Evaluation of the Effect of SPIDER System Therapy on Weight Shifting Symmetry in Chronic Stroke Patients—A Randomized Controlled Trial
Abstract
1. Introduction
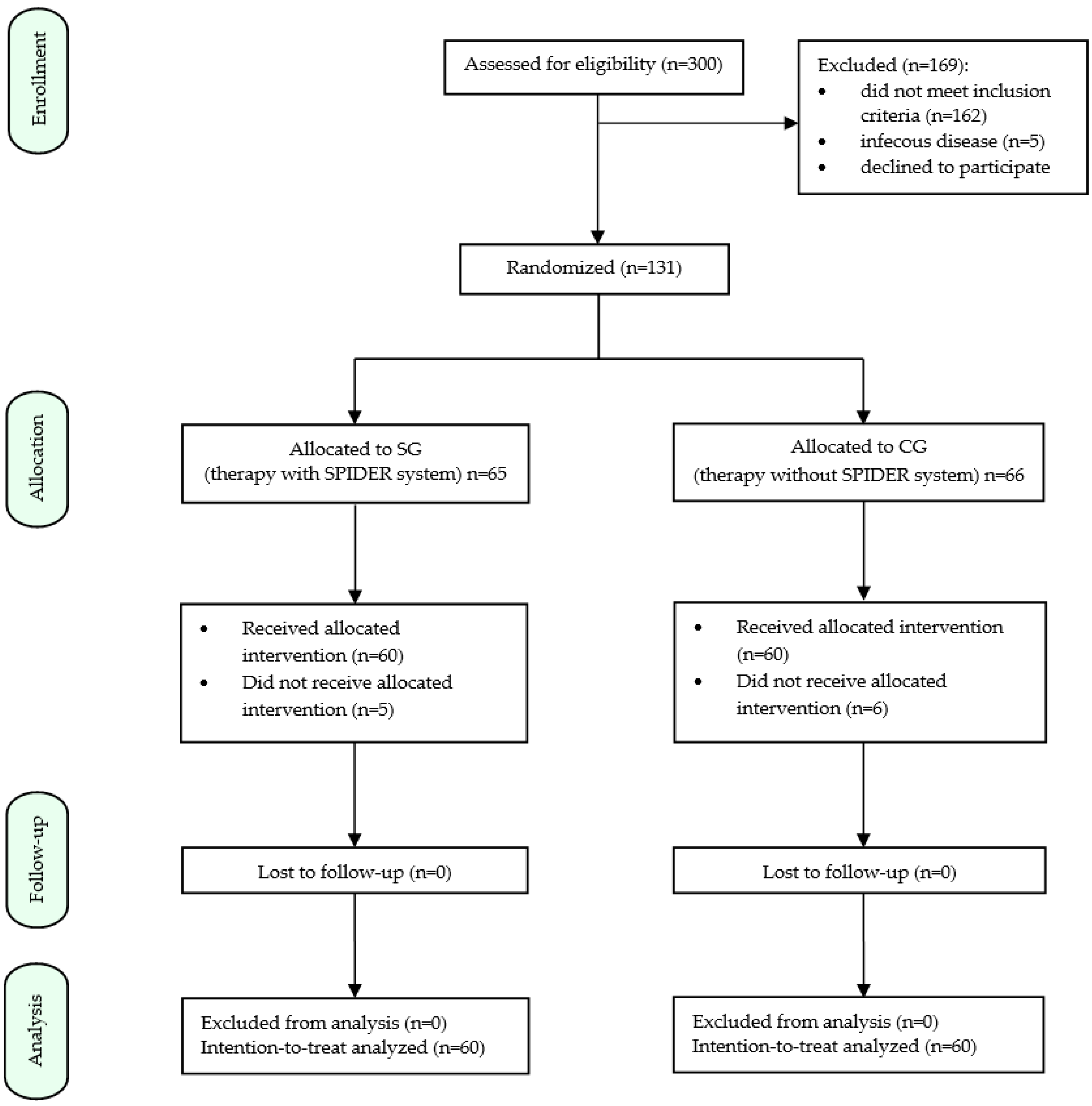
2. Materials and Methods
2.1. Subject of the Study
2.2. Assessment Protocol
- ICF-based functional testing:
- -
- Change from sitting to standing (d4103)—the patient performs a transition from sitting with feet placed on the floor to standing (without any assistance);
- -
- Holding the standing position (d4154)—the patient stays in the required standing position for a specified period of time (without any assistance);
- -
- Test to assess the risk of falls in walking (TUG—d4500)—the patient stands up from a sitting position (e.g., on a chair) and walks a distance of 3 m, then turns around and returns to the starting point by sitting down again. The time is measured from standing up to taking up a sitting position again. Norms: healthy people—less than 10 s, elderly people—14 s, patients at risk of falling—more than 30 s [22,23].
- Modified Ashworth Scale: a six-point numerical scale used to assess increased muscle tone (spasticity). Muscle tension in the lower limbs is tested in the supine position, symmetrically on both sides. The speed of passive movement, which should last approximately 1 s in full flexion or extension, proves to be important [14,24].
- Barthel scale: an international scale for assessing a patient’s functional abilities and care needs. The index assesses the patient’s level of dexterity and independence in ten basic areas: eating, moving and sitting, maintaining personal hygiene, using the toilet, washing and bathing the entire body, moving on flat surfaces, going up and down stairs, dressing and undressing, controlling feces and anal sphincter, controlling urine and bladder sphincter. Interpretation of scores: 0–20 pts.—complete dependence, 21–80 pts.—help from others needed, 81–100 pts.—patient can function independently with support from others [25].
