Abstract
Frailty is a complex interplay between several factors, including physiological changes in ageing, multimorbidities, malnutrition, living environment, genetics, and lifestyle. Early screening for frailty risk factors in community-dwelling older people allows for preventive interventions on the clinical and social determinants of frailty, which allows adverse events to be avoided. By conducting a narrative review of the literature employing the International Narrative Systematic Assessment tool, the authors aimed to develop an updated framework for the main measurement tools to assess frailty risks in older adults, paying attention to use in the community and primary care settings. This search focused on the biopsychosocial domains of frailty that are covered in the SUNFRAIL tool. The study selected 178 reviews (polypharmacy: 20; nutrition: 13; physical activity: 74; medical visits: 0; falls: 39; cognitive decline: 12; loneliness: 15; social support: 5; economic constraints: 0) published between January 2010 and December 2021. Within the selected reviews, 123 assessment tools were identified (polypharmacy: 15; nutrition: 15; physical activity: 25; medical visits: 0; falls: 26; cognitive decline: 18; loneliness: 9; social support: 15; economic constraints: 0). The narrative review allowed us to evaluate assessment tools of frailty domains to be adopted for multidimensional health promotion and prevention interventions in community and primary care.
1. Introduction
The demographic changes taking place in Europe and worldwide will have profound implications in the planning and delivery of social and health services, due to the concomitant increase in the complexity of the health needs of the older adult population [1]. The COVID-19 pandemic has been imposing health challenges of unknown dimensions, both for the increased risk of an adverse outcome in the case of infection in older adults with multimorbidity [2] and for the deterioration in their quality of life as a result of loneliness and impaired access to health and social services. Mortality from COVID-19 has been increasing especially among vulnerable subgroups of populations [3], meaning that frail older adults are exposed to an increased risk of adverse outcomes. Although it is not an inevitable condition of aging, frailty is characterized particularly in those over 65 years old by a dynamic state of vulnerability with weakness and reduction in the physiological reserve which entails an increased risk of lower quality of life, falling, institutionalization, disability, and death [4]. The prevalence of frailty is age related [5] and is therefore linked to the demographic development patterns of the population, with different implications in terms of health needs that must be managed differently depending on the time of onset and the socioeconomic context of individuals. The older adult at risk of frailty presents losses in one or more domains of human functioning (physical, psychological, social) caused by the influence of a number of variables and which increase the risk of adverse outcomes [6]. A proactive and integrated approach to frailty prevention is therefore essential, strengthening the interaction between all professionals involved in health and the social system, and also engaging informal caregivers.
The involvement of multiple stakeholders of the European Innovation Partnership on Active and Healthy Aging (EIP on AHA) in the A3 Action Group [7] allowed researchers to identify and implement innovative approaches to the prevention and management of frailty, such as the one proposed by the SUNFRAIL European project (Grant n. 664291), integrating the biomedical paradigm of frailty [8] with the “biopsychosocial” paradigm [9]. This allowed researchers to take into account all influences of the individual health trajectory towards frailty, namely environmental, physical, educational, socioeconomic, and psychological factors. The SUNFRAIL project produced, as a result, a tool for the early identification of older adults at risk of frailty in different settings [10], and aims to develop, validate, and test an innovative model of intervention to improve the prevention and treatment of frailty and the management of multimorbidity [11,12,13]. The screening tool, which consists of only nine items, can be used by General Practitioners or other health service professionals and community actors, such as Community Nurses. In the case of risk, SUNFRAIL items can be connected to other in-depth tools for the assessment of specific dimensions (Figure 1). The SUNFRAIL tool aims to identify frailty risk factors in the older adult population earlier to generate alerts orienting subsequent diagnostic assessments for health promotion, disease prevention, and other targeted interventions. SUNFRAIL allows health professionals to carry out a very early screening based on the first contact of the older adult with primary care services, communities, and specialists to guide him/her to provide subsequent insights concerning the identified frailty domains (physical, neuropsychological, social). The SUNFRAIL questions were selected from tools used internationally: specifically, the Edmonton Frailty Scale [14], the Tilburg Frailty Indicator [15], and the Gerontopole Frailty Screening Tool [16].
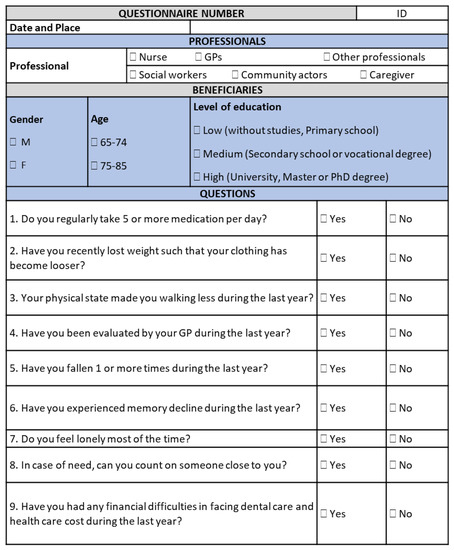
Figure 1.
The SUNFRAIL screening tool.
The early diagnosis of frailty and the identification of its risk factors can enable health professionals to prevent or delay the occurrence of the adverse outcomes through targeted and timely interventions [11]. The SUNFRAIL is the first gateway between patients/beneficiaries and formal/informal services. It supports professionals to identify risk factors of frailty in apparently robust individuals. The objective of this narrative review is to identify assessment tools, to be coherently connected to each of the SUNFRAIL tool items, and further evaluate the corresponding dimensions, in case of risk in one or more biopsychosocial frailty domains. Such a more in-depth assessment is intended to develop targeted intervention strategies for frailty prevention dedicated to community-dwelling older persons, acting on the dimension identified as being at risk or compromised.
Indeed, the identification of further scales for each of the SUNFRAIL domain brings the potential of providing a dataset on the over 65 population, which is useful for the implementation of personalized prevention and health promotion interventions for the independent and healthy living of community-dwelling older adults, based on an in-depth risk factors assessment. The next step is to link specific domains with further clinical and diagnostic evaluations to allow for the coherent integration of innovative approaches and health interventions of care and cure addressing the compromised domains [17].
2. Materials and Methods
This review uses the International Narrative Systematic Assessment (INSA) [18] tool for narrative reviews as a method. This narrative review uses the following Population Implementation Comparator Outcome Study (PICOS) approach:
- Population: older people, aged 65 years or older;
- Implementation/indicator: frailty domains tool(s) (polypharmacy, weight loss, physical activity, medical visits, falls, memory loss, loneliness, support network, economic constraints);
- Comparator: n/a;
- Outcome: multidimensional frailty screening or frailty prevention in community-dwelling older adults;
- Study: review, systematic review, and meta-analysis.
The review selection was performed using the Embase and PubMed databases. A side search was performed to identify other relevant articles, especially in those domains where the search did not yield satisfactory results.
The narrative review focused on the 9 domains of frailty that are covered in the SUNFRAIL screening tool. The search strings considered are included in Table 1.

Table 1.
Search strings based on the SUNFRAIL’s frailty domain.
The search was restricted to reviews published between January 2010 and December 2021 and was limited to the English language.
The inclusion criteria of this narrative review were frailty domains measurement in older people, aged 65 years or over.
The following papers were excluded: articles focused on the definition and models of frailty; observational, cross-sectional, or randomized controlled trials; and papers focused on a specific topic or illness or adverse outcome.
The review articles on the domains of frailty that did not include a comparison of the assessment tools used in the literature were excluded on the basis of the full text.
In step 2, an independent reviewer listed the available assessment tools for each item on the y-axis and compared the tools according to the needs of health systems for IT-supported frailty screening intervention in primary and community care on the x-axis. Each tool was evaluated according to the match against the following needs:
- used on older adults living at home;
- validation through a peer-reviewed study;
- number of items, as completeness of questions and time required for administration;
- specificity as the ability of the instrument to clearly distinguish healthy subjects from those in which the specific domain is impaired. This was assessed by the reviewer by analyzing the nature of the questions (qualitative/quantitative) and the presence or absence of a final score.;
- ease of use as the need for specific preparation in order to administer the instrument;
- usability by different professionals (doctors, nurses, social workers, etc.).
The reviewer scored each match against the need through a three-point Likert scale (1 being “barely addresses the need”; 2 being “partially addresses the need”; 3 being “fully addresses the need”).
3. Results
3.1. Polypharmacy
The process of the review paper selection is summarized in the flowchart of Figure 2.
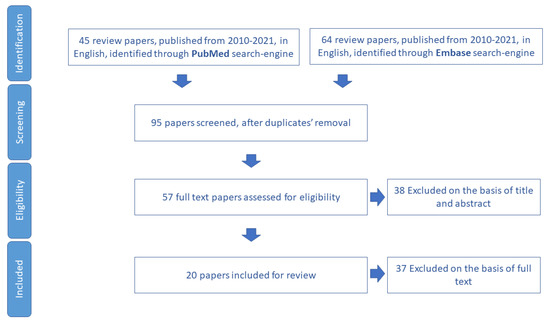
Figure 2.
Process of study selection for polypharmacy domain.
In total, we extracted 15 tools for the assessment of prescription appropriateness and adherence in the older adult population (Table S1).
The tools we most commonly found were the screening tool of older people’s prescriptions and the screening tool to alert the right treatment (STOPP/START) [19] (n = 17 review papers) followed by the Beers Criteria [20] (n = 12), Medication Appropriateness Index (MAI) [21] (n = 4), and Fit fOR The Aged list (FORTA) [22] (n = 4). These tools, as well as STOPPFrail [23], the Norwegian General Practice criteria (NORGEP) [24], the (EU)(7)-PIM list [25], the PRISCUS list [26], the Systematic Tool to Reduce Inappropriate Prescribing (STRIP) [27], Good Palliative–Geriatric Practice (GP-GP) [28], the Individualized Medication Assessment and Planning program (IMAP) [29], and the Zhan Criteria [30] are the main criteria used to review drug therapy in light of identified iterations. They are used by professionals, mainly physicians and pharmacists, to identify potential drug interactions, which may have a negative impact on the health of the patient. Other tools, such as the Drug Regimen Unassisted Grading Scale (DRUGS) [31], Medication Management Ability Assessment (MMAA) [32], and Self-Efficacy for Appropriate Medication Use Scale (SEAMS) [33] are used to assess the older adult’s ability to adhere to prescriptions and to independently take care of themselves. Table 2 shows the prescription appropriateness and adherence assessment tools scores.

Table 2.
Prescription appropriateness and adherence assessment tools scores.
3.2. Weight Loss
We included for review 13 papers (Figure 3) and we extracted 15 tools aimed to assess malnutrition in older adults (Table S2).
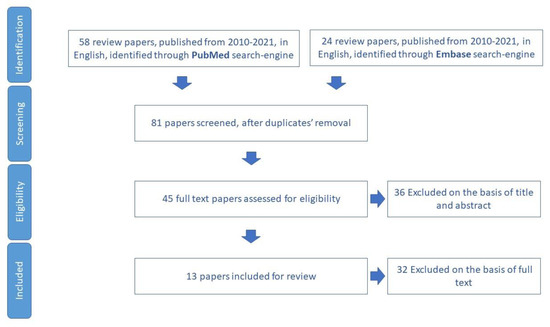
Figure 3.
Process of study selection for nutrition domain.
The best-rated nutrition screening tools are the PREDIMED [34] and the 10-point Mediterranean diet scale [35]. They are easy to use and measure adherence to the Mediterranean diet. The Mini Nutritional Assessment (MNA) [36], including the Mini Nutrition Assessment Short Form (MNA-SF) [37], is a more reliable tool for nutritional screening, but needs assessments that cannot be performed by nonmedical professionals. The Nutritional Form for the Elderly (NUFFE) [38] is a self-assessment questionnaire developed for the hospital setting, but it takes into account all aspects considered important for nutritional assessment. The Malnutrition Universal Screening Tool (MUST) [39] is a very thorough and complex tool to submit. The Canadian Nutrition Screening Tool (CNST) [40], the Chapman Nutritional screening [41], and the Malnutrition Screening Tool (MST) [42] measure the nutritional status of the patient in the hospital setting. The DETERMINE Checklist [43] is not specific. The Seniors in the Community Risk Evaluation for Eating and Nutrition (SCREEN I) or (SCREEN II) questionnaire [44] places more emphasis on clinical judgement.
The Council on Nutrition Appetite Questionnaire (CNAQ) [42] and the Simplified Nutritional Appetite Questionnaire (SNAQ) [45] are not specific, as they measure appetite level. The SNAQ65+ [46] combines self-rated questions and anthropometric measurements. The SCALES (Sadness, Cholesterol, Albumin, Loss of weight, Eating, Shopping) questionnaire [47] measures weight loss associated with other risk factors and requires specific clinical expertise. Table 3 shows malnutrition assessment tools scores.

Table 3.
Malnutrition assessment tools scores.
3.3. Physical Activity
The study selected 74 papers for review (Figure 4). In total, 25 tools aimed to assess physical activity in older adults were identified (Table S3).
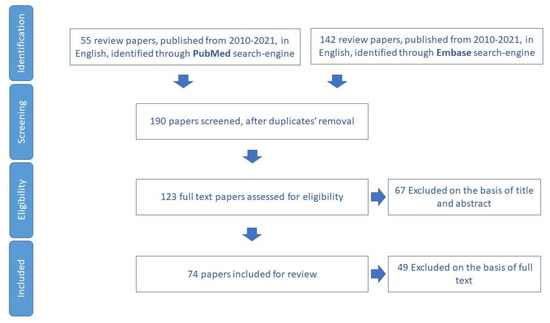
Figure 4.
Process of study selection for physical activity domain.
The tool that was found most often was the Short Physical Performance Battery (SPPB) (n = 40) [49]. It assesses the older adult’s ability to walk, get up from the chair, and remain balanced. Other instruments such as the five-chair stand test [50], Timed Up and Go Test [51] (n = 30), Four Square Step test [52], and Alternate Step Test (ATS) [53] measure several aspects of physical function individually. The SPPB is also used to assess fall risk. The 6-Minute Walking Test (6MWT) (n = 33) [54] and 5-Meter Walking Test (5MWT) [55] instruments are easy to use in both hospital and community settings to assess the older adult’s ability to walk. The Incremental Shuttle Walk Test (ISWT) [56] aims to simulate a cardiopulmonary exercise test using a field walking test. There have been several self-report questionnaires found to quantify the amount of daily physical activity of older adults. The widely used measures are the Physical Activity Scale for the Elderly (PASE) (n = 8) [57], Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire [58], Zutphen Physical Activity Questionnaire [59], Physical Activity and Sedentary Behavior Questionnaire (PASB-Q) [60], EPIC Physical Activity Questionnaire (EPAQ2) [61], International Physical Activity Questionnaire (IPAQ) [62], General Practice Physical Activity Questionnaire (GPPAQ) [63], and Longitudinal Aging Study Amsterdam Physical Activity Questionnaire (LAPAQ) [64], which aim to assess the duration, frequency, exertion level, and amount of physical activity undertaken over a seven-day period by individuals 65 years and older. The Stanford Brief Activity Survey [65], Women’s Health Initiative physical activity questionnaire (WHI-PAQ) [66], Physical Activity Scale for Individuals with Physical Disabilities (PASIPD) [67], and Duke Activity Status Index [68] assess recreational activities or exercises (mild, moderate, and strenuous) as well as household and yard activities.
