Non-Nitrogen-Fixers or Nitrogen-Fixers? Factors Distinguishing the Dominance of Chroococcal and Diazotrophic Cyanobacterial Species
Abstract
1. Introduction
2. Materials and Methods
| Type of Reservoir | Geographical Coordinates | Supply by River | Max Depth [m] | Surface [ha] | TSI Index | Dominated Cyanobacteria | |
|---|---|---|---|---|---|---|---|
| Piekary | Oxbow lake | 50°00′50.1′′ N, 19°47′35.7′′ E | Vistula | 4 | 1.6 | 64.7 eutrophy | Dolichospermum spp. |
| Tyniec | Oxbow lake | 50°01′47′′ N, 19°49′39.8′′ E | Vistula | 3 | 5.75 | 66.1 eutrophy | Microcystis ichthyoblabe and Woronichinia naegeliana |
| Podkamycze 1 | Artificial pond | 50°05′11′′ N, 19°50′01.6′′ E | Rudawa | 3 | 16.82 | 57.8 eutrophy | Aphanizomenon flos-aqaue |
| Podkamycze 2 | Artificial pond | 50°04′59.6′′ N, 19°50′05.4′′ E | Rudawa | 2 | 17.28 | 65.1 eutrophy | Aphanizomenon flos-aqaue |
Statistical Analyses
3. Results
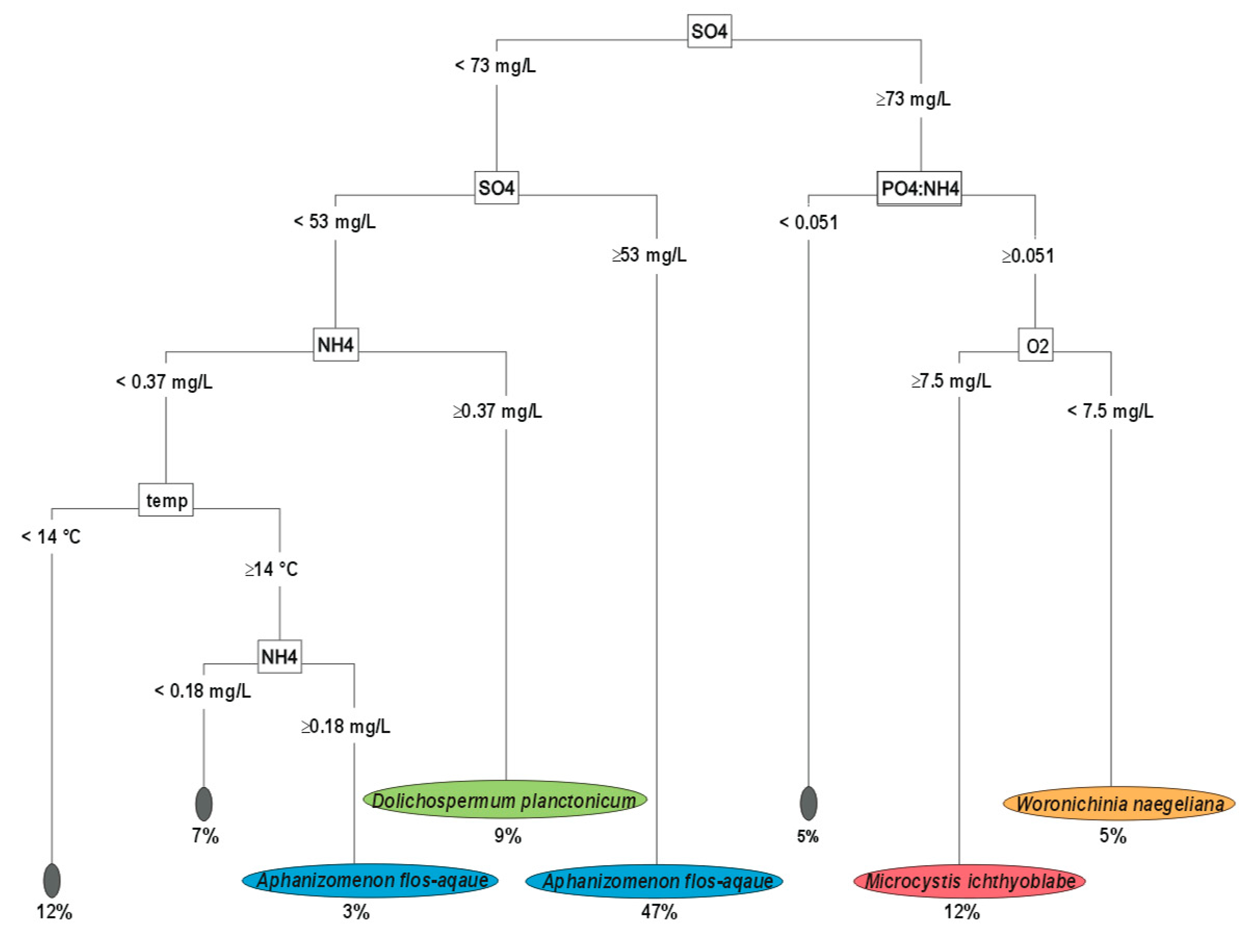
3.1. Upper Layer-Decision Tree
3.2. Decision Tree- Near-Bottom Layer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carey, C.C.; Ibelings, B.W.; Hoffmann, E.P.; Hamilton, D.P.; Brookes, J.D. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 2012, 46, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.O.; Trick, C.G.; Kudela, R.M.; Ishikawag, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the Past and Present to Forecast the Future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Havens, K.E.; Hall, N.S.; Otten, T.G.; Zhu, M.; Xu, H.; Zhu, G.; Qin, B. Mitigating a global expansion of toxic cyanobacterial blooms: Confounding effects and challenges posed by climate change. Mar. Freshw. Res. 2020, 71, 579–592. [Google Scholar] [CrossRef]
- Urrutia-Cordero, P.; Zhang, H.; Chaguaceda, F.; Geng, H.; Hansson, L.-A. Climate warming and heat waves alter harmful cyanobacterial blooms along the benthic-pelagic interface. Ecology 2020, 101, e03025. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, A.; Quesada, A.; Salmaso, N. Global expansion of toxic and non-toxic cyanobacteria: Effect on ecosystem functioning. Biodivers. Conserv. 2015, 24, 889–908. [Google Scholar] [CrossRef]
- Paerl, H. Nutrient and other environmental controls of harmful cyanobacterial blooms along the freshwater–marine continuum. Adv. Exp. Med. Biol. 2008, 619, 217–237. [Google Scholar]
