed]
- Kagan, K.O.; Hoopmann, M.; Abele, H.; Alkier, R.; Lüthgens, K. First-trimester combined screening for trisomy 21 with different combinations of placental growth factor, free β-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet. Gynecol. 2012, 40, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Grande, M.; Cararach, V.; Casals, E.; Borrell, A. First-trimester Down syndrome screening in renal-transplanted pregnant women: A model for adjusting the false-positives rates. Prenat. Diagn. 2013, 33, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Cararach, V.; Casals, E.; Martínez, S.; Carmona, F.; Aibar, C.; Quintó, L.I.; Alonso, P.; Fortuny, A. Abnormal renal function as a cause of false-positive biochemical screening for Down’s syndrome. Lancet 1997, 350, 1295. [Google Scholar] [CrossRef] [PubMed]
- Karidas, C.N.; Michailidis, G.D.; Spencer, K.; Economides, D.L. Biochemical screening for Down syndrome in pregnancies following renal transplantation. Prenat. Diagn. 2002, 22, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.; Enofe, O.; Cowans, N.J.; Stamatopoulou, A. Is maternal renal disease a cause of elevated free beta-hCG in first trimester aneuploidy screening? Prenat. Diagn. 2009, 29, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Valentin, M.; Muller, F.; Beaujard, M.P.; Dreux, S.; Czerkiewicz, I.; Meyer, V.; Leruez, M.; Ville, Y.; Salomon, L.J. First-trimester combined screening for trisomy 21 in women with renal disease. Prenat. Diagn. 2015, 35, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Meloni, P.; D’Angeli, I.; Piazze, J.; Cerekya, A.; Simari, T.; Pala, A.; Anceschi, M.M.; Guglietta, M.; Izzo, P.; Izzo, L. First Trimester PAPP-A Levels Associated with Early Prediction of Pregnancy Induced Hypertension. Hypertens. Pregnancy 2009, 28, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Kalousová, M.; Tesaíř, V.; Muravská, A.; Zima, T. Pregnancy-associated plasma protein A: Spotlight on kidney diseases. Clin. Chem. Lab. Med. 2012, 50, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Filippone, E.J.; Farber, J.L. The Monitoring of Donor-derived Cell-free DNA in Kidney Transplantation. Transplantation 2021, 105, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Attini, R.; Grati, F.R.; Menato, G.; Todros, T.; Colla, L.; Rossetti, M.; Malvestiti, B.; Alemanno, M.G.; Masturzo, B.; Piccoli, G.B.; et al. Pregnancy on dialysis and with a failing kidney graft: A double challenge for non-invasive prenatal testing. Prenat. Diagn. 2020, 40, 387–389. [Google Scholar] [CrossRef] [PubMed]