The SF-36 (physical component scale) [69] measures self-perceived physical function and role limitations, including physical, bodily pain. The WOMAC physical function subscale [70] is a widely employed patient-reported measurement instrument for difficulty in physical function due to pain and discomfort in knee osteoarthritis patients. The Tinetti Performance-Oriented Mobility Assessment (POMA) [71], Mini Motor Test (MMT) [72], and Elderly Mobility Scale [73] evaluate physical performance by means of gait, balance, and stand-up tasks. Table 4 shows physical activity assessment tool scores.

Table 4.
Physical activity assessment tools scores.
3.4. Adherence to Medical Visits
The scientific production on the topic of adherence to medical visits is not extensive. The search only concerned reviews and meta-analyses on this specific topic, so it did not produce any results (Figure 5).
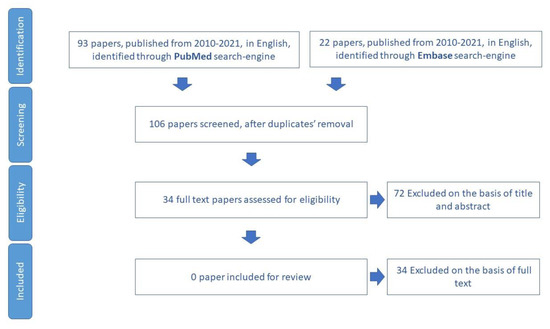
Figure 5.
Process of study selection for assessment of adherence to medical visits.
However, the analysis of the eligible papers highlighted several studies that have predominantly used checklists or single oral questions. Among the excluded papers, there is a study in which an eight-item tool was used to collect information on older adults’ access to primary care, continuity, and connectedness, which were considered to be enabling factors for the use of the emergency room [74].
3.5. Falls
The narrative review for fall items included 39 papers for review (Figure 6), through which 26 tools aimed to assess fall, and the risk of falling in older adults was identified (Table S4).
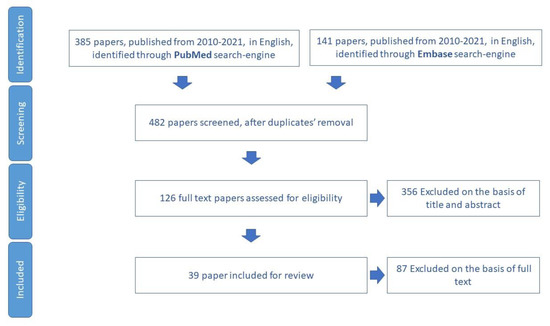
Figure 6.
Process of study selection for fall risk assessment.
The Falls Efficacy Scale (FES) [75] and its revised versions, the Modified Falls Efficacy Scale (MFES) [76] and the Short Falls Efficacy Scale—International (FES-I) [77] are the most common instruments for assessing confidence in older adults’ ability to perform Activities of Daily Living (ADL) without falling.
The Survey of Activities and Fear of Falling in the Elderly (SAFE) [78], Mobility Efficacy Scale (MES) [79], and University of Illinois at Chicago Fear of Falling Measure (UIC FFM) [80] measure the self-reported fear of falling. The Falls Risk Assessment Questionnaire (FRAQ) [81] aims to assess awareness and perception of falls risk.
The Fall Risk Index (FRI-21) [82] has been used to detect older adults’ decline in basic activities of daily living (BADL). The Fracture Risk Assessment Tool (FRAX) [83] estimates the risk of having a hip or other major fracture.
Tinetti POMA [71], Berg Balance Scale (BBS) [84], and Fullerton Advanced Balance (FAB) Scale [85] assess static and dynamic balance and fall risk in adults.
The Activities-specific Balance Confidence (ABS) scale [86] is a questionnaire developed to assess older individual’s self-perceived balance confidence in performing daily activities.
The Fall risk assessment tool (FRAT) [87], Morse Fall Scale [88], St. Thomas Risk Assessment Tool in Falling Elderly Inpatients (STRATIFY) [89], Conley Scale [90], Johns Hopkins Fall Risk Assessment Tool [91], Hendrich II Fall Risk Model tool [92], Austin Health Falls Risk Screening Tool (AHFRST) [93], and Falls Risk For Hospitalized Older People (FRHOP) [94] identify the risk factors of falls in hospitalized patients.
Falls Risk for Older People in the Community (FROP-Com) [95] and the Downton Fall Risk Index (DFRI) [96] aim to predict postdischarge injuries, identifying people who require further assessment and management. The Elderly Fall Screening Test (EFST) [97] aims to stratify the community-dwelling older adults population into a low and high risk of fall based on the fall history and observations of walking speed and gait style. The Home-Screen Scale [98] and Safety House Checklist [99] aim to identify environmental safety hazards in older adults’ homes. Table 5 shows the fall assessment tools scores.

Table 5.
Falls assessment tools scores.
3.6. Memory Loss
Regarding memory loss items, the study selected 12 papers for review (Figure 7). In total, 18 tools that aim to assess cognitive function in older adults were identified (Table S5).
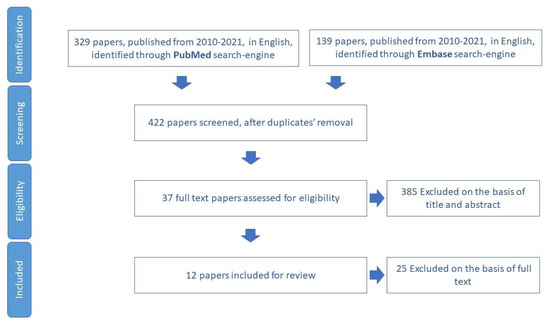
Figure 7.
Process of study selection for the assessment of cognitive decline.
Our research showed that the most used tool is the Mini Mental State Examination (MMSE) [100] and its modified version (3MS) [101], which are questionnaires that healthcare professionals commonly use to check for problems with thinking, communication, understanding, and memory. The Montreal Cognitive Assessment (MoCA) [102] was designed as a tool for the rapid screening of mild cognitive impairment. The Cognitive Abilities Screening Instrument (CASI) [103], with a 0 to 100 score range, assesses several cognitive domains: attention and concentration, executive functions, memory, language, visual constructive skills, abstraction, calculation, and orientation. The short portable mental status questionnaire (SPMSQ or Pfeiffer) [104] aims to define the presence and intensity (mild, moderate, and severe) of cognitive disturbances of organic origin in elderly patients. The Abbreviated Mental Test (AMT) [105] is a quick-to-use screening test in general hospital usage. The 6-item cognitive impairment test (6-CIT) [106] is a useful dementia screening tool in primary care. The Clifton Assessment Procedures for the Elderly (CAPE) [107] evaluates the presence and severity of impairment in mental and behavioral functioning in long-term psychiatric patients. The Clinical Dementia Rating (CDR) [108] is used to quantify the severity of dementia using a semistructured interview. The Controlled Oral Word Association Test (COWAT) [109] and Isaacs Set Test (IST) [110] are neuropsychological measures of verbal fluency. The Trail Making Test A & B (TMT) [111] assesses spatial planning ability in a visual–motor task. The Rey Auditory Verbal Learning Test (RAVLT) [112] measures the immediate memory span and provides an assessment of learning. The clock-drawing test (CDT) [113] is a quick screening test for cognitive dysfunction, but it lacks sensitivity for the diagnosis of early or mild dementia. The Brief Cognitive Screening Battery (BCSB) [114] includes the MMSE, Verbal Fluency, clock-drawing, and Figure Memory Tests. The Mini-Cog [115] consists of a three-item recall test for memory and a simply scored clock-drawing test. The Telephone Interview for Cognitive Status (TICS) [116] is designed to be administered over the telephone. The Community Screening Interview for Dementia (CSID) [117] consists of a cognitive test and an interview on performance in everyday living. Table 6 shows the cognitive decline assessment tool scores.

Table 6.
Cognitive decline assessment tools scores.
3.7. Loneliness
We included for review 15 papers (Figure 8) and we extracted nine tools aimed to assess loneliness in older adults (Table S6).
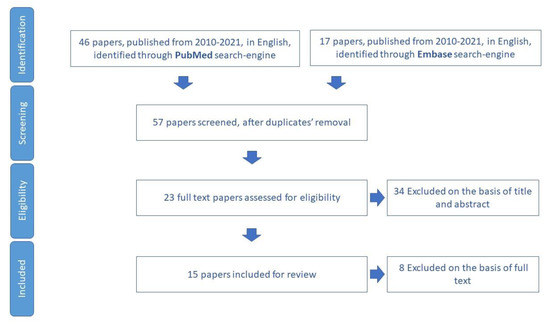
Figure 8.
Process of study selection for the assessment of loneliness.
The UCLA Loneliness Scale [118] and the De Jong Gierveld scale [119] are the most common tools to measure subjective feelings of loneliness as well as feelings of social isolation in older adults. The Three-Item Loneliness Scale [120] is an interviewer-administered questionnaire developed from the Revised UCLA Loneliness Scale. The Questionnaire to define Social Frailty Status (QSFS) [121] aims to evaluate the state of social frailty through simple questions on daily social activity, social role, and social relationships. The Social Frailty Index (SFI) [122] assesses loneliness through self-reported survey questionnaires on topics such as living alone, having no education, the absence of a confident, infrequent contact, infrequent social activities, financial difficulty, and socioeconomic depravation. The Steptoe Social Isolation Index [123] measures family status, monthly contacts with children, family and friends, and participation in social groups. The 11-item Duke Social Support Index [124] includes subscales for social interaction and subjective support. Table 7 shows the loneliness assessment tool scores.

Table 7.
Loneliness assessment tools scores.
3.8. Social Support
We included for review five papers (Figure 9) and we extracted 15 tools to assess social support networks (Table S7).
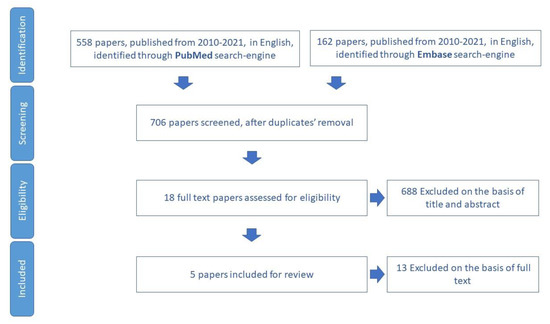
Figure 9.
Process of study selection for social support domain.
The UCLA Loneliness Scale [118] and the De Jong Gierveld scale [119] are also very frequently used to assess older adults’ support network and the presence of people who can take care of him/her in case of need. The Older Americans Resources and Services Program (OARS) and the Multidimensional Functional Assessment of Older Adults (MFAQ) [125] allows researchers to stratify the older adult population according to levels of social resources. The Inventory of Social Supportive Behaviors (ISSB) [126] assesses how often individuals received various forms of assistance during the preceding month. The Social Provision Scale [127] contains four subscales for each of the four social dispositions: attachment, social integration, material help, and value reassurance. The social support behaviors (SS-B) scale [128] and Medical Outcome Study Social Support Survey (MOS-SSS) [129] assess supportive behavior available from family and from friends in terms of emotional support, socializing, practical assistance, financial assistance, and advice/guidance. The Duke Social Support Index [124] assesses older adults’ social interaction and their satisfaction. The Multidimensional Scale of Perceived Social Support [130] and the Lubben Social Network Scale [131] measure the perceived adequacy of social support from three sources: family, friends, and significant others. The Berkman–Syme Social Network Index [132] allows researchers to categorize individuals into four levels of social connection: socially isolated, moderately isolated, moderately integrated, and socially integrated. The Personal Resource Questionnaire (PRQ) [133] provides descriptive information about the person’s resources and measures the respondent’s level of perceived social support. The six social support deficits [134] is a checklist including living alone, seeing a relative less often than once a week, lack of reciprocity with neighbors, lack of reciprocity between extended family members, relationship difficulty with one or more relatives, and dissatisfaction with support from family. The 2-Way Social Support Scale [135] assesses the emotional and instrumental support given and received. The Philadelphia Geriatric Center Morale Scale (PGCMS) [136] lonely dissatisfaction subscale measures the older person’s acceptance or dissatisfaction with the amount of social interaction they are presently experiencing. Table 8 shows support network assessment tool scores.

Table 8.
Support network assessment tools scores.
3.9. Economic Conditions
The search for reviews, systematic reviews, and meta-analyses on the assessment of the economic conditions of community-dwelling older people did not yield any results (Figure 10). The reason is that this domain belongs to a research field more related to the social sciences than to medicine and healthcare service organizations.
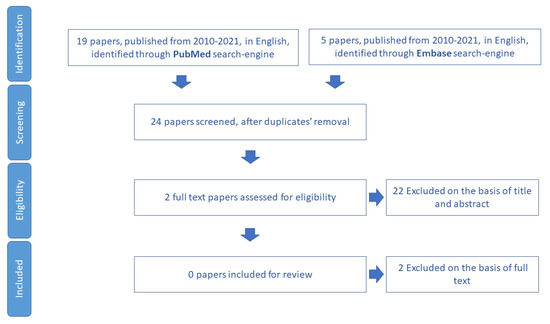
Figure 10.
Process of study selection for assessment of economic constraints.
The side-search allowed us to identify two tools that can be used to assess economic conditions of older adults. Tarasenko and Schoenberg, 2017 [138], adopted a self-perceived income sufficiency questionnaire to assess resources in an underserved population. The Subjective Status Scale of Adler et al., 2000 [139], measures self-rated socioeconomic status by means of a 10-step scale whose extremes represent people who are better off—those who have more money, more education, and the most respected jobs—and those who are worse off—people who have less money, less education, and fewer or no respected jobs.
