Umbilical Cord Stump Infections in Central Uganda: Incidence, Bacteriological Profile, and Risk Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
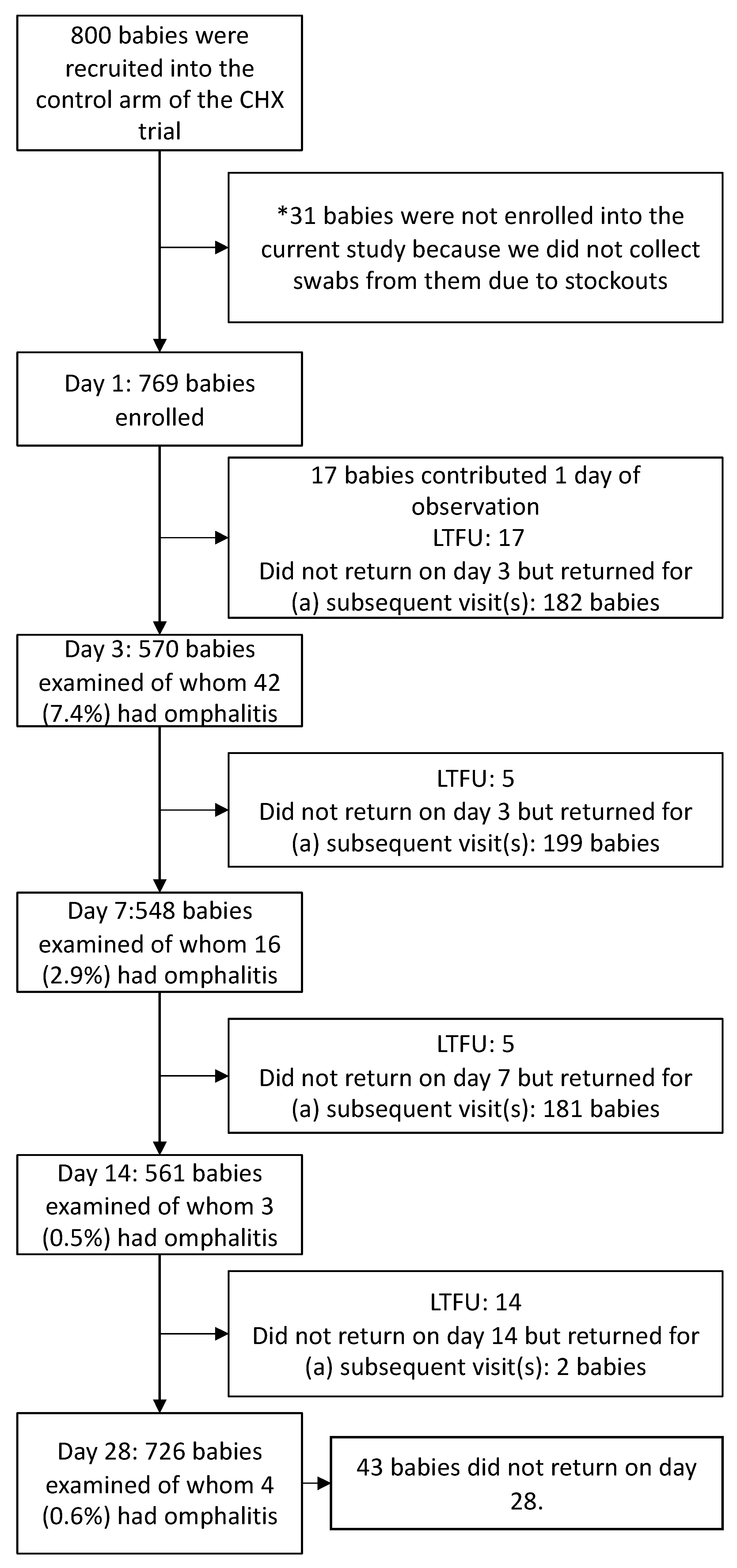
2.2. Recruitment, Enrollment, and Follow-Up
2.3. Data Collection, Management, and Quality Control
2.4. Specimen Collection
2.5. Bacterial Isolation and Identification
2.6. Antimicrobial Susceptibility Testing
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Incidence of Omphalitis
3.3. Bacteriological Profile of Omphalitis
3.4. Antimicrobial Resistance Profiles
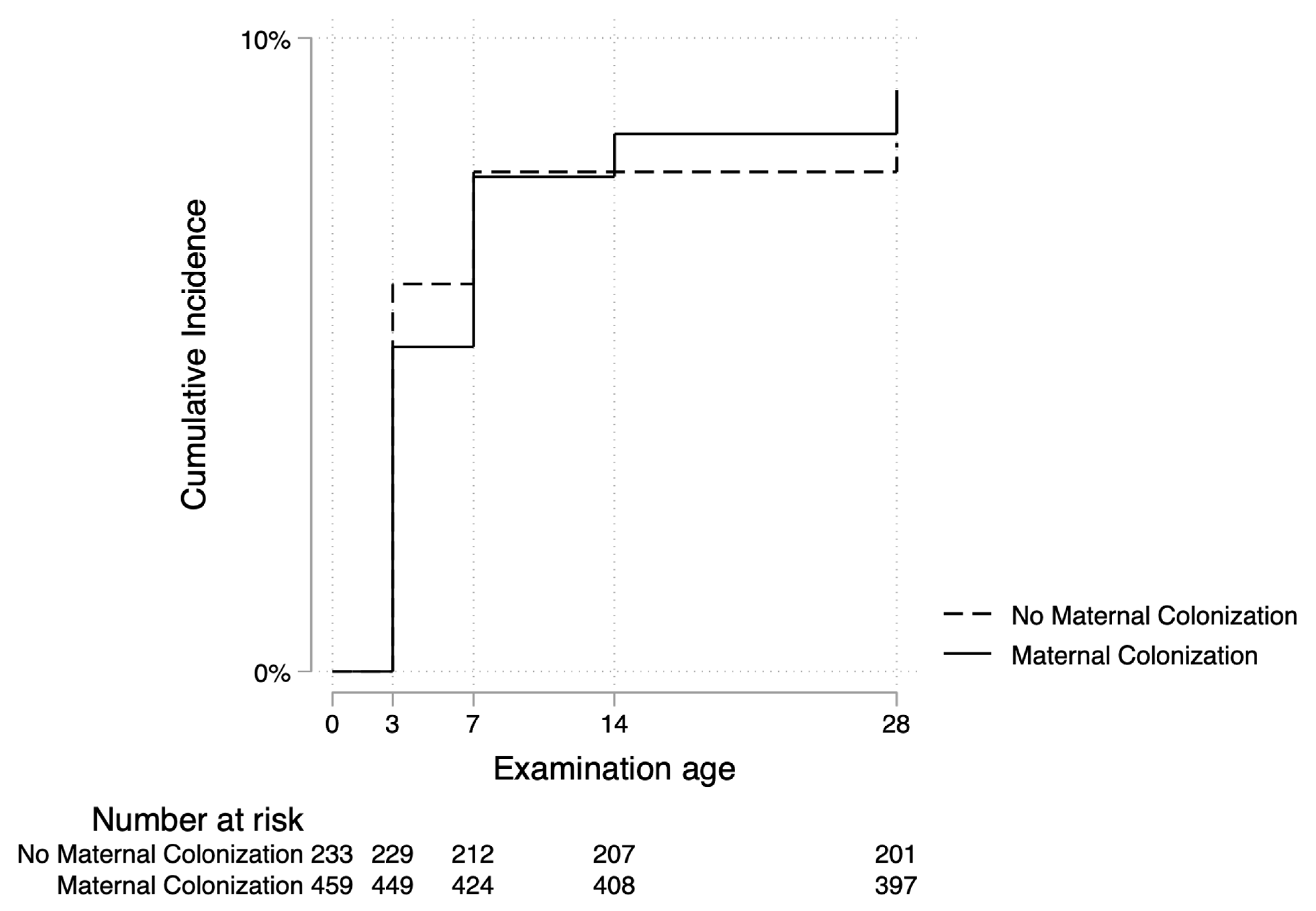
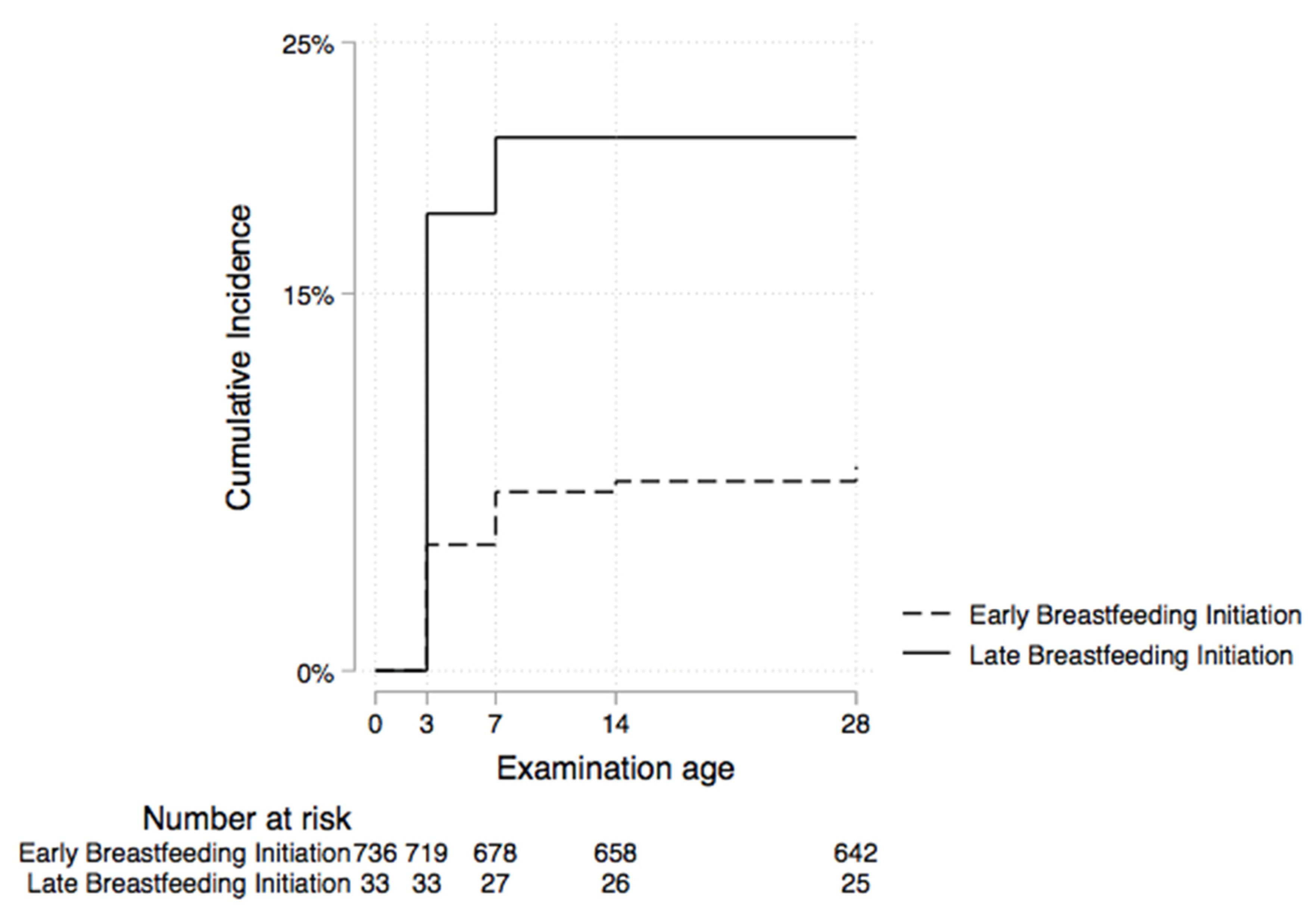
3.5. Factors Associated with Omphalitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Under-5 Mortality Collaborators. Global, regional, and national progress towards Sustainable Development Goal 3.2 for neonatal and child health: All-cause and cause-specific mortality findings from the Global Burden of Disease Study 2019. Lancet 2021, 398, 870–905. [Google Scholar] [CrossRef] [PubMed]
- Uganda Bureau of Statistcs (UBOS) and ICF. Uganda Demographic and Health Survey 2016: Key Indicators Report; UBOS: Kampala, Uganda, 2017. [Google Scholar]
- Okomo, U.; Akpalu, E.N.K.; Le Doare, K.; Roca, A.; Cousens, S.; Jarde, A.; Sharland, M.; Kampmann, B.; Lawn, J.E. Aetiology of invasive bacterial infection and antimicrobial resistance in neonates in sub-Saharan Africa: A systematic review and meta-analysis in line with the STROBE-NI reporting guidelines. Lancet Infect. Dis. 2019, 19, 1219–1234. [Google Scholar] [CrossRef] [PubMed]
- Boos, M.D., Sr. Avery’s Diseases of the Newborn, 10th ed.; UN General Assembly: New York, NY, USA, 2018. [Google Scholar]
- Mullany, L.C.; Darmstadt, G.L.; Khatry, S.K.; Katz, J.; LeClerq, S.C.; Shrestha, S.; Adhikari, R.; Tielsch, J.M. Topical applications of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in southern Nepal: A community-based, cluster-randomised trial. Lancet 2006, 367, 910–918. [Google Scholar] [CrossRef] [PubMed]