- Bucka, H. The mass invasion of several blue-green alga in two drinking water supply reservoirs in southern Poland. In Management of Lakes and Reservoirs during Global Climate Change; George, D.G., Jones, J., Reynolds, C.S., Sutcliffe, D., Eds.; NATO ASI Series, 2, Environment; Kluwer Academic Publisher: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 1998; pp. 145–151. [Google Scholar]
- Vu, H.P.; Nguyen, L.N.; Zdarta, J.; Nga, T.T.V.; Nghiem, L.D. Blue-Green Algae in Surface Water: Problems and Opportunities. Curr. Pollut. Rep. 2020, 6, 105–122. [Google Scholar] [CrossRef]
- Jewel, M.A.S.; Affan, M.A.; Khan, S. Fish mortality due to cyanobacterial bloom in an aquaculture pond in Bangladesh. Pak. J. Biol. Sci. 2003, 6, 1046–1050. [Google Scholar] [CrossRef]
- Wilk-Woźniak, E. An introduction to the ‘micronet’of cyanobacterial harmful algal blooms (CyanoHABs): Cyanobacteria, zooplankton and microorganisms: A review. Mar. Freshw. Res. 2020, 71, 636–643. [Google Scholar] [CrossRef]
- Krztoń, W.; Kosiba, J.; Pociecha, A.; Wilk-Woźniak, E. The effect of cyanobacterial blooms on bio-and functional diversity of zooplankton communities. Biodivers. Conserv. 2019, 28, 1815–1835. [Google Scholar] [CrossRef]
- Krztoń, W.; Kosiba, J. Variations in zooplankton functional groups density in freshwater ecosystems exposed to cyanobacterial blooms. Sci. Total Environ. 2020, 730, 139044. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Pearson, L.A.; Crosbie, N.D.; Neilan, B.A. Distribution and conservation of known secondary metabolite biosynthesis gene clusters in the genomes of geographically diverse Microcystis aeruginosa strains. Mar. Freshw. Res. 2020, 71, 701–716. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Duelling ‘CyanoHABs’: Unravelling the environmental drivers controlling dominance and succession among diazotrophic and non-N2 fixing harmful cyanobacteria. Environ. Microbiol. 2016, 18, 316–324. [Google Scholar] [CrossRef]
- Paerl, H.W.; Havens, K.E.; Xu, H.; Zhu, G.; McCarthy, M.J.; Newell, S.E.; Scott, J.T.; Hall, N.S.; Otten, T.G.; Qin, B. Mitigating eutrophication and toxic cyanobacterial blooms in large lakes: The evolution of a dual nutrient (N and P) reduction paradigm. Hydrobiologia 2020, 847, 4359–4375. [Google Scholar] [CrossRef]
- Howarth, R.W.; Marino, R.; Lane, J.; Cole, J.J. Nitrogen fixation in freshwater, estuarine, and marine ecosystems. 1. Rates and importance. Limnol. Oceanogr. 1988, 33, 669–8738. [Google Scholar] [CrossRef]