- NIHSS scale: an international scale to assess the state of consciousness, orientation, response to commands, associated gaze, visual field range, facial and limb muscle paresis, sensation and language function of the stroke patient. Scores range from 0 (normal) to 42 points (where 1–4—minor stroke, 5–15—moderate stroke, 16–20—moderate to severe stroke, 21–42—severe stroke) [27].
- MMSE scale: a mental status evaluation scale that allows quantitative assessment of many aspects of cognitive function: orientation in time and place, memory, attention and counting, remembering, language function, repetition, following complex oral or written instructions and visuospatial ability. The maximum score that can be obtained in the test is 30 points (a score below 24 points is indicative of varying degrees of Dementia) [28].
- FMA-LE test: an index to assess lower limb sensorimotor dysfunction in post-stroke patients. The scale includes an assessment of: reflex activity, voluntary movements within and beyond synergies, ability to perform isolated movements, speed and coordination. A total score of 34 points indicates normal lower limb function [29,30].
- Mirror test: a test used to investigate deep sensation [31].
- Berg Balance Test: a quantitative test to assess balance and fall risk. It focuses on static and dynamic balance. It includes 14 tasks with a maximum score of 56 points. A score of 0–20 points suggests total wheelchair dependence; 21–40 points are assigned to a patient who moves with assistance, 41–56 points—independent patient [29,32].
2.3. Physiotherapy Treatment Program
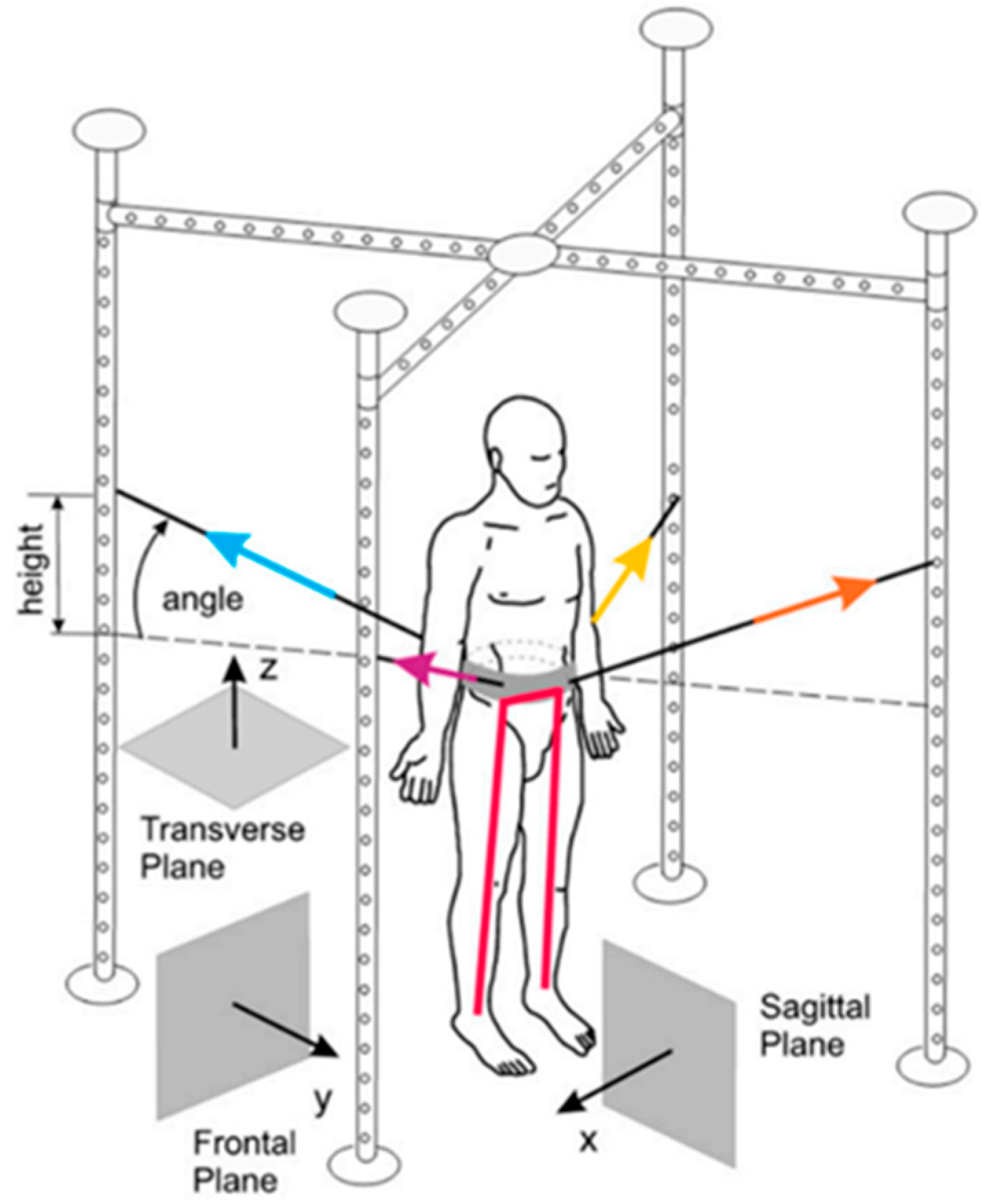
2.3.1. TYMO® platform
- -
- The length of time (in seconds) that balance can be maintained;
- -
- The range of motion (ROM) exceeded;
- -
- The center of force track (COFT), or the total length of the center of force track (COF) during each measurement. Medio-Lateral indicates the total range of lateral sway of the body (right-mid-left), and Anterior-Posterior indicates the total range of forward and backward sway of the body (front-mid-backward);
- -
- The COF movement area (the ellipse area, which contains 95% of all COF positions);
- -
- The Stability Dynamics Index (STDI), which analyzes the relationship between the distance traveled by the COF and the area of ROM. The higher the value, the more unstable the patient;
- -
- An average speed and the ratio of maximum speed to average speed. The speed value describes the number of fast movements that need to be made to maintain balance;
- -
- A posture, viz., the distribution of weight and/or force from front to back and left to right [33].