- UN General Assembly, New York USA. Transforming Our World: The 2030 Agenda for Sustainable Development. 21 October 2015. A/RES/70/1. Available online: https://www.refworld.org/docid/57b6e3e44.html (accessed on 6 May 2019).
- Celebi Celik, F.; Tuzun, F.; Duman, N.; Keskinoglu, P.; Kumral, A.; Ozkan, H. Current factors affecting the risk of omphalitis in newborns: A prospective case-control study. Int. J. Clin. Pract. 2021, 75, e14071. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; McClure, E.M.; Saleem, S. A Review of Studies with Chlorhexidine Applied Directly to the Umbilical Cord. Am. J. Perinatol. 2012, 30, 699–702. [Google Scholar] [CrossRef]
- Faridi, M.; Rattan, A.; Ahmad, S.H. Omphalitis neonatorum. J. Indian Med. Assoc. 1993, 91, 283–285. [Google Scholar]
- Sazawal, S.; Dhingra, U.; Ali, S.M.; Dutta, A.; Deb, S.; Ame, S.M.; Mkasha, M.H.; Yadav, A.; Black, R.E. Efficacy of chlorhexidine application to umbilical cord on neonatal mortality in Pemba, Tanzania: A community-based randomised controlled trial. Lancet Glob. Health 2016, 4, e837–e844. [Google Scholar] [CrossRef]
- El Arifeen, S.; Mullany, L.C.; Shah, R.; Mannan, I.; Rahman, S.M.; Talukder, M.R.R.; Begum, N.; Al-Kabir, A.; Darmstadt, G.L.; Santosham, M.; et al. The effect of cord cleansing with chlorhexidine on neonatal mortality in rural Bangladesh: A community-based, cluster-randomised trial. Lancet 2012, 379, 1022–1028. [Google Scholar] [CrossRef]
- Semrau, K.E.A.; Herlihy, J.; Grogan, C.; Musokotwane, K.; Yeboah-Antwi, K.; Mbewe, R.; Banda, B.; Mpamba, C.; Hamomba, F.; Pilingana, P.; et al. Effectiveness of 4% chlorhexidine umbilical cord care on neonatal mortality in Southern Province, Zambia (ZamCAT): A cluster-randomised controlled trial. Lancet Glob. Health 2016, 4, e827–e836. [Google Scholar] [CrossRef]