4. Discussion
In order to be effectively used for frailty screening and the implementation of prevention interventions on community-dwelling older adults, the assessment tools to be connected to SUNFRAIL should be aimed at the identification of risk factors that may lead to the impairment of one or more of the domains of frailty. The limitation of the search for reviews, systematic reviews, and meta-analyses did not allow for the inclusion of the most recent instruments in the results. As in the case of the Quick Mild Cognitive Impairment (QMCI) Screen for the assessment of mild cognitive decline, it is particularly useful for dementia prevention interventions [140]. As a consequence of the COVID-19 pandemic, many health systems are implementing a Family and Community Nurse (FCN) to meet the twin challenges of an ageing population and the increasing incidence of chronic conditions in order to prevent adverse events and enable healthcare services to encourage and ensure health improvement and wellbeing, instead of focusing exclusively on treating illness [141]. In the case of medication review support tools for older adults in polypharmacy, the assessment tools that are often used require specialist expertise and therefore cannot be administered by an FCN. Therefore, screening and prevention interventions should focus on adherence to drug therapies, just as assessment tools should measure the older adult’s ability to take care of themselves and manage their disease.
5. Conclusions
This narrative review made it possible to identify tools for the assessment of biopsychosocial frailty dimensions in community-dwelling older adults. These tools, appropriately selected and integrated, can represent the dataset for the implementation of targeted and timely health promotion, prevention, education, and information services in primary care. Further studies are needed to develop and validate a new service model for frailty screening in community-dwelling older adults by means of an IT tool that could support the healthcare providers by linking the elements of the SUNFRAIL tool to additional scales aimed at assessing impaired domains, thereby enabling professionals to develop appropriate intervention strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph192316050/s1: Table S1: List of articles on assessment tools of prescription appropriateness and adherence in older adult population; Table S2: List of articles on assessment tools of nutritional habits; Table S3: List of articles on assessment tools of physical activity; Table S4: List of articles on fall and the risk of falling assessment tools; Table S5: List of articles on assessment tools for cognitive function; Table S6: List of articles on loneliness assessment tools; Table S7: List of articles on assessment tools for social support network. Refs. [142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306] cited in Supplementary Materials.
Author Contributions
Conceptualization, V.D.L. and M.I.; methodology, V.D.L., V.F., G.L. (Grazia Lorusso), C.R., F.D.L., G.L. (Giuseppe Liotta), L.M., T.R., E.S. and M.I.; writing—original draft preparation, V.D.L., G.D.F. and R.P.; writing—review and editing, V.D.L., J.A., R.C.S., N.K.Y., C.D., W.H.v.S., G.L. (Giuseppe Liotta), G.I., M.T. and M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by European Cooperation in Science and Technology (Grant n. CA 19136).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This article is based upon work from the COST Action 19136 International Interdisciplinary Network on Smart Healthy Age-friendly Environments(NET4Age-Friendly), supported by COST (European Cooperation in Science and Technology).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ageing Europe—Looking at the Lives of Older People in the EU. Available online: https://ec.europa.eu/eurostat/documents/3217494/10166544/KS-02-19%E2%80%91681-EN-N.pdf/c701972f-6b4e-b432-57d2-91898ca94893 (accessed on 18 October 2022).
- Iaccarino, G.; Grassi, G.; Borghi, C.; Ferri, C.; Salvetti, M.; Volpe, M. SARS-RAS Investigators. Age and Multimorbidity Predict Death Among COVID-19 Patients: Results of the SARS-RAS Study of the Italian Society of Hypertension. Hypertension 2020, 76, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Marois, G.; Muttarak, R.; Scherbov, S. Assessing the potential impact of COVID-19 on life expectancy. PLoS ONE 2020, 15, e0238678. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of frailty in community-dwelling older persons: A systematic review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Gobbens, R.J.; Luijkx, K.G.; Wijnen-Sponselee, M.T.; Schols, J.M. Toward a conceptual definition of frail community dwelling older people. Nurs. Outlook 2010, 58, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Liotta, G.; Ussai, S.; Illario, M.; O’Caoimh, R.; Cano, A.; Holland, C.; Roller-Winsberger, R.; Capanna, A.; Grecuccio, C.; Ferraro, M.; et al. Frailty as the Future Core Business of Public Health: Report of the Activities of the A3 Action Group of the European Innovation Partnership on Active and Healthy Ageing (EIP on AHA). Int. J. Environ. Res. Public Health 2018, 15, 2843. [Google Scholar] [CrossRef]
- Fried, L.P.; Ferrucci, L.; Darer, J.; Williamson, J.D.; Anderson, G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 255–263. [Google Scholar] [CrossRef]
- Gobbens, R.J.; van Assen, M.A.; Luijkx, K.G.; Wijnen-Sponselee, M.T.; Schols, J.M. Determinants of frailty. J. Am. Med. Dir. Assoc. 2010, 11, 356–364. [Google Scholar] [CrossRef]
- Maggio, M.; Barbolini, M.; Longobucco, Y.; Barbieri, L.; Benedetti, C.; Bono, F.; Cacciapuoti, I.; Donatini, A.; Iezzi, E.; Papini, D.; et al. A Novel Tool for the Early Identification of Frailty in Elderly People: The Application in Primary Care Settings. J. Frailty Aging 2020, 9, 101–106. [Google Scholar] [CrossRef]
- Gobbens, R.J.J.; Maggio, M.; Longobucco, Y.; Barbolini, M. The Validity of the SUNFRAIL Tool: A Cross-Sectional Study among Dutch Community-Dwelling Older People. J. Frailty Aging 2020, 9, 219–225. [Google Scholar] [CrossRef]
- Cardoso, A.F.; Bobrowicz-Campos, E.; Couto, F.; Cardoso, D.; Barata, A.; Apóstolo, J. Feasibility, appropriateness and meaningfulness analysis of the Sunfrail Tool to the European Portuguese population during cross-cultural adaptation process. Int. J. Evid. Based Healthc. 2019, 17 (Suppl. S1), S26–S28. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.F.; Bobrowicz-Campos, E.; Teixeira-Santos, L.; Cardoso, D.; Couto, F.; Apóstolo, J. Validation and Screening Capacity of the European Portuguese Version of the SUNFRAIL Tool for Community-Dwelling Older Adults. Int. J. Environ. Res. Public Health 2021, 18, 1394. [Google Scholar] [CrossRef] [PubMed]
- Rolfson, D.B.; Majumdar, S.R.; Tsuyuki, R.T.; Tahir, A.; Rockwood, K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006, 35, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Gobbens, R.J.; van Assen, M.A.; Luijkx, K.G.; Wijnen-Sponselee, M.T.; Schols, J.M. The Tilburg Frailty Indicator: Psychometric properties. J. Am. Med. Dir. Assoc. 2010, 11, 344–355. [Google Scholar] [CrossRef]
- Tavassoli, N.; Guyonnet, S.; Abellan Van Kan, G.; Sourdet, S.; Krams, T.; Soto, M.E.; Subra, J.; Chicoulaa, B.; Ghisolfi, A.; Balardy, L.; et al. Description of 1,108 older patients referred by their physician to the "Geriatric Frailty Clinic (G.F.C) for Assessment of Frailty and Prevention of Disability" at the gerontopole. J. Nutr. Health Aging 2014, 18, 457–464. [Google Scholar] [CrossRef]
- Illario, M.; De Luca, V.; Onorato, G.; Tramontano, G.; Carriazo, A.M.; Roller-Wirnsberger, R.E.; Apostolo, J.; Eklund, P.; Gosami, N.; Iaccarino, G.; et al. Interactions Between EIP on AHA Reference Sites and Action Groups to Foster Digital Innovation of Health and Care in European Regions. Clin. Interv. Aging 2022, 17, 343–358. [Google Scholar] [CrossRef]
- La Torre, G.; Backhaus, I.; Mannocci, A. Rating for narrative reviews: Concept and development of the International Narrative Systematic Assessment tool. Senses Sci. 2015, 2, 31–35. [Google Scholar]
- O’Mahony, D.; O’Sullivan, D.; Byrne, S.; O’Connor, M.N.; Ryan, C.; Gallagher, P. STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 2015, 44, 213–218, Erratum in Age Ageing 2018, 47, 489. [Google Scholar] [CrossRef]
- 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef]
- Samsa, G.P.; Hanlon, J.T.; Schmader, K.E.; Weinberger, M.; Clipp, E.C.; Uttech, K.M.; Lewis, I.K.; Landsman, P.B.; Cohen, H.J. A summated score for the medication appropriateness index: Development and assessment of clinimetric properties including content validity. J. Clin. Epidemiol. 1994, 47, 891–896. [Google Scholar] [CrossRef]
- Pazan, F.; Gercke, Y.; Weiss, C.; Wehling, M.; Marcum, Z.A.; Gokula, M.; Nathan, K.T.; Cheng, H.Y.; Tantipinichwong, N.; Gray, S.L.; et al. The US-FORTA (Fit fOR The Aged) List: Consensus Validation of a Clinical Tool to Improve Drug Therapy in Older Adults. J. Am. Med. Dir. Assoc. 2020, 21, 439.e9–439.e13. [Google Scholar] [CrossRef] [PubMed]
- Lavan, A.H.; Gallagher, P.; Parsons, C.; O’Mahony, D. STOPPFrail (Screening Tool of Older Persons Prescriptions in Frail adults with limited life expectancy): Consensus validation. Age Ageing 2017, 46, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Rognstad, S.; Brekke, M.; Fetveit, A.; Spigset, O.; Wyller, T.B.; Straand, J. The Norwegian General Practice (NORGEP) criteria for assessing potentially inappropriate prescriptions to elderly patients. A modified Delphi study. Scand. J. Prim. Health Care 2009, 27, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Renom-Guiteras, A.; Meyer, G.; Thürmann, P.A. The EU(7)-PIM list: A list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur. J. Clin. Pharmacol. 2015, 71, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.; Schmiedl, S.; Thürmann, P.A. Potentially inappropriate medications in the elderly: The PRISCUS list. Dtsch. Arztebl. Int. 2010, 107, 543–551. [Google Scholar] [CrossRef]
- Maanen, A.C.D.; Leendertse, A.J.; Jansen, P.A.F.; Knol, W.; Keijsers, C.J.P.W.; Meulendijk, M.C.; Van Marum, R.J.; Jansen, P.A.F.; Keijsers, C.J.P.W.; Van Marum, R.J. The Systematic Tool to Reduce Inappropriate Prescribing (STRIP): Combining implicit and explicit prescribing tools to improve appropriate prescribing. J. Eval. Clin. Pract. 2018, 24, 317–322. [Google Scholar] [CrossRef]
- Bilek, A.J.; Levy, Y.; Kab, H.; Andreev, P.; Garfinkel, D. Teaching physicians the GPGP method promotes deprescribing in both inpatient and outpatient settings. Ther. Adv. Drug Saf. 2019, 10, 2042098619895914. [Google Scholar] [CrossRef]
- Nightingale, G.; Hajjar, E.; Pizzi, L.T.; Wang, M.; Pigott, E.; Doherty, S.; Prioli, K.M.; Swartz, K.; Chapman, A.E. Implementing a pharmacist-led, individualized medication assessment and planning (iMAP) intervention to reduce medication related problems among older adults with cancer. J. Geriatr. Oncol. 2017, 8, 296–302. [Google Scholar] [CrossRef]
- Zhan, C.; Sangl, J.; Bierman, A.S.; Miller, M.R.; Friedman, B.; Wickizer, S.W.; Meyer, G.S. Potentially inappropriate medication use in the community-dwelling elderly: Findings from the 1996 Medical Expenditure Panel Survey. JAMA 2001, 286, 2823–2829. [Google Scholar] [CrossRef]
- Edelberg, H.K.; Shallenberger, E.; Wei, J.Y. Medication management capacity in highly functioning community-living older adults: Detection of early deficits. J. Am. Geriatr. Soc. 1999, 47, 592–596. [Google Scholar] [CrossRef]
- Hutchison, L.C.; Jones, S.K.; West, D.S.; Wei, J.Y. Assessment of medication management by community-living elderly persons with two standardized assessment tools: A cross-sectional study. Am. J. Geriatr. Pharmacother. 2006, 4, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Risser, J.; Jacobson, T.A.; Kripalani, S. Development and psychometric evaluation of the Self-efficacy for Appropriate Medication Use Scale (SEAMS) in low-literacy patients with chronic disease. J. Nurs. Meas. 2007, 15, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Orfanos, P.; Norat, T.; Bueno-De-Mesquita, B.; Ocké, M.C.; Peeters, P.H.; Van Der Schouw, Y.T.; Boeing, H.; Hoffmann, K.; Boffetta, P.; et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ 2005, 330, 991. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E. Mini nutritional assessment. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment Short-Form (MNA®-SF): A practical tool for identificationof nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef]
- Söderhamn, U.; Soderhamn, O. Reliability and validity of the nutritional form for the elderly (NUFFE). J. Adv. Nurs. 2002, 37, 28–34. [Google Scholar] [CrossRef]
- Elia, M. The ‘MUST’ Report, Nutritional Screening of Adults: A multidisciplinary Responsibility. Development and Use of the ‘Malnutrition Universal Screening Tool’ (‘MUST’) for Adults; British Alliance for Enteral and Parenteral Nutrition (BAPEN): London, UK, 2003; Available online: https://eprints.soton.ac.uk/362499 (accessed on 18 October 2022).