2.3.2. SPIDER System
2.4. Statistical Analysis
3. Results
3.1. Demographic and Functional Characteristics of Patients
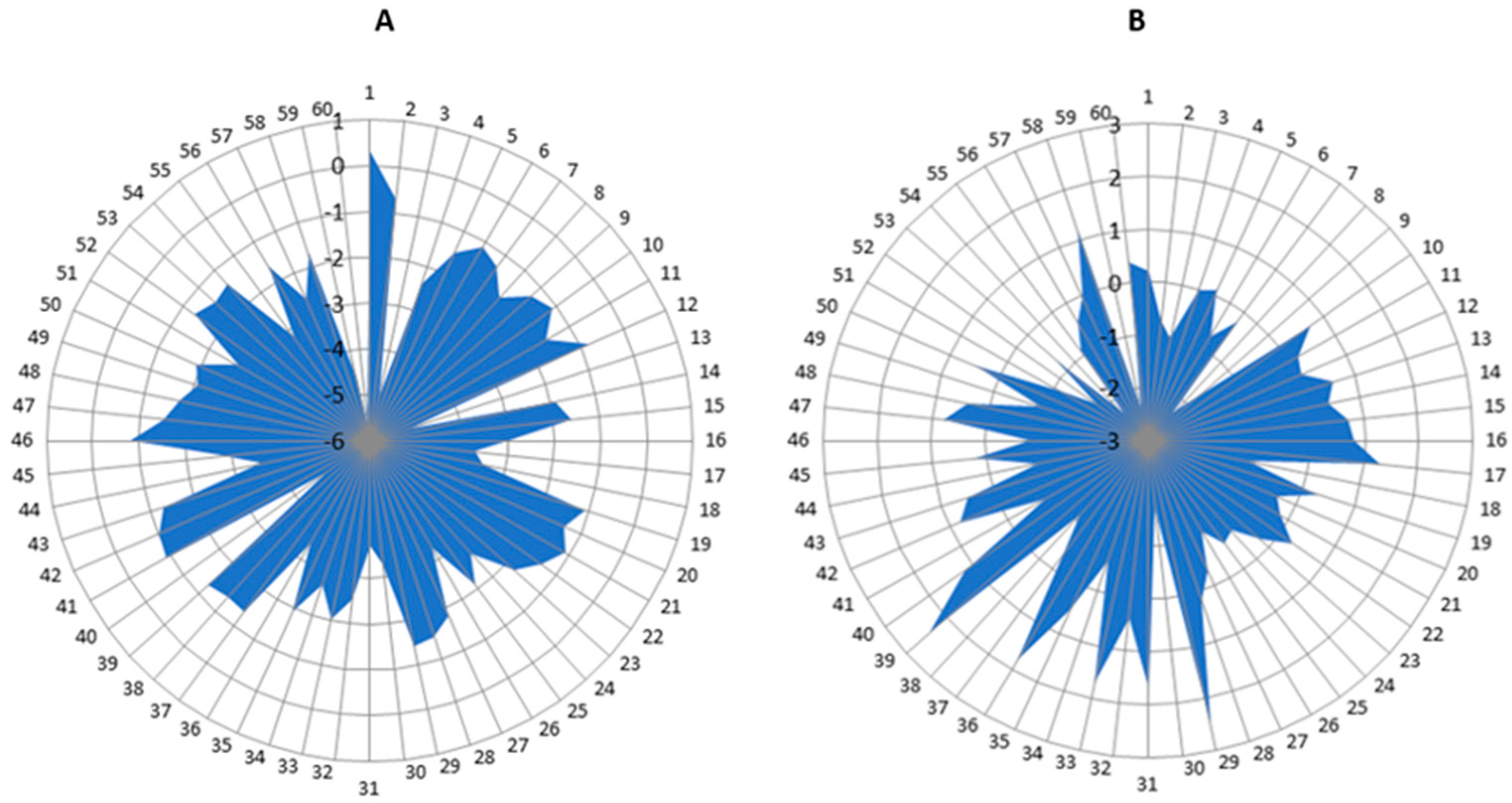
3.2. Description of Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations from Article: | |
| SPIDER | Strengthening Program for Intensive Developmental Exercises and Activities for Reaching Health Capability |
| PNF | Proprioceptive neuromuscular facilitation |
| NDT-Bobath | Neurodevelopmental treatment according to the Bobath concept |
| SG | Study (SPIDER) group |
| CG | Control group |
| BBS | Berg Balance Scale |
| TUG | Timed up and go |
| FMA (LE) | Fugl–Meyer assessment (lower extremity) |
| ICF | International Classification of Functioning, Disability and Health |
| NIHSS | National Institutes of Health Stroke Scale |
| MMSE | Mini-Mental State Examination |
| ROM | Range of motion |
| COF | Center of force |
| COFT | Center of force track |
| F | Female |
| M | Male |
| R | Right-sided hemiparesis |
| L | Left-sided hemiparesis |
| N | Hypoesthesia |
| P | Hyperesthesia |
| df | Degrees of freedom |
References
- Aruin, A.S.; Rao, N.; Sharma, A.; Chaudhuri, G. Compelled Body Weight Shift Approach in Rehabilita-tion of Individuals with Chronic Stroke. Top. Stroke Rehabil. 2012, 19, 556–563. [Google Scholar] [CrossRef]
- van Dijk, M.M.; Meyer, S.; Sandstad, S.; Wiskerke, E.; Thuwis, R.; Vandekerckhove, C.; Myny, C.; Ghosh, N.; Beyens, H.; Dejaeger, E.; et al. A cross-sectional study comparing lat-eral and diagonal maximum weight shift in people with stroke and healthy controls and the correlation with balance, gait and fear of falling. PLoS ONE 2017, 12, e0183020. [Google Scholar] [CrossRef]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke care 2, Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Verheyden, G.; Ruesen, C.; Gorissen, M.; Brumby, V.; Moran, R.; Burnett, M.; Ashburn, A. Postural alignment is altered in people with chronic stroke and related to motor and functional performance. J. Neurol. Phys. Ther. 2014, 38, 239–245. [Google Scholar] [CrossRef]
- Tyson, S.F.; Hanley, M.; Chillala, J.; Selley, A.; Tallis, R.C. Balance disability after stroke. Phys Ther. 2006, 86, 30–38. [Google Scholar] [CrossRef]
- Genthon, N.; Gissot, A.-S.; Froger, J.; Rougier, P.; Pérennou, D. Posturography in patients with stroke, estimating the percentage of body weight on each foot from a single force platform. Stroke 2008, 39, 489–491. [Google Scholar] [CrossRef]
- Nardone, A.; Godi, M.; Grasso, M.; Guglielmetti, S.; Schieppati, M. Stabilometry is a predictor of gait performance in chronic hemiparetic stroke patients. Gait Posture 2009, 30, 5–10. [Google Scholar] [CrossRef]
- Mansfield, A.; Danells, C.J.; Inness, E.; Mochizuki, G.; McIlroy, W.E. Between-limb synchronization for control of standing bal-ance in individuals with stroke. Clin. Biomech. 2011, 26, 312–317. [Google Scholar] [CrossRef]
- Weerdesteyn, V.; de Niet, M.; van Duijnhoven, H.J.R.; Geurts, A.C.H. Falls in individuals with stroke. J. Rehabil. Res. Dev. 2008, 45, 1195–1214. [Google Scholar] [CrossRef]