- Soofi, S.; Cousens, S.; Imdad, A.; Bhutto, N.; Ali, N.; Bhutta, Z.A. Topical application of chlorhexidine to neonatal umbilical cords for prevention of omphalitis and neonatal mortality in a rural district of Pakistan: A community-based, cluster-randomised trial. Lancet 2012, 379, 1029–1036. [Google Scholar] [CrossRef]
- Mullany, L.C.; El Arifeen, S.; Winch, P.J.; Shah, R.; Mannan, I.; Rahman, S.M.; Rahman, M.R.; Darmstadt, G.L.; Ahmed, S.; Santosham, M.; et al. Impact of 4.0% chlorhexidine cleansing of the umbilical cord on mortality and omphalitis among newborns of Sylhet, Bangladesh: Design of a community-based cluster randomized trial. BMC Pediatr. 2009, 9, 67. [Google Scholar] [CrossRef]
- Mir, F.; Tikmani, S.S.; Shakoor, S.; Warraich, H.J.; Sultana, S.; Ali, S.A.; Zaidi, A.K.M. Incidence and etiology of omphalitis in Pakistan: A community-based cohort study. J. Infect. Dev. Ctries. 2011, 5, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Mullany, L.C.; Faillace, S.; Tielsch, J.M.; Stoltzfus, R.J.; Nygaard, K.E.; Kavle, J.A.; Farag, T.H.; Haji, H.J.; Khalfan, S.S.; Ali, N.S.; et al. Incidence and Risk Factors for Newborn Umbilical Cord Infections on Pemba Island, Zanzibar, Tanzania. Pediatr. Infect. Dis. J. 2009, 28, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Nankabirwa, V.; Tylleskär, T.; Tumuhamye, J.; Tumwine, J.K.; Ndeezi, G.; Martines, J.C.; Sommerfelt, H. Efficacy of umbilical cord cleansing with a single application of 4% chlorhexidine for the prevention of newborn infections in Uganda: Study protocol for a randomized controlled trial. Trials 2017, 18, 322. [Google Scholar] [CrossRef]
- Tumuhamye, J.; Steinsland, H.; Bwanga, F.; Tumwine, J.K.; Ndeezi, G.; Mukunya, D.; Namugga, O.; Kasede, A.N.; Sommerfelt, H.; Nankabirwa, V. Vaginal colonization with antimicrobial-resistant bacteria among women in labor in central Uganda: Prevalence and associated factors. Antimicrob. Resist. Infect. Control 