- Savadova-Ratkus, K.; Mazur-Marzec, H.; Karosienė, J.; Kasperovičienė, J.; Paškauskas, R.; Vitonytė, I.; Koreiviene, J. Interplay of nutrients, temperature, and competition of native and alien cyanobacteria species growth and cyanotoxin production in temperate lakes. Toxins 2021, 13, 23. [Google Scholar] [CrossRef]
- Cole, J.J.; Lane, J.M.; Marino, R.; Howarth, R.W. Molybdenum assimilation by cyanobacteria and phytoplankton in freshwater and salt water. Limnol. Oceanogr. 1993, 38, 25–35. [Google Scholar] [CrossRef]
- Leung, T.; Wilkinson, G.M.; Swanner, E.D. Iron availability allows sustained cyanobacterial blooms: A dual-lake case study. Inland Waters 2021, 11, 417–429. [Google Scholar] [CrossRef]
- Molot, L.A.; Watson, S.B.; Creed, I.F.; Trick, C.G.; McCabe, S.K.; Verschoor, M.J.; Sorichetti, R.J.; Powe, C.; Venkiteswaran, J.J.; Schiff, S.L. A novel model for cyanobacteria bloom formation: The critical role of anoxia and ferrous iron. Freshw. Biol. 2014, 59, 1323–1340. [Google Scholar] [CrossRef]
- Klar, J.K.; Homoky, W.B.; Statham, P.J.; Birchill, A.J.; Harris, E.L.; Woodward, E.M.S.; Silburn, B.; Cooper, M.J.; James, R.H.; Connelly, D.P.; et al. Stability of dissolved and soluble Fe (II) in shelf sediment pore waters and release to an oxic water column. Biogeochemistry 2017, 135, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 1. Chroococcales. In Süsswasserflora von Mitteleuropa; Gustav Fischer Verlag: Jena, Germany, 1999; Volume 19. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 2. Oscillatoriales. In Süsswasserflora von Mitteleuropa; Elsevier: München, Germany; Spektrum Akademischer Verlag: Heidelberg, Germany, 2005; Volume 19. [Google Scholar]
- Komárek, J. Cyanoprokaryota 3. Heterocytous Genera. In Süsswasserflora von Mitteleuropa; Springer Spektrum: Berlin, Germany, 2013; Volume 19. [Google Scholar]
- Rott, E. Some results from phytoplankton counting intercalibrations. Schweiz. Z. Hydrol. 1981, 43, 34–62. [Google Scholar] [CrossRef]
- Carlson, R.E. A trophic state index for lakes 1. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- Carlson, R.E.; Simpson, J. A coordinator’s guide to volunteer lake monitoring methods. N. Am. Lake Manag. Soc. 1996, 96, 305. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 23 April 2020).