- Patterson, K.K.; Parafianowicz, I.; Danells, C.J.; Closson, V.; Verrier, M.C.; Staines, W.R.; Black, S.E.; McIlroy, W.E. Gait asymmetry in community-ambulating stroke survivors. Arch. Phys. Med. Rehabil. 2008, 89, 304–310. [Google Scholar] [CrossRef]
- Park, S.H. Assessment of Weight Shift Direction in Chronic Stroke Patients. Osong Public Heal. Res. Perspect. 2018, 9, 118–121. [Google Scholar] [CrossRef]
- Nam, S.H.; Son, S.M.; Kim, K. Changes of gait parameters following constrained-weight shift training in patients with stroke. J. Phys. Ther. Sci. 2017, 29, 673–676. [Google Scholar] [CrossRef]
- Beckwée, D.; Lefeber, N.; Bautmans, I.; Cuypers, L.; De Keersmaecker, E.; De Raedt, S.; Kerckhofs, E.; Nagels, G.; Njemini, R.; Perkisas, S.; et al. Muscle changes after stroke and their impact on recovery: Time for a paradigm shift? Review and commentary. Top. Stroke Rehabil. 2021, 28, 104–111. [Google Scholar] [CrossRef]
- Tanikawa, H.; Mukaino, M.; Itoh, S.; Kondoh, H.; Fujimura, K.; Teranishi, T.; Ohtsuka, K.; Hirano, S.; Kagaya, H.; Saitoh, E.; et al. Development of a simple mechanical measurement method to measure spasticity based on an analysis of a clinical maneuver and its concurrent validity with the modified Ash-worth scale. Bioeng. Biotechnol. 2022, 10, 911249. [Google Scholar] [CrossRef]
- Yasuda, K.; Saichi, K.; Kitaji, Y.; Harashima, H.; Iwata, H.; Yasuda, K.; Saichi, K.; Kitaji, Y.; Harashima, H.; Iwata, H. Development of an implicit method for directing weight shifting to the affected side in patients with stroke: A proof of concept study. Robomech. J. 2017, 4, 26. [Google Scholar] [CrossRef]
- Szopa, A.; Domagalska-Szopa, M.; Lasek-Bal, A.; Żak, A. The link between weight shift asym-metry and gait disturbances in chronic hemiparetic stroke patients. Clin. Interv. Aging 2017, 12, 2055–2062. [Google Scholar] [CrossRef]
- Jung, K.; Kim, Y.; Chung, Y.; Hwang, S. Weight-Shift Training Improves Trunk Control, Propriocep-tion, and Balance in Patients with Chronic Hemiparetic Stroke. Tohoku J. Exp. Med. 2014, 232, 195–199. [Google Scholar] [CrossRef]
- Geurts, A.C.; de Haart, M.; van Nes, I.J.; Duysens, J. A review of standing balance recovery from stroke. Gait Posture 2005, 22, 267–281. [Google Scholar] [CrossRef]
- Bruyneel, A.-V.; Dubé, F. Best Quantitative Tools for Assessing Static and Dynamic Standing Balance after Stroke: A Systematic Review. Physiother. Can. 2020, 73, 329–340. [Google Scholar] [CrossRef]
- Glowinski, S.; Blazejewski, A.; Krzyzynski, T. Inertial Sensors and Wavelets Analysis as a Tool for Pathological Gait Identification. Innov. Biomed. Eng. 2016, 16, 106–114. [Google Scholar]
- Bamberg, S.J.M.; Benbasat, A.Y.; Scarborough, D.M.; Krebs, D.E.; Paradiso, J.A. Gait Analysis Using a Shoe-Integrated Wireless Sensor System. IEEE Trans. Inf. Technol. Biomed. 2008, 12, 413–423. [Google Scholar] [CrossRef]
- WHO. International Classification of Functioning, Disability and Health (ICF); WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Guidelines of the National Council of Physiotherapists for the provision of physiotherapy health services and their de-scription in medical records. In Proceedings of the Resolution No. 142/I Krf Of The National Council Of Physiotherapeu-Tes of 1 March 2018, Warsaw, Poland, 1 March 2018.