2021, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Tumuhamye, J.; Steinsland, H.; Tumwine, J.K.; Namugga, O.; Mukunya, D.; Bwanga, F.; Sommerfelt, H.; Nankabirwa, V. Vaginal colonisation of women in labour with potentially pathogenic bacteria: A cross sectional study at three primary health care facilities in Central Uganda. BMC Infect. Dis. 2020, 20, 98. [Google Scholar] [CrossRef]
- Open Data Kit: The Standard for Mobile Data Collection. Available online: https://opendatakit.org (accessed on 16 July 2022).
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests, 10th ed.; Approved Standard; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
- Rothman, K.J. Epidemiology: An Introduction, 2nd ed.; Oxford University Press, Inc.: New York, NY, USA, 2012. [Google Scholar]
- Senaviratna NA, M.R.; Cooray TM, J.A. Diagnosing Multicollinearity of Logistic Regression Model. Asian J. Probab. Stat. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Nangia, S.; Dhingra, U.; Dhingra, P.; Dutta, A.; Menon, V.P.; Black, R.E.; Sazawal, S. Effect of 4 % chlorhexidine on cord colonization among hospital and community births in India: A randomized controlled study. BMC Pediatr. 2016, 16, 121. [Google Scholar] [CrossRef]
- Bin Zaman, S.; Siddique, A.B.; Ruysen, H.; Kc, A.; Peven, K.; Ameen, S.; Thakur, N.; Rahman, Q.S.-U.; Salim, N.; Gurung, R.; et al. Chlorhexidine for facility-based umbilical cord care: EN-BIRTH multi-country validation study. BMC Pregnancy Childbirth 2021, 21, 239. [Google Scholar] [CrossRef]
- Hodgins, S. Chlorhexidine and newborn omphalitis and mortality. Lancet Glob. Health 2017, 5, e270–e271. [Google Scholar] [CrossRef][Green Version]
- Mason, W.H.; Andrews, R.; Ross, L.A.; Wright, H.T., Jr. Omphalitis in the newborn infant. Pediatr. Infect. Dis. J. 1989, 8, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, M.; Banerjee, S.; Banerjee, P.; Guchhait, P. Outstanding Prevalence of Methicillin Resistant Staphylococcus aureus in Neonatal Omphalitis. J. Clin. Diagn. Res. 2016, 10, Dm01–Dm03. [Google Scholar] [CrossRef] [PubMed]