- Laporte, M.; Keller, H.H.; Payette, H.; Allard, J.P.; Duerksen, D.R.; Bernier, P.; Jeejeebhoy, K.N.; Gramlich, L.; Davidson, B.R.; Vesnaver, E.; et al. Validity and reliability of the new Canadian Nutrition Screening Tool in the ’real-world’ hospital setting. Eur. J. Clin. Nutr. 2015, 69, 558–564. [Google Scholar] [CrossRef]
- Visvanathan, R.; Penhall, I. Chapman Nutritional screening of older people in a sub-acute care facility in Australia and its relation to discharge outcomes. Age Ageing 2004, 33, 260–265. [Google Scholar] [CrossRef]
- Wilson, M.-M.G.; Thomas, D.R.; Rubenstein, L.; Chibnall, J.T.; Anderson, S.; Baxi, A.; Diebold, M.R.; Morley, J. Appetite assessment: Simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am. J. Clin. Nutr. 2005, 82, 1074–1081. [Google Scholar] [CrossRef]
- DeGroot, L.C.; Beck, A.M.; Schroll, M.; Staveren, W.A. Evaluating the DETERMINE your nutritional health checklist and the mini nutritional assessment as tools to identify nutritional problems in elderly Europeans. Eur. J. Clin. Nutr. 1998, 52, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Keller, H.H.; Goy, R.; Kane, S.L. Validity and reliability of SCREEN II (Seniors in the community: Risk evaluation for eating and nutrition, Version II). Eur. J. Clin. Nutr. 2005, 59, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Kruizenga, H.M.; Seidell, J.C.; de Vet, H.C.; Wierdsma, N.J.; van Bokhorst-de van der Schueren, M.A. Development and validation of a hospital screening tool for malnutrition: The short nutritional assessment questionnaire (SNAQ). Clin. Nutr. 2005, 24, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Wijnhoven, H.A.; Schilp, J.; de Vet, H.C.; Kruizenga, H.M.; Deeg, D.J.; Ferrucci, L.; Visser, M. Development and validation of criteria for determining undernutrition in community-dwelling older men and women: The Short Nutritional Assessment Questionnaire 65+. Clin. Nutr. 2012, 31, 351–358. [Google Scholar] [CrossRef]
- Morley, J.E. Why do physicians fail to recognize and treat malnutrition in older persons? J. Am. Geriatr. Soc. 1991, 39, 1139–1140. [Google Scholar] [CrossRef]
- Ferguson, M.; Capra, S.; Bauer, J.; Banks, M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999, 15, 458–464. [Google Scholar] [CrossRef]
- Welch, S.A.; Ward, R.E.; Beauchamp, M.K.; Leveille, S.G.; Travison, T.; Bean, J.F. The Short Physical Performance Battery (SPPB): A Quick and Useful Tool for Fall Risk Stratification Among Older Primary Care Patients. J. Am. Med. Dir. Assoc. 2021, 22, 1646–1651. [Google Scholar] [CrossRef]
- Kim, M.; Won, C.W. Cut Points of Chair Stand Test for Poor Physical Function and Its Association With Adverse Health Outcomes in Community-Dwelling Older Adults: A Cross-Sectional and Longitudinal Study. J. Am. Med. Dir. Assoc. 2021, 23, 1375–1382.e3. [Google Scholar] [CrossRef]
- Herman, T.; Giladi, N.; Hausdorff, J.M. Properties of the ’timed up and go’ test: More than meets the eye. Gerontology 2010, 57, 203–210. [Google Scholar] [CrossRef]
- De Aquino, M.P.M.; de Oliveira Cirino, N.T.; Lima, C.A.; de Miranda Ventura, M.; Hill, K.; Perracini, M.R. The Four Square Step Test is a useful mobility tool for discriminating older persons with frailty syndrome. Exp. Gerontol. 2022, 161, 111699. [Google Scholar] [CrossRef]
- Chung, M.; Chan, R.; Fung, Y.; Fong, S.; Lam, S.; Lai, C.; Ng, S. Reliability and validity of Alternate Step Test times in subjects with chronic stroke. J. Rehabil. Med. 2014, 46, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Danks, K.A.; Pohlig, R.; Reisman, D.S. Combining fast-walking training and a step-activity monitoring program to improve daily walking activity after stroke: A preliminary study. Arch. Phys. Med. Rehabil. 2016, 97, S185–S193. [Google Scholar] [CrossRef]
- Wilson, C.M.; Kostsuca, S.R.; Boura, J.A. Utilization of a 5-Meter Walk Test in Evaluating Self-selected Gait Speed during Preoperative Screening of Patients Scheduled for Cardiac Surgery. Cardiopulm. Phys. Ther. J. 2013, 24, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Probst, V.S.; Hernandes, N.A.; Teixeira, D.C.; Felcar, J.M.; Mesquita, R.B.; Gonçalves, C.G.; Hayashi, D.; Singh, S.; Pitta, F. Reference values for the incremental shuttle walking test. Respir. Med. 2012, 106, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Washburn, R.A.; McAuley, E.; Katula, J.; Mihalko, S.L.; Boileau, R.A. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J. Clin. Epidemiol. 1993, 46, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.L.; Mills, K.M.; King, A.C.; Haskell, W.L.; Gillis, D.; Ritter, P.L. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Med. Sci. Sports Exerc. 2001, 33, 1126–1141. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Bloemberg, B.P.; Saris, W.H.; Merritt, R.K.; Kromhout, D. The prevalence of selected physical activities and their relation with coronary heart disease risk factors in elderly men: The Zutphen Study, 1985. Am. J. Epidemiol. 1991, 133, 1078–1092. [Google Scholar] [CrossRef]
- Canadian Society for Exercise Physiology. Canadian Society for Exercise Physiology-Physical Activity Training for Health (CSEP-PATH); Canadian Society for Exercise Physiology: Ottawa, ON, Canada, 2013. [Google Scholar]
- España-Romero, V.; Golubic, R.; Martin, K.R.; Hardy, R.; Ekelund, U.; Kuh, D.; Wareham, N.J.; Cooper, R.; Brage, S.; NSHD Scientific and Data Collection Teams. Comparison of the EPIC Physical Activity Questionnaire with combined heart rate and movement sensing in a nationally representative sample of older British adults. PLoS ONE 2014, 9, e87085. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Ahmad, S.; Harris, T.; Limb, E.; Kerry, S.; Victor, C.R.; Ekelund, U.; Iliffe, S.; Whincup, P.H.; Beighton, C.; Ussher, M.; et al. Evaluation of reliability and validity of the General Practice Physical Activity Questionnaire (GPPAQ) in 60–74 year old primary care patients. BMC Fam. Pract. 2015, 16, 113. [Google Scholar] [CrossRef]
- Stel, V.S.; Smit, J.H.; Pluijm, S.M.; Visser, M.; Deeg, D.J.; Lips, P. Comparison of the LASA Physical Activity Questionnaire with a 7-day diary and pedometer. J. Clin. Epidemiol. 2004, 57, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Piliae, R.E.; Fair, J.M.; Haskell, W.L.; Varady, A.N.; Iribarren, C.; Hlatky, M.; Go, A.S.; Fortmann, S.P. Validation of the Stanford Brief Activity Survey: Examining psychological factors and physical activity levels in older adults. J. Phys. Act. Health 2010, 7, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.M.; Evenson, K.R.; Morimoto, L.; Siscovick, D.; White, E. Test-retest reliability of the Women’s Health Initiative physical activity questionnaire. Med. Sci. Sports Exerc. 2009, 41, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Frost, Y.; Weingarden, H.; Zeilig, G.; Nota, A.; Rand, D. Self-Care Self-Efficacy Correlates with Independence in Basic Activities of Daily Living in Individuals with Chronic Stroke. J. Stroke Cerebrovasc. Dis. 2015, 24, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Polese, J.; da Silva, S.; Faria-Fortini, I.; Faria, C.; Teixeira-Salmela, L. Duke Activity Status Index cut-off scores for assessing functional capacity after stroke. Disabil. Rehabil. 2021, 43, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C. The SF-36 physical and mental health summary measures: An example of how to interpret scores. J. Health Serv. Res. Policy 1998, 3, 92–96. [Google Scholar] [CrossRef]
- Salaffi, F.; Leardini, G.; Canesi, B.; Mannoni, A.; Fioravanti, A.; Caporali, R.; Lapadula, G.; Punzi, L.; GOnorthrosis and Quality Of Life Assessment (GOQOLA). Reliability and validity of the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index in Italian patients with osteoarthritis of the knee. Osteoarthr. Cartil. 2003, 11, 551–560. [Google Scholar] [CrossRef]
- Canbek, J.; Fulk, G.; Nof, L.; Echternach, J. Test-retest reliability and construct validity of the tinetti performance-oriented mobility assessment in people with stroke. J. Neurol. Phys. Ther. 2013, 37, 14–19. [Google Scholar] [CrossRef]
- Mourey, F.; Camus, A.; D’Athis, P.; Blanchon, M.-A.; Martin-Hunyadi, C.; de Rekeneire, N.; Pfitzenmeyer, P. Mini motor test: A clinical test for rehabilitation of patients showing psychomotor disadaptation syndrome (PDS). Arch. Gerontol. Geriatr. 2005, 40, 201–211. [Google Scholar] [CrossRef]
- Prosser, L.; Canby, A. Further validation of the Elderly Mobility Scale for measurement of mobility of hospitalized elderly people. Clin. Rehabil. 1997, 11, 338–343. [Google Scholar] [CrossRef]
- Cunningham, A.; Mautner, D.; Ku, B.; Scott, K.; LaNoue, M. Frequent emergency department visitors are frequent primary care visitors and report unmet primary care needs. J. Eval. Clin. Pract. 2017, 23, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E.; Richman, D.; Powell, L. Falls efficacy as a measure of fear of falling. J. Gerontol. 1990, 45, P239–P243. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.D.; Schwarz, J.A.; Kalogeropoulos, A.J.; Gibson, S.J. Fear of falling revisited. Arch. Phys. Med. Rehabil. 1996, 77, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Kempen, G.I.; Todd, C.J.; Van Haastregt, J.C.; Rixt Zijlstra, G.A.; Beyer, N.; Freiberger, E.; Hauer, K.A.; Piot-Ziegler, C.; Yardley, L. Cross-cultural validation of the falls efficacy scale international (FES-I) in older people: Results from Germany, the Netherlands and the UK were satisfactory. Disabil. Rehabil. 2007, 29, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-W.; Ng, S.S.M. The reliability and validity of the Survey of Activities and Fear of Falling in the Elderly for assessing fear and activity avoidance among stroke survivors. PLoS ONE 2019, 14, e0214796. [Google Scholar] [CrossRef] [PubMed]
- Lusardi, M.M.; Smith, E.V., Jr. Development of a scale to assess concern about falling and applications to treatment programs. J. Outcome Meas. 1997, 1, 34–55. [Google Scholar]
- Velozo, C.A.; Peterson, E.W. Developing meaningful fear of falling measures for community dwelling elderly. Am. J. Phys. Med. Rehabil. 2001, 80, 662–673. [Google Scholar] [CrossRef]
- Wiens, C.A.; Koleba, T.; Jones, C.A.; Feeny, D.F. The Falls Risk Awareness Questionnaire: Development and validation for use with older adults. J. Gerontol. Nurs. 2006, 32, 43–50. [Google Scholar] [CrossRef]
- Ishimoto, Y.; Wada, T.; Kasahara, Y.; Kimura, Y.; Fukutomi, E.; Chen, W.; Hirosaki, M.; Nakatsuka, M.; Fujisawa, M.; Sakamoto, R.; et al. Fall Risk Index predicts functional decline regardless of fall experiences among community-dwelling elderly. Geriatr. Gerontol. Int. 2012, 12, 659–666. [Google Scholar] [CrossRef]
- Polovina, S.; Micić, D.; Miljić, D.; Milić, N.; Micić, D.; Popović, V. The Fracture Risk Assessment Tool (FRAX score) in subclinical hyperthyroidism. Vojn. Pregl. 2015, 72, 510–516. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, Y.S. The Diagnostic Accuracy of the Berg Balance Scale in Predicting Falls. West. J. Nurs. Res. 2017, 39, 1502–1525. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.J.; Fiedler, R.C.; Rose, D.J. Rasch Analysis of the Fullerton Advanced Balance (FAB) Scale. Physiother. Can. 2011, 63, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Powell, L.E.; Myers, A.M. The Activities-specific Balance Confidence (ABC) Scale. J. Gerontol. Ser. A 1995, 50, M28–M34. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, C.; Hough, P.; Bull, K.; Hill, K.; Greenwood, K.; Oldmeadow, L. A 4-item falls-risk screening tool for sub-acute and residential care: The first step in falls prevention. Australas. J. Ageing 2009, 28, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Schwendimann, R.; De Geest, S.; Milisen, K. Evaluation of the Morse Fall Scale in hospitalised patients. Age Ageing 2006, 35, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.; Kamar, J.; Graco, M.; Lawlor, V.; Hill, K. Adding value to the STRATIFY falls risk assessment in acute hospitals. J. Adv. Nurs. 2011, 67, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Conley, D.; Schultz, A.A.; Selvin, R. The challenge of predicting patients at risk for falling: Development of the Conley Scale. Medsurg Nurs. 1999, 8, 348–354. [Google Scholar]
- Poe, S.S.; Dawson, P.B.; Cvach, M.; Burnett, M.; Kumble, S.; Lewis, M.; Thompson, C.B.; Hill, E.E. The Johns Hopkins Fall Risk Assessment Tool: A Study of Reliability and Validity. J. Nurs. Care Qual. 2018, 33, 10–19. [Google Scholar] [CrossRef]
- Hendrich, A.L.; Bufalino, A.; Groves, C. Validation of the Hendrich II Fall Risk Model: The imperative to reduce modifiable risk factors. Appl. Nurs. Res. 2020, 53, 151243. [Google Scholar] [CrossRef]
- Said, C.M.; Churilov, L.; Shaw, K. Validation and inter-rater reliability of a three item falls risk screening tool. BMC Geriatr. 2017, 17, 273. [Google Scholar] [CrossRef]