- Freire, B.; Valle, M.B.D.; Lanferdini, F.J.; Foschi, C.V.S.; Abou, L.; Pietta-Dias, C. Cut-off score of the modified Ashworth scale corresponding to walking ability and functional mobility in in-dividuals with chronic stroke. Disabil. Rehabil. 2002, 1–5. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, M.; Zhao, J.; Gao, Y.; Wang, Y.; Zhou, J.; Wan, L.; Nie, G.; Wang, Y. Functional Independence and Disability Evaluation in Stroke Patients: Optimal Cutoff Scores for a Pictorial-Based Long-shi Scale, Barthel Index, and Modified Rankin Scale. Front. Neurol. 2022, 13, 710852. [Google Scholar] [CrossRef]
- Chye, A.; Hackett, M.L.; Hankey, G.J.; Lundström, E.; Almeida, O.P.; Gommans, J.; Dennis, M.; Jan, S.; Mead, G.E.; Ford, A.H.; et al. Repeated Measures of Modified Rankin Scale Scores to As-sess Functional Recovery From Stroke: AFFINITY Study Findings. J. Am. Heart Assoc. 2022, 11, e025425. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Zhang, S.; Liu, H.; Yuan, X.; Yang, X.; Gu, P. Value of Barthel, PLAN and NIHSS scores for predicting the death of patients with acute ischemic stroke during their 5-year follow-up. J. Clin. Neurosci. 2021, 90, 94–98. [Google Scholar] [CrossRef]
- Ito, D.; Kawakami, M.; Narita, Y.; Yoshida, T.; Mori, N.; Kondo, K. Cognitive Function is a Predictor of the Daily Step Count in Patients with Subacute Stroke With Independent Walking Ability: A Prospective Cohort Study. Arch. Rehabil. Res. Clin. Transl. 2021, 3, 100132. [Google Scholar] [CrossRef]
- Goliwas, M.; Małecka, J.; Lewandowski, J.; Kamińska, E.; Adamczewska, K.; Kocur, P. Analysis of Dependencies Between Fugl-Meyer and Berg Balance Scale Tests as Evaulation of Increased Muscle Tone in Chronic-Phase Patients After Ischaemic Stroke. Med. Rehabil. 2022, 26, 4–9. [Google Scholar] [CrossRef]
- Hernández, E.D.; Forero, S.M.; Galeano, C.P.; Barbosa, N.E.; Sunnerhagen, K.S.; Murphy, M.A. Intra- and inter-rater reliability of Fugl-Meyer Assessment of Lower Extremity early after stroke. Braz. J. Phys. Ther. 2021, 25, 709–718. [Google Scholar] [CrossRef]
- Ofek, H.; Alperin, M.; Laufer, Y. Lower Extremity Position Test: A new clinical quantitative assess-ment tool of proprioception post stroke. NeuroRehabilitation 2019, 44, 479–484. [Google Scholar] [CrossRef]
- Fiedorová, I.; Mrázková, E.; Zádrapová, M.; Tomášková, H. Receiver Operating Characteristic Curve Analysis of the Somatosensory Organization Test, Berg Balance Scale, and Fall Efficacy Scale-International for Predicting Falls in Discharged Stroke Patients. Int. J. Environ. Res. Public Health 2022, 19, 9181. [Google Scholar] [CrossRef]
- TYROMOTION GmbH. TYMO® therapy plategebrauchsanweisung/manual; TYROMOTION GmbH: Graz, Austria, 2015; pp. 5–6. [Google Scholar]
- Glowinski, S.; Blazejewski, A. SPIDER as A Rehabilitation Tool for Patients with Neurological Disabilities: The Preliminary Research. J. Pers. Med. 2020, 10, 33. [Google Scholar] [CrossRef]
- Afzal, F.; Gulraiz; Qurratulain; Manzoor, S. Role of Spider Cage in Motor Control in Cerebral Palsy. Int. J. Phys. Med. Rehabil. 2017, 5, 4. [Google Scholar] [CrossRef]
- Patent Office of the Republic of Poland. Wiadomości Urzędu Patentowego, No 3 MARCH 1998, Publishing House of Patent Office of the Republic of Poland: Warsaw, Poland, 1998; p. 255, INDEKS 38135. ISSN 0043-5201.