- Sawardekar, K.P. Changing spectrum of neonatal omphalitis. Pediatr. Infect. Dis. J. 2004, 23, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Edmond, K.M.; Zandoh, C.; Quigley, M.A.; Amenga-Etego, S.; Owusu-Agyei, S.; Kirkwood, B.R. Delayed Breastfeeding Initiation Increases Risk of Neonatal Mortality. Pediatrics 2006, 117, e380–e386. [Google Scholar] [CrossRef] [PubMed]
- NEOVITA Study Group. Timing of initiation, patterns of breastfeeding, and infant survival: Prospective analysis of pooled data from three randomised trials. The Lancet Global health. 2016, 4, e266–e275. [Google Scholar]
- Westreich, D.; Greenland, S. The Table 2 Fallacy: Presenting and Interpreting Confounder and Modifier Coefficients. Am. J. Epidemiol. 2013, 177, 292–298. [Google Scholar] [CrossRef]
| Mothers Characteristics | No Omphalitis N = 704 (%) | Omphalitis N = 65 (%) |
|---|---|---|
| Age | ||
| 19 years or less | 94 (13.4) | 11 (16.9) |
| 20–24 years | 281 (39.9) | 21 (32.3) |
| ≥25 years | 329 (46.7) | 33 (50.8) |
| Level of education | ||
| Primary | 228 (32.4) | 24 (36.9) |
| Secondary and Tertiary | 476 (67.6) | 41 (63.1) |
| Socioeconomic status | ||
| Quintile 1 | 224 (31.8) | 17 (26.2) |
| Quintile 2 | 80 (11.4) | 9 (13.9) |
| Quintile 3 | 124 (17.6) | 9 (13.9) |
| Quintile 4 | 136 (19.3) | 15 (23.1) |
| Quintile 5 | 140 (19.9) | 15 (23.1) |
| Mode of delivery | ||
| Spontaneous vaginal delivery | 637 (90.5) | 59 (90.8) |
| Assisted vaginal delivery | 64 (9.1) | 6 (9.2) |
| Caesarian section | 3 (0.4) | 0 (0) |
| Gravidity | ||
| One pregnancy | 212 (30.1) | 18 (27.7) |
| Two or more pregnancies | 492 (69.9) | 47 (72.3) |
| Vaginal colonization | ||
| No | 214 (33.9) | 19 (31.7) |
| Yes | 418 (66.1) | 41 (68.3) |
| * Missing data | 72 (10.2) | 5 (7.7) |
| Health facility | ||
| Kawaala HC III | 265 (37.6) | 10 (15.4) |
| Kitebi HC III | 248 (35.3) | 28 (43.1) |
| Mukono general hospital | 191 (27.1) | 27 (41.5) |