- Chang, Y.W.; Chang, Y.H.; Pan, Y.L.; Kao, T.W.; Kao, S. Validation and reliability of Falls Risk for Hospitalized Older People (FRHOP): Taiwan version. Medicine 2017, 96, e7693. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.A.; Hill, K.D.; Day, L.M.; Blackberry, I.; Gurrin, L.C.; Dharmage, S.C. Development of the Falls Risk for Older People in the Community (FROP-Com) screening tool. Age Ageing 2009, 38, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Mojtaba, M.; Alinaghizadeh, H.; Rydwik, E. Downton Fall Risk Index during hospitalisation is associated with fall-related injuries after discharge: A longitudinal observational study. J. Physiother. 2018, 64, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Julie, G.; Cwikel, A. Veronica Fried, Aya Biderman & David Galinsky (1998) Validation of a fall-risk screening test, the Elderly Fall Screening Test (EFST), for community-dwelling elderly. Disabil. Rehabil. 1998, 20, 161–167. [Google Scholar] [CrossRef]
- Johnson, M.; Cusick, A.; Chang, S. Home-screen: A short scale to measure fall risk in the home. Public Health Nurs. 2001, 18, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.E.; Campbell, E.M.; Sanson-Fisher, R.W.; Redman, S.; Gillespie, W.J. Environmental hazards in the homes of older people. Age Ageing 1997, 26, 195–202. [Google Scholar] [CrossRef]
- Kukull, W.A.; Larson, E.B.; Teri, L.; Bowen, J.; McCormick, W.; Pfanschmidt, M.L. The Mini-Mental State Examination score and the clinical diagnosis of dementia. J. Clin. Epidemiol. 1994, 47, 1061–1067. [Google Scholar] [CrossRef]
- Teng, E.L.; Chui, H.C. The Modified Mini-Mental State (3MS) examination. J. Clin. Psychiatry 1987, 48, 314–318. [Google Scholar]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699, published correction appears in J. Am. Geriatr. Soc. 2019, 67, 1991. [Google Scholar] [CrossRef]
- Teng, E.L.; Hasegawa, K.; Homma, A.; Imai, Y.; Larson, E.; Graves, A.; Sugimoto, K.; Yamaguchi, T.; Sasaki, H.; Chiu, D.; et al. The Cognitive Abilities Screening Instrument (CASI): A practical test for cross-cultural epidemiological studies of dementia. Int. Psychogeriatr. 1994, 6, 45–62. [Google Scholar] [CrossRef]
- Pfeiffer, E. A short portable mental status questionnaire for the assessment of organic brain deficits in the elderly. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Jitapunkul, S.; Pillay, I.; Ebrahim, S. The abbreviated mental test: Its use and validity. Age Ageing 1991, 20, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Brooke, P.; Bullock, R. Validation of a 6 item cognitive impairment test with a view to primary care usage. Int. J. Geriatr. Psychiatry 1999, 14, 936–940. [Google Scholar] [CrossRef]
- Pattie, A.H. A survey version of the Clifton Assessment Procedures for the Elderly (CAPE). Br. J. Clin. Psychol. 1981, 20, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C. Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int. Psychogeriatr. 1997, 9 (Suppl. 1), 173–178. [Google Scholar] [CrossRef]
- Malek-Ahmadi, M.; Small, B.J.; Raj, A. The diagnostic value of controlled oral word association test-FAS and category fluency in single-domain amnestic mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2011, 32, 235–240. [Google Scholar] [CrossRef]
- Isaacs, B.; Kennie, A.T. The Set test as an aid to the detection of dementia in old people. Br. J. Psychiatry 1973, 123, 467–470. [Google Scholar] [CrossRef]
- Llinàs-Reglà, J.; Vilalta-Franch, J.; López-Pousa, S.; Calvó-Perxas, L.; Torrents Rodas, D.; Garre-Olmo, J. The Trail Making Test. Assessment 2016, 24, 183–196. [Google Scholar] [CrossRef]
- Ricci, M.; Graef, S.; Blundo, C.; Miller, L.A. Using the Rey Auditory Verbal Learning Test (RAVLT) to differentiate alzheimer’s dementia and behavioural variant fronto-temporal dementia. Clin. Neuropsychol. 2012, 26, 926–941. [Google Scholar] [CrossRef]
- Nishiwaki, Y.; Breeze, E.; Smeeth, L.; Bulpitt, C.J.; Peters, R.; Fletcher, A.E. Validity of the Clock-Drawing Test as a screening tool for cognitive impairment in the elderly. Am. J. Epidemiol. 2004, 160, 797–807. [Google Scholar] [CrossRef]
- Fichman-Charchat, H.; Miranda, C.V.; Fernandes, C.S.; Mograbi, D.; Oliveira, R.M.; Novaes, R.; Aguiar, D. Brief Cognitive Screening Battery (BCSB) is a very useful tool for diagnosis of probable mild Alzheimer´s disease in a geriatric clinic. Arq. Neuro-Psiquiatr. 2016, 74, 149–154. [Google Scholar] [CrossRef][Green Version]
- Borson, S.; Scanlan, J.; Brush, M.; Vitaliano, P.; Dokmak, A. The mini-cog: A cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int. J. Geriatr. Psychiatry. 2000, 15, 1021–1027. [Google Scholar] [CrossRef]
- Fong, T.G.; Fearing, M.A.; Jones, R.N.; Shi, P.; Marcantonio, E.R.; Rudolph, J.L.; Yang, F.M.; Kiely, K.D.; Inouye, S.K. Telephone interview for cognitive status: Creating a crosswalk with the Mini-Mental State Examination. Alzheimer’s Dement. 2009, 5, 492–497. [Google Scholar] [CrossRef]
- Hall, K.S.; Gao, S.; Emsley, C.L.; Ogunniyi, A.O.; Morgan, O.; Hendrie, H.C. Community screening interview for dementia (CSI ’D’); performance in five disparate study sites. Int. J. Geriatr. Psychiatry 2000, 15, 521–531. [Google Scholar] [CrossRef]
- Russell, D.W. UCLA Loneliness Scale (Version 3): Reliability, validity, and factor structure. J. Pers. Assess. 1996, 66, 20–40. [Google Scholar] [CrossRef]
- De Jong-Gierveld, J.; Kamphuis, F. The development of a Rasch-type loneliness scale. Appl. Psychol. Meas. 1985, 9, 289–299. [Google Scholar] [CrossRef]
- Hughes, M.E.; Waite, L.J.; Hawkley, L.C.; Cacioppo, J.T. A Short Scale for Measuring Loneliness in Large Surveys: Results From Two Population-Based Studies. Res. Aging 2004, 26, 655–672. [Google Scholar] [CrossRef]
- Makizako, H.; Shimada, H.; Tsutsumimoto, K.; Lee, S.; Doi, T.; Nakakubo, S.; Hotta, R.; Suzuki, T. Social Frailty in Community-Dwelling Older Adults as a Risk Factor for Disability. J. Am. Med. Dir. Assoc. 2015, 16, 1003.e7–1003.e11. [Google Scholar] [CrossRef]
- Teo, N.; Gao, Q.; Nyunt, M.S.Z.; Wee, S.L.; Ng, T.P. Social Frailty and Functional Disability: Findings From the Singapore Longitudinal Ageing Studies. J. Am. Med. Dir. Assoc. 2017, 18, 637.e13–637.e19. [Google Scholar] [CrossRef]
- Steptoe, A.; Shankar, A.; Demakakos, P.; Wardle, J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc. Natl. Acad. Sci. USA 2013, 110, 5797–5801. [Google Scholar] [CrossRef]
- Koenig, H.G.; Westlund, R.E.; George, L.K.; Hughes, D.C.; Blazer, D.G.; Hybels, C. Abbreviating the Duke Social Support Index for use in chronically ill elderly individuals. Psychosomatics 1993, 34, 61–69. [Google Scholar] [CrossRef]
- Fillenbaum, G.G.; Smyer, M.A. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J. Gerontol. 1981, 36, 428–434. [Google Scholar] [CrossRef]
- Barrera, M.; Sandler, I.N.; Ramsey, T.B. Preliminary development of a scale of social support: Studies on college students. Am. J. Community Psychol. 1981, 9, 435–447. [Google Scholar] [CrossRef]
- Caron, J. L’Échelle de provisions sociales: La validation québécoise du Social Provisions Scale. St. Ment. Au Québec 1996, 21, 158–180. [Google Scholar] [CrossRef]
- Resnick, H.E.; Fries, B.E.; Verbrugge, L.M. Windows to their world: The effect of sensory impairments on social engagement and activity time in nursing home residents. J. Gerontol. B Psychol. Sci. Soc. Sci. 1997, 52, S135–S144. [Google Scholar] [CrossRef]
- Sherbourne, C.D.; Stewart, A.L. The MOS social support survey. Soc. Sci. Med. 1991, 32, 705–714. [Google Scholar] [CrossRef]
- Zimet, G.D.; Powell, S.S.; Farley, G.K.; Werkman, S.; Berkoff, K.A. Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. J. Pers. Assess. 1990, 55, 610–617. [Google Scholar] [CrossRef]
- Lubben, J.E. Assessing social networks among elderly populations. Fam. Community Health 1988, 11, 42–52. [Google Scholar] [CrossRef]
- Loucks, E.; Sullivan, L.; D’Agostino Sr, R.; Larson, M.; Berkman, L.; Benjamin, E. Social networks and inflammatory markers in the Framingham heart study. J. Biosoc. Sci. 2006, 38, 835–842. [Google Scholar] [CrossRef]
- Weinert, C. Evaluation of the Personal Resource Questionnaire: A social support measure. Birth Defects Orig. Artic. Ser. 1984, 20, 59–97. [Google Scholar]
- Suttajit, S.; Punpuing, S.; Jirapramukpitak, T.; Tangchonlatip, K.; Darawuttimaprakorn, N.; Stewart, R.; Dewey, M.E.; Prince, M.; Abas, M.A. Impairment, disability, social support and depression among older parents in rural Thailand. Psychol. Med. 2010, 40, 1711–1721. [Google Scholar] [CrossRef][Green Version]
- Shakespeare-Finch, J.; Obst, P.L. The development of the 2-Way Social Support Scale: A measure of giving and receiving emotional and instrumental support. J. Pers. Assess. 2011, 93, 483–490. [Google Scholar] [CrossRef]
- Lawton, M.P. The Philadelphia Geriatric Center Morale Scale: A revision. J. Gerontol. 1975, 30, 85–89. [Google Scholar] [CrossRef]
- Lem, K.; McGilton, K.S.; Aelick, K.; Iaboni, A.; Babineau, J.; Hewitt Colborne, D.; Edwards, C.; Bretzlaff, M.; Lender, D.; Gibson, J.L.; et al. Social connection and physical health outcomes among long-term care home residents: A scoping review. BMC Geriatr. 2021, 21, 722. [Google Scholar] [CrossRef]
- Tarasenko, Y.N.; Schoenberg, N.E. Self-perceived Income Sufficiency and Self-reported Income Level among a Health Inequity Population. J. Health Care Poor Underserved 2017, 28, 812–828. [Google Scholar] [CrossRef]
- Adler, N.E.; Epel, E.S.; Castellazzo, G.; Ickovics, J.R. Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy white women. Health Psychol. 2000, 19, 586–592. [Google Scholar] [CrossRef]
- Glynn, K.; Coen, R.; Lawlor, B.A. Is the Quick Mild Cognitive Impairment Screen (QMCI) more accurate at detecting mild cognitive impairment than existing short cognitive screening tests? A systematic review of the current literature. Int. J. Geriatr. Psychiatry 2019, 34, 1739–1746. [Google Scholar] [CrossRef]
- Dellafiore, F.; Caruso, R.; Cossu, M.; Russo, S.; Baroni, I.; Barello, S.; Vangone, I.; Acampora, M.; Conte, G.; Magon, A.; et al. The State of the Evidence about the Family and Community Nurse: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 4382. [Google Scholar] [CrossRef]
- Sadowski, C.A.; Bharadia, M.; Bowles, S.K.; Isenor, J.E.; Patel, T. Review of the top 5 geriatrics studies of 2018–2019. Can. Pharm. J. 2021, 154, 256–261. [Google Scholar] [CrossRef]
- Saeed, D.; Carter, G.; Parsons, C. A systematic review of interventions to improve medicines optimisation in frail older patients in secondary and acute care settings. Int. J. Pharm. Pract. 2021, 29 (Suppl. S1), i22–i23. [Google Scholar] [CrossRef]
- Amorim, W.W.; Passos, L.C.; Gama, R.S.; Souza, R.M.; Graia, L.T.; Macedo, J.C.; Santos, D.B.; Oliveira, M.G. Physician and patient-related factors associated with inappropriate prescribing to older patients within primary care: A cross-sectional study in brazil. Sao Paulo Med. J. 2021, 139, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Loste, C.; Moltó, J.; Pérez-Álvarez, N.; Puig, J.; Echeverría, P.; Bonjoch, A.; Fumaz, C.R.; Lemos, B.; Estany, C.; Clotet, B.; et al. Potential prescribing issues among older HIV-infected subjects in a Mediterranean cohort: Does the current prevalence give cause for concern? Br. J. Clin. Pharmacol. 2021, 87, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Pazan, F.; Petrovic, M.; Cherubini, A.; Onder, G.; Cruz-Jentoft, A.J.; Denkinger, M.; van der Cammen, T.J.M.; Stevenson, J.M.; Ibrahim, K.; Rajkumar, C.; et al. Current evidence on the impact of medication optimization or pharmacological interventions on frailty or aspects of frailty: A systematic review of randomized controlled trials. Eur. J. Clin. Pharmacol. 2021, 77, 1–12, Erratum in Eur. J. Clin. Pharmacol. 2021, 77, 1593–1594. [Google Scholar] [CrossRef]
- De Oliveira, L.M.; Diel, J.D.A.C.; Nunes, A.; Dal Pizzol, T.D.S. Prevalence of drug interactions in hospitalised elderly patients: A systematic review. Eur. J. Hosp. Pharm. 2021, 28, 4–9. [Google Scholar] [CrossRef]
- Williams, S.; Miller, G.; Khoury, R.; Grossberg, G.T. Rational deprescribing in the elderly. Ann. Clin. Psychiatry 2019, 31, 144–152. [Google Scholar] [PubMed]
- Muth, C.; Blom, J.W.; Smith, S.M.; Johnell, K.; Gonzalez-Gonzalez, A.I.; Nguyen, T.S.; Brueckle, M.-S.; Cesari, M.; Tinetti, M.E.; Valderas, J.M. Evidence supporting the best clinical management of patients with multimorbidity and polypharmacy: A systematic guideline review and expert consensus. J. Intern. Med. 2019, 285, 272–288. [Google Scholar] [CrossRef]
- Kok, R.M.; Reynolds, C.F. Management of Depression in Older Adults: A Review. JAMA 2017, 317, 2114–2122. [Google Scholar] [CrossRef]
- Huiskes, V.J.; Burger, D.M.; van den Ende, C.H.; van den Bemt, B.J. Effectiveness of medication review: A systematic review and meta-analysis of randomized controlled trials. BMC Fam. Pract. 2017, 18, 5. [Google Scholar] [CrossRef]
- Ulley, J.; Harrop, D.; Ali, A.; Alton, S.; Fowler Davis, S. Deprescribing interventions and their impact on medication adherence in community-dwelling older adults with polypharmacy: A systematic review. BMC Geriatr. 2019, 19, 15. [Google Scholar] [CrossRef]
- Storms, H.; Marquet, K.; Aertgeerts, B.; Claes, N. Prevalence of inappropriate medication use in residential long-term care facilities for the elderly: A systematic review. Eur. J. Gen. Pract. 2017, 23, 69–77. [Google Scholar] [CrossRef]