- Mohapatra, S.; Eviota, A.C.; Ringquist, K.L.; Muthukrishnan, S.R.; Aruin, A.S. Compelled Body Weight Shift Technique to Facilitate Rehabilitation of Individuals with Acute Stroke. ISRN Rehabil. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Andersson, P.; Franzén, E. Effects of weight-shift training on walking ability, ambulation, and weight distribution in individuals with chronic stroke: A pilot study. Top. Stroke Rehabil. 2016, 22, 437–443. [Google Scholar] [CrossRef]
- Perry, J.; Garrett, M.; Gronley, J.K.; Mulroy, S.J. Classification of walking handicap in the stroke population. Stroke 1995, 26, 982–989. [Google Scholar] [CrossRef]
- Park, S.H.; Hsu, C.; Lin, J.; Dee, W.; Roth, E.J.; Rymer, W.Z.; Wu, M. Increased motor variability facilitates motor learning in weight shift toward the paretic side during walking in post-stroke individuals. Eur. J. Neurosci. 2021, 53, 3490–3506. [Google Scholar] [CrossRef]
- Liao, W.-C.; Lai, C.-L.; Hsu, P.-S.; Chen, K.-C.; Wang, C.-H. Different weight shift trainings can improve the balance performance of patients with a chronic stroke. A randomized controlled trial. Medicine 2018, 97, 45. [Google Scholar] [CrossRef]
- Park, S.H.; Hsu, C.-J.; Dee, W.; Roth, E.J.; Rymer, W.Z.; Wu, M. Enhanced error facilitates motor learning in weight shift and increases use of the paretic leg during walking at chronic stage after stroke. Exp. Brain Res. 2021, 239, 3327–3341. [Google Scholar] [CrossRef]
- Hendrickson, J.; Patterson, K.K.; Inness, E.L.; McIlroy, W.E.; Mansfield, A. Relationship between asymmetry of quiet standing balance control and walking post-stroke. Gait Posture 2014, 39, 177–181. [Google Scholar] [CrossRef]
- Hung, J.-W.; Chou, C.-X.; Hsieh, Y.-W.; Wu, W.-C.; Yu, M.-Y.; Chen, P.-C.; Chang, H.-F.; Ding, S.-E. A Randomized Comparison Trial of Balance Training by Using Exergaming and Conventional Weight-Shifting Therapy in Patients with Chronic Stroke. Arch. Phys. Med. Rehabil. 2014, 95, 1629–1637. [Google Scholar] [CrossRef]
- Roerdink, M.; Geurts, A.C.H.; De Haart, M.; Beek, P.J. On the relative contribution of the paretic leg to the control of posture after stroke. Neurorehabil. Neural Repair. 2009, 23, 267–274. [Google Scholar] [CrossRef]
- Eng, J.J.; Chu, K.S. Reliability and comparison of weight-bearing ability during standing tasks for individuals with chronic stroke. Arch. Phys. Med. Rehabil. 2002, 83, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- de Haart, M.; Geurts, A.C.; Dault, M.C.; Nienhuis, B.; Duysens, J. Restoration of weight-shifting capacity in patients with postacute stroke: A rehabilitation cohort study. Arch. Phys. Med. Rehabil. 2005, 86, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Hsu, C.