| Initiation of breastfeeding | ||
| Early (within 1 h of birth) | 678 (96.3) | 58 (89.2) |
| Late | 26 (3.7) | 7 (10.8) |
| First breast milk | ||
| Gave it to baby | 699 (99.3) | 63 (96.9) |
| Threw it away | 5 (0.7) | 2 (3.1) |
| Birth weight | ||
| Low | 25 (3.5) | 2 (3.3) |
| Normal | 679 (96.5) | 63 (96.9) |
| Home remedy substance applied on the cord | ||
| No | 610 (86.7) | 53 (81.5) |
| Yes | 94 (13.3) | 12 (18.5) |
| Sex of baby | ||
| Male | 375 (53.3) | 30 (46.2) |
| Female | 329 (46.7) | 35 (53.8) |
| Number (%) of the 65 Neonates Whose Umbilical Cord Stumps Were Colonized with Potentially Pathogenic Bacteria Resistant to One or More Antiobiotics | ||||
|---|---|---|---|---|
| Antibiotic | Escherichia coli (n = 18) | Klebsiella pneumoniae (n = 10) | Citrobacter species (n = 5) | Enterobacter species (n = 4) |
| Ampicillin | 14 (21.5) | 10 (15.4) | 5 (7.7) | 4 (6.2) |
| Ampicillin-Clavulanic acid | 13 (20.0) | 8 (12.3) | 4 (6.2) | 3 (4.6) |
| Trimethoprim-Sulfamethoxazole | 11 (16.9) | 7 (10.8) | 4 (6.2) | 1 (1.5) |
| Ciprofloxacin | 2 (3.1) | 3 (4.6) | 1 (1.5) | 1 (1.5) |
| Chloramphenicol | 2 (3.1) | 3 (4.6) | 2 (3.1) | 0 |
| Gentamicin | 1 (1.5) | 3 (4.6) | 0 | 0 |
| Amikacin | 2 (3.1) | 6 (9.2) | 0 | 1 (1.5) |
| Ceftriaxone | 3 (4.6) | 2 (3.1) | 0 | 0 |
| Cefuroxime | 5 (7.7) | 2 (3.1) | 1 (1.5) | 0 |
| Ceftazidime | 1 (1.5) | 2 (3.1) | 0 | 0 |
| Imipenem | 0 | 2 (3.1) | 0 | 0 |
| Mothers Characteristics | Unadjusted HR 95% CI | Adjusted HR 95% CI N = 692 |
|---|---|---|
| Vaginal colonization | ||
| No | 1 | 1 |
| Yes | 1.1 (0.63, 1.9) | 1.1 (0.63, 1.9) |
| Age | ||
| 19 years or less | 1.5 (0.73, 3.1) | 1.4 (0.63, 3.0) |
| 20–24 years | 1 | 1 |
| 25 years and above | 1.3 (0.76, 2.3) | 1.1 (0.63, 2.0) |
| Level of education | ||
| Primary | 1 | 1 |
| Secondary and Tertiary | 0.83 (0.50, 1.4) | 0.83 (0.49, 1.4) |
| Socioeconomic status | ||
| Quintile 1 | 1 | 1 |