- Mohamed, M.R.; Ramsdale, E.; Loh, K.P.; Arastu, A.; Xu, H.; Obrecht, S.; Castillo, D.; Sharma, M.; Holmes, H.M.; Nightingale, G.; et al. Associations of Polypharmacy and Inappropriate Medications with Adverse Outcomes in Older Adults with Cancer: A Systematic Review and Meta-Analysis. Oncologist 2020, 25, e94–e108. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.J.; Elliott, R.A.; Petrie, K.; Kuruvilla, L.; George, J. Interventions for improving medication-taking ability and adherence in older adults prescribed multiple medications. Cochrane Database Syst. Rev. 2020, 2020, CD012419. [Google Scholar] [CrossRef]
- Palmer, K.; Villani, E.R.; Vetrano, D.L.; Cherubini, A.; Cruz-Jentoft, A.J.; Curtin, D.; Denkinger, M.; Gutiérrez-Valencia, M.; Guðmundsson, A.; Knol, W.; et al. Association of polypharmacy and hyperpolypharmacy with frailty states: A systematic review and meta-analysis. Eur. Geriatr. Med. 2019, 10, 9–36. [Google Scholar] [CrossRef] [PubMed]
- Muhlack, D.C.; Hoppe, L.K.; Weberpals, J.; Brenner, H.; Schöttker, B. The Association of Potentially Inappropriate Medication at Older Age With Cardiovascular Events and Overall Mortality: A Systematic Review and Meta-Analysis of Cohort Studies. J. Am. Med. Dir. Assoc. 2017, 18, 211–220. [Google Scholar] [CrossRef]
- Hasan Ibrahim, A.S.; Barry, H.E.; Hughes, C.M. A systematic review of general practice-based pharmacists’ services to optimize medicines management in older people with multimorbidity and polypharmacy. Fam. Pract. 2021, 38, 509–523. [Google Scholar] [CrossRef]
- Kua, C.H.; Mak, V.S.L.; Huey Lee, S.W. Health Outcomes of Deprescribing Interventions Among Older Residents in Nursing Homes: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2019, 20, 362–372.e11. [Google Scholar] [CrossRef]
- Ibrahim, K.; Cox, N.J.; Stevenson, J.M.; Lim, S.; Fraser, S.D.S.; Roberts, H.C. A systematic review of the evidence for deprescribing interventions among older people living with frailty. BMC Geriatr. 2021, 21, 258. [Google Scholar] [CrossRef]
- Chang, C.T.; Ang, J.Y.; Islam, M.A.; Chan, H.K.; Cheah, W.K.; Gan, S.H. Prevalence of Drug-Related Problems and Complementary and Alternative Medicine Use in Malaysia: A Systematic Review and Meta-Analysis of 37,249 Older Adults. Pharmaceuticals 2021, 14, 187. [Google Scholar] [CrossRef]
- Suárez, M.; Boqué, N.; Del Bas, J.M.; Mayneris-Perxachs, J.; Arola, L.; Caimari, A. Mediterranean Diet and Multi-Ingredient-Based Interventions for the Management of Non-Alcoholic Fatty Liver Disease. Nutrients 2017, 9, 1052. [Google Scholar] [CrossRef]
- Laur, C.V.; McNicholl, T.; Valaitis, R.; Keller, H.H. Malnutrition or frailty? Overlap and evidence gaps in the diagnosis and treatment of frailty and malnutrition. Appl. Physiol. Nutr. Metab. 2017, 42, 449–458. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Gahche, J.J.; Weiler, M.; Arensberg, M.B. Screening Community-Living Older Adults for Protein Energy Malnutrition and Frailty: Update and Next Steps. J. Community Health 2020, 45, 640–660. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S. Protein-energy malnutrition in the rehabilitation setting: Evidence to improve identification. Maturitas 2016, 86, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zupo, R.; Castellana, F.; Bortone, I.; Griseta, C.; Sardone, R.; Lampignano, L.; Lozupone, M.; Solfrizzi, V.; Castellana, M.; Giannelli, G.; et al. Nutritional domains in frailty tools: Working towards an operational definition of nutritional frailty. Ageing Res. Rev. 2020, 64, 101148. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; Quattrocchi, A.; Degrassi, F.; Catalano, F.; Basile, G.; Agodi, A. The Effects of Diet and Dietary Interventions on the Quality of Life among Breast Cancer Survivors: A Cross-Sectional Analysis and a Systematic Review of Experimental Studies. Cancers 2020, 12, 322. [Google Scholar] [CrossRef] [PubMed]
- Oktaviana, J.; Zanker, J.; Vogrin, S.; Duque, G. The effect of protein supplements on functional frailty in older persons: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2020, 86, 103938. [Google Scholar] [CrossRef]
- Robertson, D.A.; Savva, G.M.; Kenny, R.A. Frailty and cognitive impairment--a review of the evidence and causal mechanisms. Ageing Res. Rev. 2013, 12, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.J.; Morrison, L.; Ibrahim, K.; Robinson, S.M.; Sayer, A.A.; Roberts, H.C. New horizons in appetite and the anorexia of ageing. Age Ageing 2020, 49, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Jadczak, A.D.; Visvanathan, R. Anorexia of Aging—An Updated Short Review. J. Nutr. Health Aging 2019, 23, 306–309. [Google Scholar] [CrossRef]
- Fernández-Pombo, A.; Rodríguez-Carnero, G.; Castro, A.I.; Cantón-Blanco, A.; Seoane, L.M.; Casanueva, F.F.; Crujeiras, A.B.; Martínez-Olmos, M.A. Relevance of nutritional assessment and treatment to counteract cardiac cachexia and sarcopenia in chronic heart failure. Clin. Nutr. 2021, 40, 5141–5155. [Google Scholar] [CrossRef]
- Ruiz, M.; Kamerman, L.A. Nutritional screening tools for HIV-infected patients: Implications for elderly patients. J. Int. Assoc. Physicians AIDS Care 2010, 9, 362–367. [Google Scholar] [CrossRef]
- Kuzuya, M. Nutritional status related to poor health outcomes in older people: Which is better, obese or lean? Geriatr. Gerontol. Int. 2021, 21, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Giannitsi, S.; Bougiakli, M.; Bechlioulis, A.; Kotsia, A.; Michalis, L.K.; Naka, K.K. 6-minute walking test: A useful tool in the management of heart failure patients. Ther. Adv. Cardiovasc. Dis. 2019, 13, 1753944719870084. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, G.; Russo, F.; De Salvatore, S.; Cortina, G.; Albo, E.; Papalia, R.; Denaro, V. Physical Activity for the Treatment of Chronic Low Back Pain in Elderly Patients: A Systematic Review. J. Clin. Med. 2020, 9, 1023. [Google Scholar] [CrossRef] [PubMed]
- Tamuleviciute-Prasciene, E.; Drulyte, K.; Jurenaite, G.; Kubilius, R.; Bjarnason-Wehrens, B. Frailty and Exercise Training: How to Provide Best Care after Cardiac Surgery or Intervention for Elder Patients with Valvular Heart Disease. BioMed Res. Int. 2018, 2018, 9849475. [Google Scholar] [CrossRef] [PubMed]
- Lynch, E.A.; Jones, T.M.; Simpson, D.B.; Fini, N.A.; Kuys, S.S.; Borschmann, K.; Kramer, S.; Johnson, L.; Callisaya, M.L.; Mahendran, N.; et al. Activity monitors for increasing physical activity in adult stroke survivors. Cochrane Database Syst. Rev. 2018, 7, CD012543. [Google Scholar] [CrossRef]
- McGettigan, M.; Cardwell, C.R.; Cantwell, M.M.; Tully, M.A. Physical activity interventions for disease-related physical and mental health during and following treatment in people with non-advanced colorectal cancer. Cochrane Database Syst. Rev. 2020, 5, CD012864. [Google Scholar] [CrossRef][Green Version]
- Gordt, K.; Gerhardy, T.; Najafi, B.; Schwenk, M. Effects of Wearable Sensor-Based Balance and Gait Training on Balance, Gait, and Functional Performance in Healthy and Patient Populations: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Gerontology 2018, 64, 74–89. [Google Scholar] [CrossRef]
- E, J.Y.; Li, T.; McInally, L.; Thomson, K.; Shahani, U.; Gray, L.; Howe, T.E.; Skelton, D.A. Environmental and behavioural interventions for reducing physical activity limitation and preventing falls in older people with visual impairment. Cochrane Database Syst. Rev. 2020, 9, CD009233. [Google Scholar] [CrossRef]
- Schwenk, M.; Howe, C.; Saleh, A.; Mohler, J.; Grewal, G.; Armstrong, D.; Najafi, B. Frailty and technology: A systematic review of gait analysis in those with frailty. Gerontology 2014, 60, 79–89. [Google Scholar] [CrossRef]
- Albrecht, M.; Koolhaas, C.M.; Schoufour, J.D.; van Rooij, F.J.; Kavousi, M.; Ikram, M.A.; Franco, O.H. Physical activity types and atrial fibrillation risk in the middle- aged and elderly: The Rotterdam Study. Eur. J. Prev. Cardiol. 2018, 25, 1316–1323. [Google Scholar] [CrossRef]
- Sattler, M.C.; Jaunig, J.; Tösch, C.; Watson, E.D.; Mokkink, L.B.; Dietz, P.; van Poppel, M.N.M. Current Evidence of Measurement Properties of Physical Activity Questionnaires for Older Adults: An Updated Systematic Review. Sports Med. 2020, 50, 1271–1315. [Google Scholar] [CrossRef] [PubMed]
- Karpman, C.; Benzo, R. Gait speed as a measure of functional status in COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 1315–1320. [Google Scholar] [CrossRef]
- Vialleron, T.; Delafontaine, A.; Ditcharles, S.; Fourcade, P.; Yiou, E. Effects of stretching exercises on human gait: A systematic review and meta-analysis. F1000Research 2020, 9, 984. [Google Scholar] [CrossRef]
- Aquilani, R.; D’Antona, G.; Baiardi, P.; Gambino, A.; Iadarola, P.; Viglio, S.; Pasini, E.; Verri, M.; Barbieri, A.; Boschi, F. Essential amino acids and exercise tolerance in elderly muscle-depleted subjects with chronic diseases: A rehabilitation without rehabilitation? BioMed Res. Int. 2014, 2014, 341603. [Google Scholar] [CrossRef] [PubMed]
- Berner, K.; Morris, L.; Baumeister, J.; Louw, Q. Objective impairments of gait and balance in adults living with HIV-1 infection: A systematic review and meta- analysis of observational studies. BMC Musculoskelet. Disord. 2017, 18, 325. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Liu, J.; Tang, M.; Wang, D.; Ma, X. Effects of Tai Chi exercise on improving walking function and posture control in elderly patients with knee osteoarthritis: A systematic review and meta-analysis. Medicine 2021, 100, e25655. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Poggiogalle, E.; Mosca, V.; Pinto, A.; Migliaccio, S.; Brunani, A.; Capodaglio, P. Critical review of the equations predicting 6-minute walking distance in obese subjects. Monaldi Arch. Chest Dis. 2016, 81, 745. [Google Scholar] [CrossRef][Green Version]
- Cheng, D.K.; Dagenais, M.; Alsbury-Nealy, K.; Legasto, J.M.; Scodras, S.; Aravind, G.; Takhar, P.; Salbach, N.M. Distance-limited walk tests post-stroke: A systematic review of measurement properties. NeuroRehabilitation 2021, 48, 413–439. [Google Scholar] [CrossRef]
- Argillander, T.E.; Heil, T.C.; Melis, R.J.F.; van Duijvendijk, P.; Klaase, J.M.; van Munster, B.C. Preoperative physical performance as predictor of postoperative outcomes in patients aged 65 and older scheduled for major abdominal cancer surgery: A systematic review. Eur. J. Surg. Oncol. 2021, 48, 570–581. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Ju, C.; Sun, Z.; Yin, H.; Zügel, M.; Otto, S.; Steinacker, J.M.; Schumann, U. Step Counter Use and Sedentary Time in Adults: A Meta-Analysis. Medicine 2015, 94, e1412. [Google Scholar] [CrossRef]
- Daami, M.; Latiri, I.; Rouatbi, S.; Sfaxi, R.; Ben Saad, H. 6-min walk-distance norms in adults Arab populations: A literature review. Tunis. Med. 2017, 95, 743–755. [Google Scholar] [PubMed]
- Keating, C.J.; Cabrera-Linares, J.C.; Párraga-Montilla, J.A.; Latorre-Román, P.A.; Del Castillo, R.M.; García-Pinillos, F. Influence of Resistance Training on Gait & Balance Parameters in Older Adults: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 1759. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, D.; Hale, M.; Todd, O.; Zaman, H.; Marques, I.; Petty, D.; Alldred, D.P.; Johnson, O.; Faisal, M.; Gardner, P.; et al. Associations Between Anticholinergic Medication Exposure and Adverse Health Outcomes in Older People with Frailty: A Systematic Review and Meta-analysis. Drugs-Real World Outcomes 2021, 8, 431–458. [Google Scholar] [CrossRef] [PubMed]
- Vavasour, G.; Giggins, O.M.; Doyle, J.; Kelly, D. How wearable sensors have been utilised to evaluate frailty in older adults: A systematic review. J. Neuroeng. Rehabil. 2021, 18, 112. [Google Scholar] [CrossRef]
- Pu, C.Y.; Batarseh, H.; Zafron, M.L.; Mador, M.J.; Yendamuri, S.; Ray, A.D. Effects of Preoperative Breathing Exercise on Postoperative Outcomes for Patients With Lung Cancer Undergoing Curative Intent Lung Resection: A Meta-analysis. Arch. Phys. Med. Rehabil. 2021, 102, 2416–2427.e4. [Google Scholar] [CrossRef]
- Baritello, O.; Salzwedel, A.; Sündermann, S.H.; Niebauer, J.; Völler, H. The Pandora’s Box of Frailty Assessments: Which Is the Best for Clinical Purposes in TAVI Patients? A Critical Review. J. Clin. Med. 2021, 10, 4506. [Google Scholar] [CrossRef]
- Anabitarte-García, F.; Reyes-González, L.; Rodríguez-Cobo, L.; Fernández-Viadero, C.; Somonte-Segares, S.; Díez-Del-Valle, S.; Mandaluniz, E.; García-García, R.; López-Higuera, J.M. Early diagnosis of frailty: Technological and non-intrusive devices for clinical detection. Ageing Res. Rev. 2021, 70, 101399. [Google Scholar] [CrossRef]
- Abou, L.; Peters, J.; Wong, E.; Akers, R.; Dossou, M.S.; Sosnoff, J.J.; Rice, L.A. Gait and Balance Assessments using Smartphone Applications in Parkinson’s Disease: A Systematic Review. J. Med. Syst. 2021, 45, 87. [Google Scholar] [CrossRef]
- Elena, P.; Demetris, S.; Christina, M.; Marios, P. Differences Between Exergaming Rehabilitation and Conventional Physiotherapy on Quality of Life in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 683385. [Google Scholar] [CrossRef]