- Glass, J.B.; Axler, R.P.; Chandra, S.; Goldman, C.R. Molybdenum limitation of microbial nitrogen assimilation in aquatic ecosystems and pure cultures. Front. Microbiol. 2012, 3, 331. [Google Scholar] [CrossRef]
- Moisander, P.H.; Ochiai, M.; Lincoff, A. Nutrient limitation of Microcystis aeruginosa in northern California Klamath River reservoirs. Harmful Algae 2009, 8, 889–897. [Google Scholar] [CrossRef]
- Evans, J.C.; Prepas, E.E. Relative importance of iron and molybdenum in restricting phytoplankton biomass in high phosphorus saline lakes. Limnol. Oceanogr. 1997, 42, 461–472. [Google Scholar] [CrossRef]
- Howarth, R.W.; Cole, J.J. Molybdenum availability, nitrogen limitation, and phytoplankton growth in natural waters. Science 1985, 229, 653–655. [Google Scholar] [CrossRef]
- Wu, Y.; Li, L.; Zheng, L.; Dai, G.; Ma, H.; Shan, K.; Wu, H.; Zhou, Q.; Song, L. Patterns of succession between bloom-forming cyanobacteria Aphanizomenon flos-aquae and Microcystis and related environmental factors in large, shallow Dianchi Lake, China. Hydrobiologia 2016, 765, 1–13. [Google Scholar] [CrossRef]
- Xiang, T.; Hong, S.; Li-Rong, S.; Xiang, T. Comparative studies on physiological responses at phosphorus stress of three water bloom-forming cyanobacteria. Acta Hydrobiol. Sin. 2007, 31, 693–699. [Google Scholar]
- Shan, K.L.; Song, L.; Chen, W.; Li, L.; Liu, L.; Wu, Y.; Jia, Y.; Zhou, Q.; Peng, L. Analysis of environmental drivers influencing interspecific variations and associations among bloom-forming cyanobacteria in large, shallow eutrophic lakes. Harmful Algae 2019, 84, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, R.J. Rola zasilania wewnętrznego w eutrofizacji zbiorników zaporowych (The role of internal loading in dam reservoir eutrophication). In Procesy Biologiczne w Ochronie i Rekultywacji Nizinnych Zbiorników Zaporowych (Biological Processes in the Protection and Reclamation of the Low-Land dam Reservoirs); Zalewski, M., Ed.; Materiały z Konferencji Grupy Roboczej Narodowego Komitetu UNESCO, MAB-5 “Ekosystemy wodne”; Bibl Monit Środ: Łódź, Poland, 1995; pp. 61–70. [Google Scholar]
- Gonsiorczyk, T.; Casper, P.; Koschel, R. Mechanisms of phosphorus release from the bottom sediment of the oligotrophic Lake Stechlin: Importance of the permanently oxic sediment surface. Arch. Hydrobiol. 2001, 151, 203–219. [Google Scholar] [CrossRef]
- Heidenreich, M.; Kleeberg, A. Phosphorus-binding in iron-rich sediments of a shallow reservoir: Spatial characterization based on sonar data. Hydrobiologia 2003, 506–509, 147–153. [Google Scholar] [CrossRef]
- Loh, P.S.; Molot, L.A.; Nowak, E.; Nürnberg, G.K.; Watson, S.B.; Ginn, B. Evaluating relationships between sediment chemistry and anoxic phosphorus and iron release across three different water bodies. Inland Waters 2013, 3, 105–118. [Google Scholar] [CrossRef]
- Wilk-Woźniak, E. Late autumn mass development of Woronichinia naegeliana (Cyanophyceae) in a dam reservoir in Southern Poland. Biologia 1998, 53, 1–5. [Google Scholar]
- Wojciechowska, W.; Poniewozik, M.; Pasztaleniec, A. Vertical distribution of dominant cyanobacteria species in three lakes-Evidence of tolerance to different turbulence and oxygen conditions. Pol. J. Ecol. 2004, 52, 347–351. [Google Scholar]