| Variable | Study Group (n = 27) | Control Group (n = 110) | p |
|---|---|---|---|
| Maternal characteristics: | |||
| Age (years) | 35.0 ± 3.5 | 34.1 ± 4.3 | 0.31 |
| BMI (kg/m2) | 24.7 ± 4.2 | 23.9 ± 7.6 | 0.19 |
| Nulliparous (%) | 13 (48.1) | 68 (61.8) | <0.0001 |
| Hypertension before pregnancy (%) | 21 (77.8) | 0 | <0.001 |
| Pregnancy complications: | |||
| Pregnancy-induced hypertension (%) | 6 (22.2) | 9 (8.2) | 0.047 |
| Gestational diabetes (%) | 5 (18.5) | 7 (6.4) | 0.06 |
| Anemia (%) | 11 (40.7) | 11 (10.0) | <0.0001 |
| Proteinuria (%) | 12 (44.4) | 3 (2.7) | <0.0001 |
| Intrahepatic cholestasis (%) | 0 | 2 (1.8) | 0.98 |
| HCV infection (%) | 1 (3.7) | 0 | 0.197 |
| HBV infection (%) | 1 (3.7) | 1 (0.9) | 0.356 |
| Perinatal outcomes: | |||
| Gestational age at delivery (weeks) | 34.8 ± 4.2 | 39.1 ± 1.6 | <0.0001 |
| Cesarean section (%) | 21 (77.8) | 37 (33.6) | <0.0001 |
| Birth weight (g) | 2350 ± 878.36 | 3404 ± 488.37 | 0.001 |
| Birth weight centiles | 38.63 ± 23.47 | 45.97 ± 27.76 | 0.171 |
| SGA (<10 percentile) (%) | 4 (14.8) | 8 (7.3) | 0.252 |
| Variables (Mean ± SD) | Study Group (n = 27) |
|---|---|
| Prepregnancy laboratory values: | |
| SCr (mg/dL) | 1.03 ± 0.24 |
| eGFR (mL/min/1.73 m2) | 65.5 ± 23.8 |
| Pregnancy (I trimester) laboratory values | |
| SCr (mg/dL) | 1.14 ± 0.38 |
| ALT (U/L) | 18.9 ± 0.11 |
| AST (U/L) | 17.04 ± 0.83 |
| Hgb (g/dL) | 10.84 ± 1.27 |
| Urea (mg/dL) | 7.24 ± 1.91 |
| Postpartum Scr (mg/dL) | 1.17 ± 0.40 |
| Variable | Study Group (n = 27) | Control Group (n = 110) | p-Value |
|---|---|---|---|
| free β-hCG (mIU/L) | 123.06 ± 76.54 | 50.76 ± 32.71 | 0.047 |
| free β-hCG MoM | 3.47 ± 2.08 | 1.38 ± 0.85 | 0.035 |
| PAPP-A (mIU/L) | 5.25 ± 4.20 | 2.48 ± 1.16 | 0.016 |
| PAPP-A MoM | 1.46 ± 0.81 | 0.98 ± 0.57 | 0.007 |
| NT (mm) | 1.77 ± 0.43 | 1.70 ± 0.47 | 0.687 |
| UtA PI | 1.06 ± 0.23 | 1.28 ± 0.53 | 0.052 |