-J.; Dee, W.; Roth, E.J.; Rymer, W.Z.; Wu, M. Gradual adaptation to pel-vis perturbation during walking reinforces motor learning of weight shift toward the paretic side in individuals post stroke. Exp. Brain Res. 2021, 239, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.W.; Kim, K.; Lee, N.K.; Kwon, J.W.; Son, S.M. Effect of constrained weight shift on the static balance and muscle activation of stroke patients. J. Phys. Ther. Sci. 2015, 27, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Tsaklis, P.; Grooten, W.J.; Franzén, E. Effects of Weight-Shift Training on Balance Control and Weight Distribution in Chronic Stroke: A Pilot Study. Top Stroke Rehabil. 2012, 19, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Wyszyński, S.; Brzęk, A.; Famuła, A.; Stiler, S.; Jarski, P.; Ziaja, D. Measurement and analysis of the distribution of body mass in patients after stroke. Acta Bio-Opt. Et Inform. Med. Biomed. Eng. 2017, 23, 1. [Google Scholar]
- Aruin, A.S.; Hanke, T.; Chaudhuri, G.; Harvey, R.; Rao, N. Compelled weightbearing in persons with hemiparesis following stroke: The effect of a lift insert and goal-directed balance exercise. J. Rehabilitation Res. Dev. 2000, 37, 65–72. [Google Scholar]




| Inclusion Criteria for the Study and Control Group: |
|
| Exclusion criteria for the study and control group: |
|
| Average/Value | Standard Deviation | Minimum | Maximum | |
|---|---|---|---|---|
| Gender: F (female) | 38 (31.67%) | |||
| M (male) | 82 (68.33%) | |||
| Age | 56.13 | 14.4 | 24 | 84 |
| Hemiparesis side: R (right) | 56 (46.6%) | |||
| L (left) | 64 (53.4%) | |||
| Modified Ashworth Scale: 2 | 112 | |||
| 1+ | 8 | |||
| Barthel Scale | 70 | 7 | 55 | 95 |
| Superficial sensation: N (hyposensitivity) | 115 | |||
| P (hypersensitivity) | 5 | |||
| Deep sensitivity: (+/−) | 50 | |||
| (+) | 64 | |||
| (−) | 6 | |||
| BBS | 25 | 2 | 21 | 32 |
| NIHSS | 7 | 2 | 3 | 11 |
| Rankin Scale | 3 | 0 | 3 | 3 |
| FMA-LE | 17 | 1 | 16 | 23 |
| SG | CG | |||||||
|---|---|---|---|---|---|---|---|---|
| Average/Value | Standard Deviation | Minimum | Maximum | Average/Value | Standard Deviation | Minimum | Maximum | |
| Gender: F | 18 (30%) | 20 (33.33%) | ||||||
| M | 42 (70%) | 40 (66.67%) | ||||||
| Age | 59 | 14.7 | 24 | 80 | 57.5 | 14.3 | 25 | 84 |
| Hemiparesis side: R | 29 (48.33%) | 29 (48.33%) | ||||||
| L | 31 (51.67%) | 31 (51.67%) | ||||||
| Modified Ashworth Scale: 2 | 56 | 56 | ||||||
| 1+ | 4 | 4 | ||||||
| Barthel Scale | 65 | 6.5 | 55 | 85 | 70 | 8.1 | 55 | 95 |
| Superficial sensation: N | 57 | 58 | ||||||
| P | 3 | 2 | ||||||
| Deep sensitivity: (+/−) | 24 | 26 | ||||||
| (+) | 32 | 32 | ||||||
| (−) | 4 | 2 | ||||||
| BBS | 25 | 1.9 | 21 | 30 | 25 | 2.2 | 21 | 32 |
| NIHSS | 7 | 1.4 | 4 | 11 | 7 | 2 | 3 | 11 |
| Rankin Scale | 3 | 0 | 3 | 3 | 3 | 0 | 3 | 3 |