- Beutel, M.W. Inhibition of ammonia release from anoxic profundal sediments in lakes using hypolimnetic oxygenation. Ecol. Eng. 2006, 28, 271–279. [Google Scholar] [CrossRef]
- Burger, D.F.; Hamilton, D.P.; Pilditch, C.A.; Gibbs, M.M. Benthic nutrient fluxes in a eutrophic, polymictic lake. Hydrobiologia 2007, 584, 13–25. [Google Scholar] [CrossRef]
- Wilk-Woźniak, E.; Mazurkiewicz-Boroń, G. The autumn dominance of cyanoprokaryotes in a deep meso-eutrophic submontane reservoir. Biologia 2003, 58, 17–24. [Google Scholar]
- Yamamoto, Y.; Nakahara, H. The formation and degradation of cyanobacterium Aphanizomenon flos-aquae blooms: The importance of pH, water temperature, and day length. Limnology 2005, 6, 1–6. [Google Scholar] [CrossRef]
- Wu, W.J.; Li, G.B.; Li, D.H.; Liu, Y.D. Temperature may be the dominating factor on the alternant succession of Aphanizomenon flos-aquae and Microcystis aeruginosa in Dianchi Lake. Fresenius Environ. Bull. 2010, 19, 846–853. [Google Scholar]
- Zapomělová, E.; Hrouzek, P.; Řeháková, K.; Šabacká, M.; Stibal, M.; Caisová, L.; Komárková, J.; Lukesová, A. Morphological variability in selected heterocystous cyanobacterial strains as a response to varied temperature, light intensity and medium composition. Folia Microbiol. 2008, 53, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewska-Madura, K.; Dondajewska, R.; Gołdyn, R. Internal phosphorus loading in selected lakes of the Cybina River valley. Oceanol. Hydrobiol. Stud. 2010, 39, 35–45. [Google Scholar] [CrossRef]
- Howarth, R.W.; Marino, R.; Cole, J.J. Nitrogen fixation in freshwater, estuarine, and marine ecosystems. 2. Biogeochemical controls 1. Limnol. Oceanogr. 1988, 33, 688–701. [Google Scholar] [CrossRef]
- Gallon, J.R. Reconciling the incompatible: N2 fixation and O2. New Phytol. 1992, 122, 571–609. [Google Scholar] [CrossRef]
- Garcia, N.S.; Fu, F.; Sedwick, P.N.; Hutchins, D.A. Iron deficiency increases growth and nitrogen-fixation rates of phosphorus-deficient marine cyanobacteria. ISME J. 2015, 9, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Baracaldo, P.; Hayes, P.K.; Blank, C.E. Morphological and habitat evolution in the Cyanobacteria using a compartmentalization approach. Geobiology 2005, 3, 145–165. [Google Scholar] [CrossRef]
- Dunalska, J.A.; Grochowska, J.; Wiśniewski, G.; Napiórkowska-Krzebietke, A. Can we restore badly degraded urban lakes? Ecol. Eng. 2015, 82, 432–441. [Google Scholar] [CrossRef]
- Kowalczewska-Madura, K.; Rosińska, J.; Dondajewska-Pielka, R.; Gołdyn, R.; Kaczmarek, L. The Effects of Limiting Restoration Treatments in a Shallow Urban Lake. Water 2020, 12, 1383. [Google Scholar] [CrossRef]

| Parameter | Layer | Water Body | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | T | P1 | P2 | P | T | P1 | P2 | P | T | P1 | P2 | ||
| Min–Max | Average | SD | |||||||||||
| Water temperature [°C] | Upper | 8.7–24.3 | 9.3–24.7 | 7.2–23.4 | 8.4–25.6 | 17.4 | 17.8 | 17.4 | 18.9 | 4.7 | 4.6 | 4.2 | 4.3 |
| Lower | 8.6–17.2 | 9.3–23.2 | 7.0–19.4 | 8.4–24.1 | 13.8 | 16.0 | 16.1 | 18.2 | 2.6 | 3.9 | 3.4 | 4.0 | |