- Mañas, A.; Gómez-Redondo, P.; Valenzuela, P.L.; Morales, J.S.; Lucía, A.; Ara, I. Unsupervised home-based resistance training for community-dwelling older adults: A systematic review and meta-analysis of randomized controlled trials. Ageing Res. Rev. 2021, 69, 101368. [Google Scholar] [CrossRef]
- Yoo, J.; Ruppar, T.; Wilbur, J.; Miller, A.; Westrick, J.C. Effects of Home-Based Exercise on Frailty in Patients With End-Stage Renal Disease: Systematic Review. Biol. Res. Nurs. 2022, 24, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Pitsillides, A.; Stasinopoulos, D.; Mamais, I. Blood flow restriction training in patients with knee osteoarthritis: Systematic review of randomized controlled trials. J. Bodyw. Mov. Ther. 2021, 27, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Van der Kruk, E.; Silverman, A.K.; Reilly, P.; Bull, A.M.J. Compensation due to age-related decline in sit-to-stand and sit-to-walk. J. Biomech. 2021, 122, 110411. [Google Scholar] [CrossRef] [PubMed]
- Williams, F.R.; Milliken, D.; Lai, J.C.; Armstrong, M.J. Assessment of the Frail Patient With End-Stage Liver Disease: A Practical Overview of Sarcopenia, Physical Function, and Disability. Hepatol. Commun. 2021, 5, 923–937. [Google Scholar] [CrossRef]
- Bortone, I.; Sardone, R.; Lampignano, L.; Castellana, F.; Zupo, R.; Lozupone, M.; Moretti, B.; Giannelli, G.; Panza, F. How gait influences frailty models and health-related outcomes in clinical-based and population-based studies: A systematic review. J. Cachex-Sarcopenia Muscle 2021, 12, 274–297. [Google Scholar] [CrossRef]
- Inoue, T.; Maeda, K.; Nagano, A.; Shimizu, A.; Ueshima, J.; Murotani, K.; Sato, K.; Hotta, K.; Morishita, S.; Tsubaki, A. Related Factors and Clinical Outcomes of Osteosarcopenia: A Narrative Review. Nutrients 2021, 13, 291. [Google Scholar] [CrossRef]
- Yang, F.; Lees, J.; Simpkins, C.; Butler, A. Interventions for preventing falls in people post-stroke: A meta-analysis of randomized controlled trials. Gait Posture 2021, 84, 377–388. [Google Scholar] [CrossRef]
- Gielen, E.; Beckwée, D.; Delaere, A.; De Breucker, S.; Vandewoude, M.; Bautmans, I.; Sarcopenia Guidelines Development Group of the Belgian Society of Gerontology and Geriatrics (BSGG). Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: An umbrella review of systematic reviews and meta-analyses. Nutr. Rev. 2021, 79, 121–147. [Google Scholar] [CrossRef]
- Gafner, S.C.; Allet, L.; Hilfiker, R.; Bastiaenen, C.H.G. Reliability and Diagnostic Accuracy of Commonly Used Performance Tests Relative to Fall History in Older Persons: A Systematic Review. Clin. Interv. Aging 2021, 16, 1591–1616. [Google Scholar] [CrossRef]
- McMillan, J.M.; Michalchuk, Q.; Goodarzi, Z. Frailty in Parkinson’s disease: A systematic review and meta-analysis. Clin. Park. Relat. Disord. 2021, 4, 100095. [Google Scholar] [CrossRef]
- Ponciano, V.; Pires, I.M.; Ribeiro, F.R.; Spinsante, S. Sensors are Capable to Help in the Measurement of the Results of the Timed-Up and Go Test? A Systematic Review. J. Med. Syst. 2020, 44, 199. [Google Scholar] [CrossRef] [PubMed]
- Lund, C.M.; Dolin, T.G.; Mikkelsen, M.K.; Juhl, C.B.; Vinther, A.; Nielsen, D.L. Effect of Exercise on Physical Function and Psychological Well-being in Older Patients With Colorectal Cancer Receiving Chemotherapy—A Systematic Review. Clin. Color. Cancer 2020, 19, e243–e257. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, G.; Wang, X.; Yin, H.; Jia, Y.; Leng, M.; Li, H.; Chen, L. Effect of exergames on physical outcomes in frail elderly: A systematic review. Aging Clin. Exp. Res. 2020, 32, 2187–2200. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Bao, D.; Manor, B.; Zhou, J. The Effects of Transcranial Direct Current Stimulation (tDCS) on Balance Control in Older Adults: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2020, 12, 275. [Google Scholar] [CrossRef]
- Byra, J.; Czernicki, K. The Effectiveness of Virtual Reality Rehabilitation in Patients with Knee and Hip Osteoarthritis. J. Clin. Med. 2020, 9, 2639. [Google Scholar] [CrossRef]
- Zampogna, B.; Papalia, R.; Papalia, G.F.; Campi, S.; Vasta, S.; Vorini, F.; Fossati, C.; Torre, G.; Denaro, V. The Role of Physical Activity as Conservative Treatment for Hip and Knee Osteoarthritis in Older People: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 1167. [Google Scholar] [CrossRef]
- Wang, D.X.M.; Yao, J.; Zirek, Y.; Reijnierse, E.M.; Maier, A.B. Muscle mass, strength, and physical performance predicting activities of daily living: A meta-analysis. J. Cachex-Sarcopenia Muscle 2020, 11, 3–25. [Google Scholar] [CrossRef]
- Chen, M.Y.; Orkaby, A.R.; Rosenberg, M.A.; Driver, J.A. Frailty, Implantable Cardioverter Defibrillators, and Mortality: A Systematic Review. J. Gen. Intern. Med. 2019, 34, 2224–2231. [Google Scholar] [CrossRef]
- Pei, G.; Tang, Y.; Tan, L.; Tan, J.; Ge, L.; Qin, W. Aerobic exercise in adults with chronic kidney disease (CKD): A meta-analysis. Int. Urol. Nephrol. 2019, 51, 1787–1795. [Google Scholar] [CrossRef]
- Bet, P.; Castro, P.C.; Ponti, M.A. Fall detection and fall risk assessment in older person using wearable sensors: A systematic review. Int. J. Med. Inform. 2019, 130, 103946. [Google Scholar] [CrossRef]
- Granic, A.; Sayer, A.A.; Robinson, S.M. Dietary Patterns, Skeletal Muscle Health, and Sarcopenia in Older Adults. Nutrients 2019, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Facal, D.; Maseda, A.; Pereiro, A.X.; Gandoy-Crego, M.; Lorenzo-López, L.; Yanguas, J.; Millán-Calenti, J.C. Cognitive frailty: A conceptual systematic review and an operational proposal for future research. Maturitas 2019, 121, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Mol, A.; Reijnierse, E.M.; Bui Hoang, P.T.S.; van Wezel, R.J.A.; Meskers, C.G.M.; Maier, A.B. Orthostatic hypotension and physical functioning in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2018, 48, 122–144. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Baldwin, C. Oral nutritional support with or without exercise in the management of malnutrition in nutritionally vulnerable older people: A systematic review and meta-analysis. Clin. Nutr. 2018, 37 Pt A, 1879–1891. [Google Scholar] [CrossRef]
- Liao, C.-D.; Lee, P.-H.; Hsiao, D.-J.; Huang, S.-W.; Tsauo, J.-Y.; Chen, H.-C.; Liou, T.-H. Effects of Protein Supplementation Combined with Exercise Intervention on Frailty Indices, Body Composition, and Physical Function in Frail Older Adults. Nutrients 2018, 10, 1916. [Google Scholar] [CrossRef]
- Doma, K.; Grant, A.; Morris, J. The Effects of Balance Training on Balance Performance and Functional Outcome Measures Following Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 2367–2385. [Google Scholar] [CrossRef]
- Lopez, P.; Pinto, R.S.; Radaelli, R.; Rech, A.; Grazioli, R.; Izquierdo, M.; Cadore, E.L. Benefits of resistance training in physically frail elderly: A systematic review. Aging Clin. Exp. Res. 2018, 30, 889–899. [Google Scholar] [CrossRef]
- Dewansingh, P.; Melse-Boonstra, A.; Krijnen, W.P.; van der Schans, C.P.; Jager-Wittenaar, H.; van den Heuvel, E.G.H.M. Supplemental protein from dairy products increases body weight and vitamin D improves physical performance in older adults: A systematic review and meta-analysis. Nutr. Res. 2018, 49, 1–22. [Google Scholar] [CrossRef]
- Luo, D.; Lin, Z.; Li, S.; Liu, S.J. Effect of nutritional supplement combined with exercise intervention on sarcopenia in the elderly: A meta-analysis. Int. J. Nurs. Sci. 2017, 4, 389–401. [Google Scholar] [CrossRef]
- Apóstolo, J.; Cooke, R.; Bobrowicz-Campos, E.; Santana, S.; Marcucci, M.; Cano, A.; Vollenbroek-Hutten, M.; Germini, F.; Holland, C. Predicting risk and outcomes for frail older adults: An umbrella review of frailty screening tools. JBI Évid. Synth. 2017, 15, 1154–1208. [Google Scholar] [CrossRef]
- Wouters, H.; van der Meer, H.; Taxis, K. Quantification of anticholinergic and sedative drug load with the Drug Burden Index: A review of outcomes and methodological quality of studies. Eur. J. Clin. Pharmacol. 2017, 73, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Van Deudekom, F.J.; Schimberg, A.S.; Kallenberg, M.H.; Slingerland, M.; van der Velden, L.A.; Mooijaart, S.P. Functional and cognitive impairment, social environment, frailty and adverse health outcomes in older patients with head and neck cancer, a systematic review. Oral Oncol. 2017, 64, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Bahureksa, L.; Najafi, B.; Saleh, A.; Sabbagh, M.; Coon, D.; Mohler, M.J.; Schwenk, M. The Impact of Mild Cognitive Impairment on Gait and Balance: A Systematic Review and Meta-Analysis of Studies Using Instrumented Assessment. Gerontology 2017, 63, 67–83. [Google Scholar] [CrossRef] [PubMed]
- McDonald, V.S.; Thompson, K.A.; Lewis, P.R.; Sise, C.B.; Sise, M.J.; Shackford, S.R. Frailty in trauma: A systematic review of the surgical literature for clinical assessment tools. J. Trauma Acute Care Surg. 2016, 80, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Bullo, V.; Bergamin, M.; Gobbo, S.; Sieverdes, J.C.; Zaccaria, M.; Neunhaeuserer, D.; Ermolao, A. The effects of Pilates exercise training on physical fitness and wellbeing in the elderly: A systematic review for future exercise prescription. Prev. Med. 2015, 75, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Tanemoto, K. Frailty in cardiothoracic surgery: Systematic review of the literature. Gen. Thorac. Cardiovasc. Surg. 2015, 63, 425–433. [Google Scholar] [CrossRef]
- McNeely, M.E.; Duncan, R.P.; Earhart, G.M. A comparison of dance interventions in people with Parkinson disease and older adults. Maturitas 2015, 81, 10–16. [Google Scholar] [CrossRef]
- Hill, K.D.; Hunter, S.W.; Batchelor, F.A.; Cavalheri, V.; Burton, E. Individualized home-based exercise programs for older people to reduce falls and improve physical performance: A systematic review and meta-analysis. Maturitas 2015, 82, 72–84. [Google Scholar] [CrossRef]
- Pamoukdjian, F.; Paillaud, E.; Zelek, L.; Laurent, M.; Lévy, V.; Landre, T.; Sebbane, G. Measurement of gait speed in older adults to identify complications associated with frailty: A systematic review. J. Geriatr. Oncol. 2015, 6, 484–496. [Google Scholar] [CrossRef]
- Stehr, M.D.; von Lengerke, T. Preventing weight gain through exercise and physical activity in the elderly: A systematic review. Maturitas 2012, 72, 13–22. [Google Scholar] [CrossRef]
- Chou, C.H.; Hwang, C.L.; Wu, Y.T. Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: A meta-analysis. Arch. Phys. Med. Rehabil. 2012, 93, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-H.; Lee, Y.-S.; Chan, D.-C. Interventions targeting geriatric frailty: A systemic review. J. Clin. Gerontol. Geriatr. 2012, 3, 47–52. [Google Scholar] [CrossRef][Green Version]
- Den Ouden, M.E.; Schuurmans, M.J.; Arts, I.E.; van der Schouw, Y.T. Physical performance characteristics related to disability in older persons: A systematic review. Maturitas 2011, 69, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.D.; Guo, L.; Kang, D.; Xiong, S. Exergame technology and interactive interventions for elderly fall prevention: A systematic literature review. Appl. Ergon. 2017, 65, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, D.; Kumar, A.; Carpenter, H.; Zijlstra, G.A.; Skelton, D.A.; Cook, J.R.; Stevens, Z.; Belcher, C.M.; Haworth, D.; Gawler, S.J.; et al. Exercise for reducing fear of falling in older people living in the community. Cochrane Database Syst. Rev. 2014, 2014, CD009848. [Google Scholar] [CrossRef] [PubMed]
- Papalia, G.F.; Papalia, R.; Diaz Balzani, L.A.; Torre, G.; Zampogna, B.; Vasta, S.; Fossati, C.; Alifano, A.M.; Denaro, V. The Effects of Physical Exercise on Balance and Prevention of Falls in Older People: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2595. [Google Scholar] [CrossRef]
- De Coninck, L.; Bekkering, G.E.; Bouckaert, L.; Declercq, A.; Graff, M.J.L.; Aertgeerts, B. Home- and Community-Based Occupational Therapy Improves Functioning in Frail Older People: A Systematic Review. J. Am. Geriatr. Soc. 2017, 65, 1863–1869. [Google Scholar] [CrossRef]
- Nunan, S.; Brown Wilson, C.; Henwood, T.; Parker, D. Fall risk assessment tools for use among older adults in long-term care settings: A systematic review of the literature. Australas. J. Ageing 2018, 37, 23–33. [Google Scholar] [CrossRef]
- Kumar, A.; Delbaere, K.; Zijlstra, G.A.; Carpenter, H.; Iliffe, S.; Masud, T.; Skelton, D.; Morris, R.; Kendrick, D. Exercise for reducing fear of falling in older people living in the community: Cochrane systematic review and meta-analysis. Age Ageing 2016, 45, 345–352. [Google Scholar] [CrossRef]
- Liau, S.J.; Lalic, S.; Visvanathan, R.; Dowd, L.A.; Bell, J.S. The FRAIL-NH Scale: Systematic Review of the Use, Validity and Adaptations for Frailty Screening in Nursing Homes. J. Nutr. Health Aging 2021, 25, 1205–1216. [Google Scholar] [CrossRef]