| Variables | Tacrolimus (n = 19) | Cyclosporin (n = 7) | p-Value |
|---|---|---|---|
| NT (mm) | 1.91 ± 0.36 | 1.96 ± 0.31 | 0.75 |
| UtA PI | 1.13 ± 0.37 | 1.12 ± 0.29 | 0.95 |
| free β-hCG (mIU/L) | 101.01 ± 68.10 | 157.32 ± 80.50 | 0.18 |
| free β-hCG MoM | 2.87 ± 1.76 | 3.40 ± 1.30 | 0.47 |
| PAPP-A (mIU/L) | 4.44 ± 3.25 | 7.88 ± 6.31 | 0.16 |
| PAPP-A MoM | 1.28 ± 0.64 | 2.02 ± 1.07 | 0.12 |
| GA at delivery (weeks) | 34.16 ± 4.60 | 36.00 ± 2.70 | 0.33 |
| Birthweight (g) | 2266.05 ± 937.35 | 2401.43 ± 654.08 | 0.73 |
| Birthweight percentile | 40.79 ± 23.26 | 26.71 ± 14.49 | 0.16 |
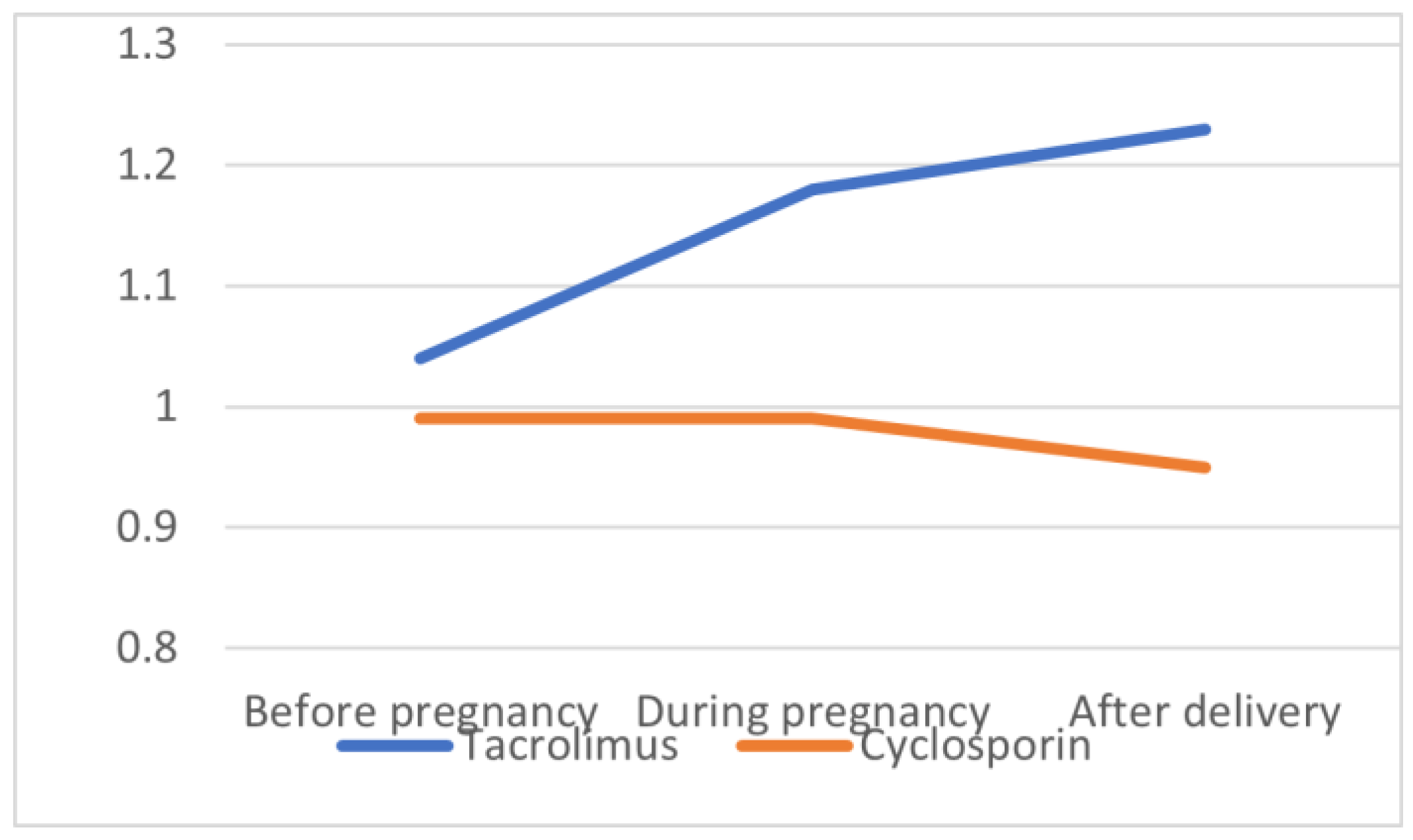
| SCr before pregnancy (mg/dL) | 1.04 ± 0.28 | 0.99 ± 0.11 | 0.70 |
| SCr during pregnancy (md/dL) | 1.18 ± 0.39 | 0.99 ± 0.32 | 0.29 |
| SCr after delivery (mg/dL) | 1.23 ± 0.44 | 0.95 ± 0.16 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazanowska, N.; Jarmużek-Orska, P.; Pietrzak, B.; Pazik, J.; Jabiry-Zieniewicz, Z.; Kosiński, P. First-Trimester Biochemical Serum Markers in Female Kidney Transplant Recipients—The Impact of Graft Function. Int. J. Environ. Res. Public Health 2022, 19, 16352. https://doi.org/10.3390/ijerph192316352
Mazanowska N, Jarmużek-Orska P, Pietrzak B, Pazik J, Jabiry-Zieniewicz Z, Kosiński P. First-Trimester Biochemical Serum Markers in Female Kidney Transplant Recipients—The Impact of Graft Function. International Journal of Environmental Research and Public Health. 2022; 19(23):16352. https://doi.org/10.3390/ijerph192316352
Chicago/Turabian StyleMazanowska, Natalia, Patrycja Jarmużek-Orska, Bronisława Pietrzak, Joanna Pazik, Zoulikha Jabiry-Zieniewicz, and Przemysław Kosiński. 2022. "First-Trimester Biochemical Serum Markers in Female Kidney Transplant Recipients—The Impact of Graft Function" International Journal of Environmental Research and Public Health 19, no. 23: 16352. https://doi.org/10.3390/ijerph192316352
APA StyleMazanowska, N., Jarmużek-Orska, P., Pietrzak, B., Pazik, J., Jabiry-Zieniewicz, Z., & Kosiński, P. (2022). First-Trimester Biochemical Serum Markers in Female Kidney Transplant Recipients—The Impact of Graft Function. International Journal of Environmental Research and Public Health, 19(23), 16352. https://doi.org/10.3390/ijerph192316352