| Oxygen saturation [%] | Upper | 53.1–100.8 | 41.0–169.6 | 88.5–214.7 | 11.8–236.6 | 74.4 | 88.3 | 141.6 | 133.1 | 17.6 | 37.8 | 34.1 | 49.1 |
| Lower | 2.6–53.3 | 2.2–61.2 | 30.3–182.1 | 16.4–226.2 | 30.7 | 27.4 | 113.4 | 123.5 | 17.2 | 19.5 | 36.5 | 49.2 | |
| pH | Upper | 6.4–8.3 | 6.8–8.3 | 7.2–8.9 | 7.4–8.6 | 7.2 | 7.4 | 7.9 | 8.0 | 0.5 | 0.5 | 0.4 | 0.3 |
| Lower | 5.5–7.5 | 6.4–7.7 | 7.5–8.6 | 7.6–8.5 | 6.5 | 7.0 | 8.0 | 8.0 | 0.6 | 0.4 | 0.3 | 0.3 | |
| NO3− [mg/L] | Upper | 0.18–1.03 | nd-1.05 | 6.53–12.11 | 0.67–5.62 | 0.39 | 0.53 | 9.37 | 2.96 | 0.24 | 0.24 | 1.63 | 1.64 |
| Lower | 0.14–3.50 | nd-4.08 | 7.57–13.65 | 0.85–6.05 | 0.56 | 0.74 | 10.72 | 3.04 | 0.98 | 1.03 | 1.24 | 1.76 | |
| NH4+ [mg/L] | Upper | 0.03–0.56 | 0.03–0.78 | 0.02–0.56 | 0.02–0.56 | 0.18 | 0.22 | 0.14 | 0.15 | 0.16 | 0.22 | 0.15 | 0.17 |
| Lower | 0.11–1.96 | 0.06–1.21 | 0.02–0.43 | 0.01–0.48 | 0.58 | 0.28 | 0.12 | 0.12 | 0.55 | 0.31 | 0.11 | 0.14 | |
| PO43− [mg/L] | Upper | 0–0.189 | 0–0.490 | 0–0.538 | 0.021–0.432 | 0.059 | 0.147 | 0.184 | 0.098 | 0.054 | 0.159 | 0.167 | 0.095 |
| Lower | 0–0.171 | 0–0.517 | 0–0.573 | 0–0.432 | 0.060 | 0.117 | 0.230 | 0.082 | 0.048 | 0.146 | 0.172 | 0.103 | |
| SO42− [mg/L] | Upper | 21.2–78.1 | 75.9–100.1 | 50.5–58.9 | 50.1–62.9 | 36.7 | 84.7 | 54.9 | 55.6 | 14.5 | 6.4 | 2.4 | 4.1 |
| Lower | 17.7–63.2 | 79.3–91.8 | 52.3–58.0 | 50.1–66.7 | 33.3 | 84.8 | 54.4 | 55.2 | 11.0 | 3.9 | 1.7 | 4.5 | |
| Fe dissolved [µg/L] | Upper | 5.6–101.0 | 3.4–160.0 | 0.8–48.0 | 1.2–45.0 | 29.3 | 55.5 | 21.8 | 22.1 | 25.9 | 50.0 | 13.3 | 13.5 |
| Lower | 4.7–159.0 | 1.9–204.4 | 1.6–66.0 | 3.0–55.0 | 40.0 | 49.2 | 23.5 | 24.9 | 41.8 | 53.3 | 17.2 | 15.0 | |
| Cyanobacterial biomass [mg/L] | 0–0.354 | 0–12.830 | 0.11–5.61 | 0.06–9.23 | 0.11 | 4.65 | 1.33 | 2.15 | 0.12 | 3.77 | 1.41 | 3.10 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilk-Woźniak, E.; Szarek-Gwiazda, E.; Walusiak, E.; Kosiba, J.; Krztoń, W. Non-Nitrogen-Fixers or Nitrogen-Fixers? Factors Distinguishing the Dominance of Chroococcal and Diazotrophic Cyanobacterial Species. Int. J. Environ. Res. Public Health 2022, 19, 15980. https://doi.org/10.3390/ijerph192315980
Wilk-Woźniak E, Szarek-Gwiazda E, Walusiak E, Kosiba J, Krztoń W. Non-Nitrogen-Fixers or Nitrogen-Fixers? Factors Distinguishing the Dominance of Chroococcal and Diazotrophic Cyanobacterial Species. International Journal of Environmental Research and Public Health. 2022; 19(23):15980. https://doi.org/10.3390/ijerph192315980
Chicago/Turabian StyleWilk-Woźniak, Elżbieta, Ewa Szarek-Gwiazda, Edward Walusiak, Joanna Kosiba, and Wojciech Krztoń. 2022. "Non-Nitrogen-Fixers or Nitrogen-Fixers? Factors Distinguishing the Dominance of Chroococcal and Diazotrophic Cyanobacterial Species" International Journal of Environmental Research and Public Health 19, no. 23: 15980. https://doi.org/10.3390/ijerph192315980
APA StyleWilk-Woźniak, E., Szarek-Gwiazda, E., Walusiak, E., Kosiba, J., & Krztoń, W. (2022). Non-Nitrogen-Fixers or Nitrogen-Fixers? Factors Distinguishing the Dominance of Chroococcal and Diazotrophic Cyanobacterial Species. International Journal of Environmental Research and Public Health, 19(23), 15980. https://doi.org/10.3390/ijerph192315980