- Zhang, J.; Dennison, E.; Prieto-Alhambra, D. Osteoporosis epidemiology using international cohorts. Curr. Opin. Rheumatol. 2020, 32, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Strini, V.; Schiavolin, R.; Prendin, A. Fall Risk Assessment Scales: A Systematic Literature Review. Nurs. Rep. 2021, 11, 430–443. [Google Scholar] [CrossRef]
- Zengin, A.; Maple-Brown, L.J.; Brennan-Olsen, S.; Center, J.R.; Eades, S.; Ebeling, P.R. Musculoskeletal health of Indigenous Australians. Arch. Osteoporos. 2018, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Geller, A.I.; Strasser, D.C. Analytical review: Focus on fall screening assessments. PM&R 2013, 5, 609–621. [Google Scholar] [CrossRef]
- Romli, M.H.; Tan, M.P.; Mackenzie, L.; Lovarini, M.; Suttanon, P.; Clemson, L. Falls amongst older people in Southeast Asia: A scoping review. Public Health 2017, 145, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Williams-Roberts, H.; Arnold, C.; Kemp, D.; Crizzle, A.; Johnson, S. Scoping Review of Clinical Practice Guidelines for Fall Risk Screening and Assessment in Older Adults across the Care Continuum. Can. J. Aging 2021, 40, 206–223. [Google Scholar] [CrossRef]
- Omaña, H.; Bezaire, K.; Brady, K.; Davies, J.; Louwagie, N.; Power, S.; Santin, S.; Hunter, S.W. Functional Reach Test, Single-Leg Stance Test, and Tinetti Performance-Oriented Mobility Assessment for the Prediction of Falls in Older Adults: A Systematic Review. Phys. Ther. 2021, 101, pzab173. [Google Scholar] [CrossRef]
- Wiseman, T.; Betihavas, V. The association between unexplained falls and cardiac arrhythmias: A scoping literature review. Aust. Crit. Care 2019, 32, 434–441. [Google Scholar] [CrossRef]
- Vilaca, T.; Salam, S.; Schini, M.; Harnan, S.; Sutton, A.; Poku, E.; Allen, I.E.; Cummings, S.R.; Eastell, R. Risks of Hip and Nonvertebral Fractures in Patients With CKD G3a-G5D: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2020, 76, 521–532. [Google Scholar] [CrossRef]
- Howcroft, J.; Kofman, J.; Lemaire, E.D. Review of fall risk assessment in geriatric populations using inertial sensors. J. Neuroeng. Rehabil. 2013, 10, 91. [Google Scholar] [CrossRef]
- Ciorba, A.; Bianchini, C.; Scanelli, G.; Pala, M.; Zurlo, A.; Aimoni, C. The impact of dizziness on quality-of-life in the elderly. Eur. Arch. Otorhinolaryngol. 2017, 274, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Lenouvel, E.; Novak, L.; Biedermann, A.; Kressig, R.W.; Klöppel, S. Preventive treatment options for fear of falling within the Swiss healthcare system: A position paper. Z. Gerontol. Geriatr. 2021, 55, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.; Robinson, K.; Logan, P.; Gordon, A.L.; Harwood, R.H.; Masud, T. Chair-based exercises for frail older people: A systematic review. BioMed Res. Int. 2013, 2013, 309506. [Google Scholar] [CrossRef]
- Aranda-Gallardo, M.; Morales-Asencio, J.M.; Canca-Sanchez, J.C.; Barrero-Sojo, S.; Perez-Jimenez, C.; Morales-Fernandez, A.; de Luna-Rodriguez, M.E.; Moya-Suarez, A.B.; Mora-Banderas, A.M. Instruments for assessing the risk of falls in acute hospitalized patients: A systematic review and meta-analysis. BMC Health Serv. Res. 2013, 13, 122. [Google Scholar] [CrossRef]
- Reeve, E.; Jordan, V.; Thompson, W.; Sawan, M.; Todd, A.; Gammie, T.M.; Hopper, I.; Hilmer, S.N.; Gnjidic, D. Withdrawal of antihypertensive drugs in older people. Cochrane Database Syst. Rev. 2020, 6, CD012572. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Z.; Song, Y. Falls in aged people of the Chinese mainland: Epidemiology, risk factors and clinical strategies. Ageing Res. Rev. 2010, 9, S13–S17. [Google Scholar] [CrossRef] [PubMed]
- Bahat, G.; Catikkas, N.M.; Yavuz, D.G.; Borman, P.; Guzel, R.; Reginster, J.Y. The current situation in the approach to osteoporosis in older adults in Turkey: Areas in need of improvement with a model for other populations. Arch. Osteoporos. 2021, 16, 179. [Google Scholar] [CrossRef]
- Senderovich, H.; Bayeva, N.; Montagnese, B.; Yendamuri, A. Managing Fall Prevention through Exercise in Older Adults Afflicted by Cognitive and Strength Impairment. Dement. Geriatr. Cogn. Disord. 2021, 50, 507–518. [Google Scholar] [CrossRef]
- Kruisbrink, M.; Crutzen, R.; Kempen, G.I.J.M.; Delbaere, K.; Ambergen, T.; Cheung, K.L.; Kendrick, D.; Iliffe, S.; Zijlstra, G.A.R. Disentangling interventions to reduce fear of falling in community-dwelling older people: A systematic review and meta-analysis of intervention components. Disabil. Rehabil. 2021, 44, 6247–6257. [Google Scholar] [CrossRef]
- Lim, S.E.R.; Cox, N.J.; Tan, Q.Y.; Ibrahim, K.; Roberts, H.C. Volunteer-led physical activity interventions to improve health outcomes for community-dwelling older people: A systematic review. Aging Clin. Exp. Res. 2021, 33, 843–853. [Google Scholar] [CrossRef]
- Sattar, S.; Kenis, C.; Haase, K.; Burhenn, P.; Stolz-Baskett, P.; Milisen, K.; Ayala, A.P.; Puts, M.T.E. Falls in older patients with cancer: Nursing and Allied Health Group of International Society of Geriatric Oncology review paper. J. Geriatr. Oncol. 2020, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kruisbrink, M.; Delbaere, K.; Kempen, G.I.J.M.; Crutzen, R.; Ambergen, T.; Cheung, K.L.; Kendrick, D.; Iliffe, S.; Zijlstra, G.A.R. Intervention Characteristics Associated With a Reduction in Fear of Falling Among Community-Dwelling Older People: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Gerontologist 2021, 61, e269–e282. [Google Scholar] [CrossRef] [PubMed]
- Stockwell-Smith, G.; Adeleye, A.; Chaboyer, W.; Cooke, M.; Phelan, M.; Todd, J.A.; Grealish, L. Interventions to prevent in-hospital falls in older people with cognitive impairment for further research: A mixed studies review. J. Clin. Nurs. 2020, 29, 3445–3460. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.; Fleury, J. External validity of physical activity interventions for community-dwelling older adults with fall risk: A quantitative systematic literature review. J. Adv. Nurs. 2012, 68, 2140–2154. [Google Scholar] [CrossRef]
- Gaspar, A.G.M.; Lapão, L.V. eHealth for Addressing Balance Disorders in the Elderly: Systematic Review. J. Med. Internet Res. 2021, 23, e22215. [Google Scholar] [CrossRef]
- Hill, A.-M.; Etherton-Beer, C.; McPhail, S.M.; Morris, M.; Flicker, L.; Shorr, R.; Bulsara, M.; Lee, D.-C.; Francis-Coad, J.; Waldron, N.; et al. Reducing falls after hospital discharge: A protocol for a randomised controlled trial evaluating an individualised multimodal falls education programme for older adults. BMJ Open 2017, 7, e013931. [Google Scholar] [CrossRef]
- Marques-Vieira CM, A.; Sousa LM, M.; Severino, S.; Sousa, L.; Caldeira, S. Cross-cultural validation of the falls efficacy scale international in elderly: Systematic literature review. J. Clin. Gerontol. Geriatr. 2016, 7, 72–76. [Google Scholar] [CrossRef]
- Visschedijk, J.; Achterberg, W.; Van Balen, R.; Hertogh, C. Fear of falling after hip fracture: A systematic review of measurement instruments, prevalence, interventions, and related factors. J. Am. Geriatr. Soc. 2010, 58, 1739–1748. [Google Scholar] [CrossRef]
- Caçador, C.; Teixeira-Lemos, E.; Martins, S.O.; Ramos, F. The Role of Nutritional Status on Polypharmacy, Cognition, and Functional Capacity of Institutionalized Elderly: A Systematic Review. Nutrients 2021, 13, 3477. [Google Scholar] [CrossRef]
- Van Aalst, F.M.; Verwijmeren, L.; van Dongen, E.P.A.; de Vries, J.P.M.; de Groot, E.; Noordzij, P.G. Frailty and functional outcomes after open and endovascular procedures for patients with peripheral arterial disease: A systematic review. J. Vasc. Surg. 2019, 71, 297–306.e1. [Google Scholar] [CrossRef]
- Borges, M.K.; Canevelli, M.; Cesari, M.; Aprahamian, I. Frailty as a Predictor of Cognitive Disorders: A Systematic Review and Meta-Analysis. Front. Med. 2019, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Lozupone, M.; Solfrizzi, V.; Sardone, R.; Dibello, V.; Di Lena, L.; D’Urso, F.; Stallone, R.; Petruzzi, M.; Giannelli, G.; et al. Different Cognitive Frailty Models and Health- and Cognitive-related Outcomes in Older Age: From Epidemiology to Prevention. J. Alzheimer’s Dis. 2018, 62, 993–1012. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Solfrizzi, V.; Tortelli, R.; Resta, F.; Sabbà, C.; Logroscino, G. Prevention of Late-life Cognitive Disorders: Diet-Related Factors, Dietary Patterns, and Frailty Models. Curr. Nutr. Rep. 2014, 3, 110–129. [Google Scholar] [CrossRef]
- Gracie, T.J.; Caufield-Noll, C.; Wang, N.Y.; Sieber, F.E. The Association of Preoperative Frailty and Postoperative Delirium: A Meta-analysis. Anesth. Analg. 2021, 133, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, G.; Gao, D.; Wang, S.; Meng, X.; Wang, C.; Yuan, H.; Chen, L. Cognitive frailty as a predictor of dementia among older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2020, 87, 103997. [Google Scholar] [CrossRef]
- Vella Azzopardi, R.; Beyer, I.; Vermeiren, S.; Petrovic, M.; Van Den Noortgate, N.; Bautmans, I.; Gorus, E.; Gerontopole Brussels Study Group. Increasing use of cognitive measures in the operational definition of frailty-A systematic review. Ageing Res. Rev. 2018, 43, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Courtin, E.; Knapp, M. Social isolation, loneliness and health in old age: A scoping review. Health Soc. Care Community 2017, 25, 799–812. [Google Scholar] [CrossRef]
- Cohen-Mansfield, J.; Perach, R. Interventions for alleviating loneliness among older persons: A critical review. Am. J. Health Promot. 2015, 29, e109–e125. [Google Scholar] [CrossRef]
- Li, J.; Erdt, M.; Chen, L.; Cao, Y.; Lee, S.Q.; Theng, Y.L. The Social Effects of Exergames on Older Adults: Systematic Review and Metric Analysis. J. Med. Internet Res. 2018, 20, e10486. [Google Scholar] [CrossRef]
- Smith, K.J.; Victor, C. The association of loneliness with health and social care utilisation in older adults in the general population: A systematic review. Gerontologist 2021, gnab177, ahead of print. [Google Scholar] [CrossRef]
- Van der Aa, H.P.; Margrain, T.H.; van Rens, G.H.; Heymans, M.W.; van Nispen, R.M. Psychosocial interventions to improve mental health in adults with vision impairment: Systematic review and meta-analysis. Ophthalmic Physiol. Opt. 2016, 36, 584–606. [Google Scholar] [CrossRef]
- McClelland, H.; Evans, J.J.; Nowland, R.; Ferguson, E.; O’Connor, R.C. Loneliness as a predictor of suicidal ideation and behaviour: A systematic review and meta-analysis of prospective studies. J. Affect. Disord. 2020, 274, 880–896. [Google Scholar] [CrossRef] [PubMed]
- Noone, C.; McSharry, J.; Smalle, M.; Burns, A.; Dwan, K.; Devane, D.; Morrissey, E.C. Video calls for reducing social isolation and loneliness in older people: A rapid review. Cochrane Database Syst. Rev. 2020, 2020, CD013632. [Google Scholar] [CrossRef]
- Chawla, K.; Kunonga, T.P.; Stow, D.; Barker, R.; Craig, D.; Hanratty, B. Prevalence of loneliness amongst older people in high-income countries: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0255088. [Google Scholar] [CrossRef] [PubMed]
- Casanova, G.; Zaccaria, D.; Rolandi, E.; Guaita, A. The Effect of Information and Communication Technology and Social Networking Site Use on Older People’s Well- Being in Relation to Loneliness: Review of Experimental Studies. J. Med. Internet Res. 2021, 23, e23588. [Google Scholar] [CrossRef]
- Ellis, S.; Sheik Ali, S.; Ahmed, W. A review of the impact of hearing interventions on social isolation and loneliness in older people with hearing loss. Eur. Arch. Otorhinolaryngol. 2021, 278, 4653–4661. [Google Scholar] [CrossRef]
- Krzeczkowska, A.; Spalding, D.M.; McGeown, W.J.; Gow, A.J.; Carlson, M.C.; Nicholls, L.A.B. A systematic review of the impacts of intergenerational engagement on older adults’ cognitive, social, and health outcomes. Ageing Res. Rev. 2021, 71, 101400. [Google Scholar] [CrossRef]
- Bessa, B.; Ribeiro, O.; Coelho, T. Assessing the social dimension of frailty in old age: A systematic review. Arch. Gerontol. Geriatr. 2018, 78, 101–113. [Google Scholar] [CrossRef]
- Landeiro, F.; Barrows, P.; Nuttall Musson, E.; Gray, A.M.; Leal, J. Reducing social isolation and loneliness in older people: A systematic review protocol. BMJ Open 2017, 7, e013778. [Google Scholar] [CrossRef]
- Tong, F.; Yu, C.; Wang, L.; Chi, I.; Fu, F. Systematic Review of Efficacy of Interventions for Social Isolation of Older Adults. Front. Psychol. 2021, 12, 554145. [Google Scholar] [CrossRef]
- Dahlberg, L.; McKee, K.J.; Frank, A.; Naseer, M. A systematic review of longitudinal risk factors for loneliness in older adults. Aging Ment. Health 2022, 26, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, M.; Bordonali, A.; Giannotti, C.; Montecucco, F.; Nencioni, A.; Odetti, P.; Monacelli, F. Social vulnerability underlying disability amongst older adults: A systematic review. Eur. J. Clin. Investig. 2020, 50, e13239. [Google Scholar] [CrossRef] [PubMed]
- Tengku Mohd, T.A.M.; Yunus, R.M.; Hairi, F.; Hairi, N.N.; Choo, W.Y. Social support and depression among community dwelling older adults in Asia: A systematic review. BMJ Open 2019, 9, e026667. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).