| FMA-LE | 16 | 0.9 | 16 | 20 | 18 | 1.5 | 16 | 23 |
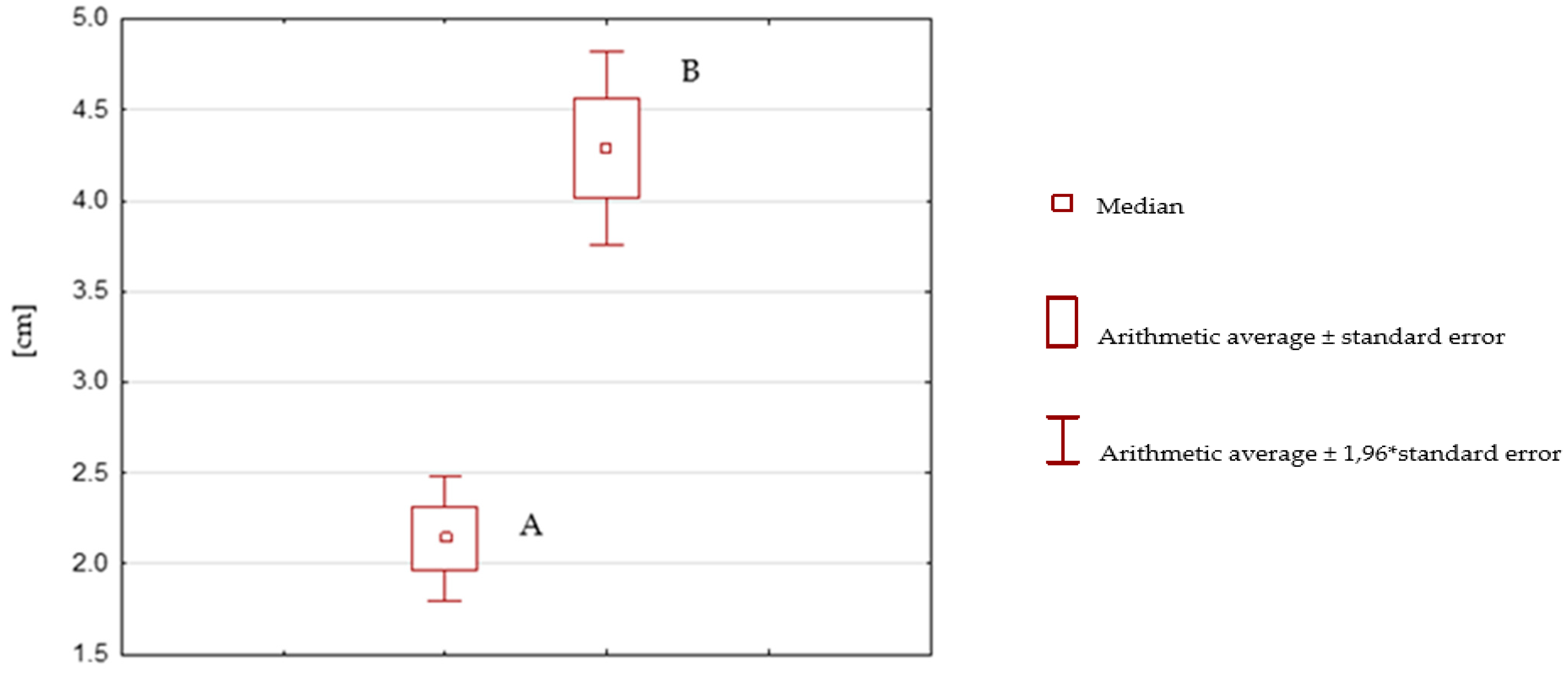
| After Rehabilitation with the SPIDER System | Before Rehabilitation with the SPIDER System | |
|---|---|---|
| Average | 2.14 | 4.29 |
| Variation | 1866169492 | 4426 |
| Population | 60 | 60 |
| Pearson correlation | 0.835873939 | |
| df | 59 | |
| t Stat | −13.65422458 | |
| P(T ≤ t) one-sided | 3.22093 × 10−20 | |
| One-sided t-test | 1.671093033 | |
| P(T ≤ t) bilateral | 6.44186 × 10−20 | |
| Two-sided t-test | 2.000995361 | |
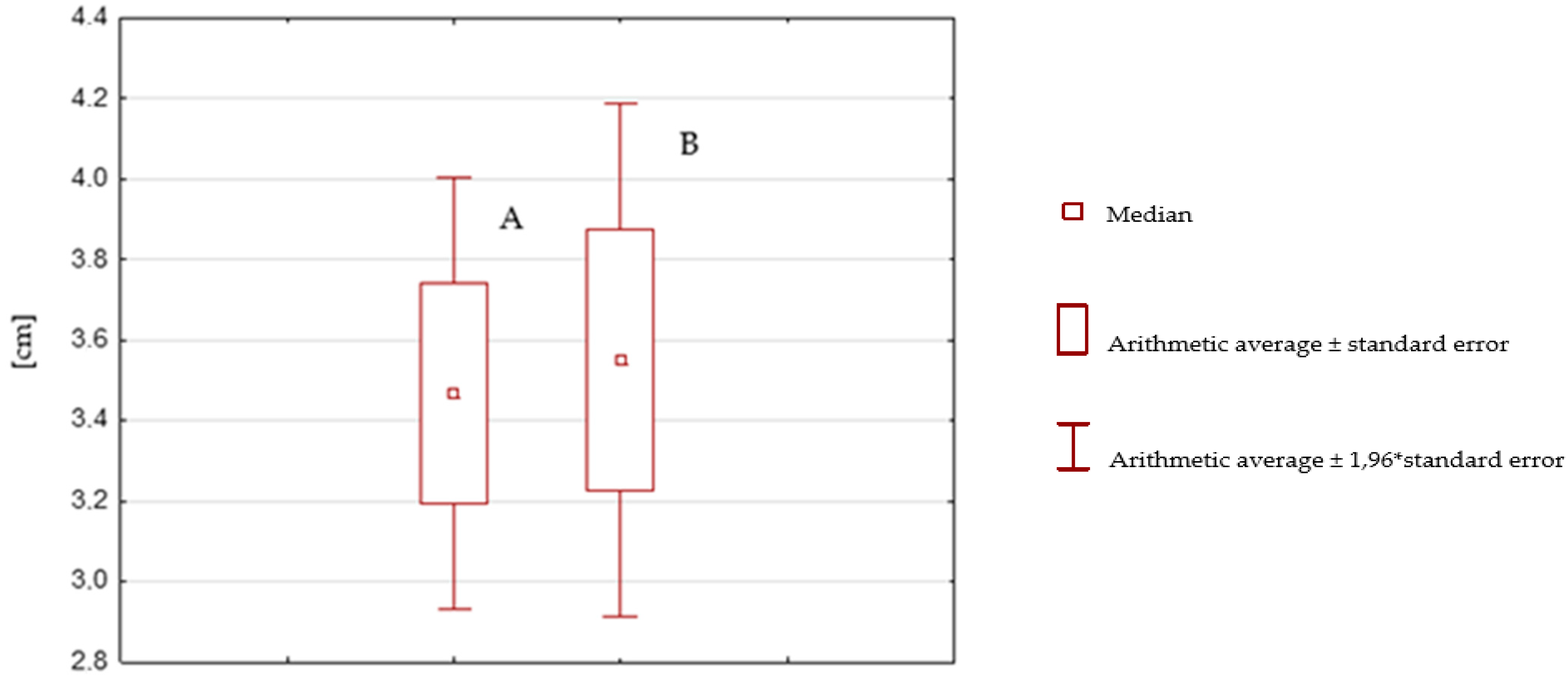
| After Rehabilitation without the SPIDER System | Before Rehabilitation without the SPIDER System | |
|---|---|---|
| Average | 3.468333333 | 3.55 |
| Variation | 4.45169209 | 6.331016949 |
| Population | 60 | 60 |
| Pearson correlation | 0.906659242 | |
| df | 59 | |
| t Stat | −0.58833267 | |
| P(T ≤ t) one-sided | 0.27927728 | |
| One-sided t-test | 1.671093033 | |
| P(T ≤ t) bilateral | 0.55855456 | |
| Two-sided t-test | 2.000995361 | |
| After rehabilitation with the SPIDER system | After rehabilitation without the SPIDER system | |
|---|---|---|
| Average | −2.15 | −0.081666667 |
| Variation | 1.487627119 | 1.15609887 |
| Population | 60 | 60 |
| Total variance | 1.321862994 | |
| df | 118 | |
| t Stat | −9.853441355 | |
| P(T ≤ t) one-sided | 2.23299 × 10−17 | |
| One-sided t-test | 1.657869523 | |
| P(T ≤ t) bilateral | 4.46597 × 10−17 | |
| Two-sided t-test | 1.980272226 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostrowska, P.M.; Studnicki, R.; Rykaczewski, M.; Spychała, D.; Hansdorfer-Korzon, R. Evaluation of the Effect of SPIDER System Therapy on Weight Shifting Symmetry in Chronic Stroke Patients—A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 16214. https://doi.org/10.3390/ijerph192316214
Ostrowska PM, Studnicki R, Rykaczewski M, Spychała D, Hansdorfer-Korzon R. Evaluation of the Effect of SPIDER System Therapy on Weight Shifting Symmetry in Chronic Stroke Patients—A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2022; 19(23):16214. https://doi.org/10.3390/ijerph192316214
Chicago/Turabian StyleOstrowska, Paulina Magdalena, Rafał Studnicki, Marcin Rykaczewski, Dawid Spychała, and Rita Hansdorfer-Korzon. 2022. "Evaluation of the Effect of SPIDER System Therapy on Weight Shifting Symmetry in Chronic Stroke Patients—A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 19, no. 23: 16214. https://doi.org/10.3390/ijerph192316214
APA StyleOstrowska, P. M., Studnicki, R., Rykaczewski, M., Spychała, D., & Hansdorfer-Korzon, R. (2022). Evaluation of the Effect of SPIDER System Therapy on Weight Shifting Symmetry in Chronic Stroke Patients—A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 19(23), 16214. https://doi.org/10.3390/ijerph192316214