| Quintile 2 | 1.4 (0.64, 3.2) | 1.3 (0.56, 3.1) |
| Quintile 3 | 0.96 (0.43, 2.1) | 0.91 (0.39, 2.2) |
| Quintile 4 | 1.4 (0.71, 2.8) | 1.4 (0.66, 2.8) |
| Quintile 5 | 1.4 (0.69, 2.8) | 1.4 (0.70, 3.0) |
| Birth weight | ||
| Normal | 1 | 1 |
| Low | 0.89 (0.22, 3.7) | 1.1 (0.26, 4.4) |
| Initiation of breastfeeding | ||
| Early | 1 | 1 |
| Late | 2.8 (1.3, 6.2) | 3.1 (1.3, 7.3) |
| Child Sex | ||
| Male | 1 | 1 |
| Female | 1.3 (0.80, 2.1) | 1.4 (0.85, 2.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumuhamye, J.; Sommerfelt, H.; Tumwine, J.K.; Mukunya, D.; Ndeezi, G.; Namugga, O.; Bwanga, F.; Steinsland, H.; Nankabirwa, V. Umbilical Cord Stump Infections in Central Uganda: Incidence, Bacteriological Profile, and Risk Factors. Int. J. Environ. Res. Public Health 2022, 19, 16055. https://doi.org/10.3390/ijerph192316055
Tumuhamye J, Sommerfelt H, Tumwine JK, Mukunya D, Ndeezi G, Namugga O, Bwanga F, Steinsland H, Nankabirwa V. Umbilical Cord Stump Infections in Central Uganda: Incidence, Bacteriological Profile, and Risk Factors. International Journal of Environmental Research and Public Health. 2022; 19(23):16055. https://doi.org/10.3390/ijerph192316055
Chicago/Turabian StyleTumuhamye, Josephine, Halvor Sommerfelt, James K. Tumwine, David Mukunya, Grace Ndeezi, Olive Namugga, Freddie Bwanga, Hans Steinsland, and Victoria Nankabirwa. 2022. "Umbilical Cord Stump Infections in Central Uganda: Incidence, Bacteriological Profile, and Risk Factors" International Journal of Environmental Research and Public Health 19, no. 23: 16055. https://doi.org/10.3390/ijerph192316055
APA StyleTumuhamye, J., Sommerfelt, H., Tumwine, J. K., Mukunya, D., Ndeezi, G., Namugga, O., Bwanga, F., Steinsland, H., & Nankabirwa, V. (2022). Umbilical Cord Stump Infections in Central Uganda: Incidence, Bacteriological Profile, and Risk Factors. International Journal of Environmental Research and Public Health, 19(23), 16055. https://doi.org/10.3390/ijerph192316055






