Influence of the Cognitive and Emotional Status of Patients with Chronic Pain on Treatment Success (Reduction in Pain Intensity and Adherence to Pharmacotherapy): A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Outcome Measure
2.3.1. Numeric Rating Scale (NRS)
2.3.2. The Montreal Cognitive Assessment (MoCA)
2.3.3. The Depression, Anxiety, and Stress Scale (DASS-21)
2.3.4. Adherence to the Physician’s Instructions
2.4. Statistical Methods
2.5. Ethical Consideration
3. Results
4. Discussion
5. Limitations
6. Implications for Practice
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosser, B.A.; McCracken, L.M.; Velleman, S.C.; Boichat, C.; Eccleston, C. Concerns about medication and medication adherence in patients with chronic pain recruited from general practice. Pain 2011, 152, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Andresen, T.; Niesters, M.; Dahan, A.; Morlion, B.; O´Brien, T.; Drewes, A.M. Pharmacological Management of Chronic Pain: How to Deal with the Catch-22 Situation. Curr. Med. Res. Opin. 2021, 4, 773–791. [Google Scholar] [CrossRef]
- NICE Medicines Optimization: The Safe and Effective Use of Medicines to Enable the Best Possible Outcomes. NICE Guideline. 2015. Available online: www.nice.org.uk/guidance/ng5/resources/medicines-optimisation-the-safe-and-effective-use-of-medicines-to-enable-the-best-possible-outcomes-pdf-51041805253 (accessed on 17 August 2022).
- Hawker, G.A.; Stewart, L.; French, M.R.; Cibere, J.; Jordan, J.; March, L.; Suarez-Almazor, M.; Gooberman-Hill, R. Understanding the pain experience in hip and knee osteoarthritis—An OARSI/OMERACT initiative. Osteoarthr. Cartil. 2008, 16, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.I.; Deyo, R.A.; Mirza, S.K.; Turner, J.A.; Comstock, B.A.; Hollingworth, W.; Sullivan, S.D. Expenditures and health status among adults with back and neck problems. JAMA 2008, 299, 656–664. [Google Scholar] [CrossRef]
- Goldberg, D.S.; McGee, S.J. Pain as a global public health priority. BMC Public Health 2011, 11, 770. [Google Scholar] [CrossRef]
- Canadian Pain Task Force. Chronic Pain in Canada: Laying a Foundation for Action. Health Canada. 2019. Available online: www.canada.ca/en/health-canada/corporate/about-health-canada/public-engagement/external-advisory-bodies/canadian-pain-task-force/report-2019 (accessed on 19 August 2022).
- Hnatešen, D.; Pavić, R.; Radoš, I.; Dimitrijević, I.; Budrovac, D.; Čebohin, M.; Gusar, I. Quality of Life and Mental Distress in Patients with Chronic Low Back Pain: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 10657. [Google Scholar] [CrossRef]
- Gaskin, D.J.; Richard, P. The economic costs of pain in the United States. J. Pain 2012, 13, 715–724. [Google Scholar] [CrossRef]
- IASP. Classification of Chronic Pain. Available online: https://www.iasp-pain.org/publications/free-ebooks/classification-ofchronic-pain-second-edition-revised/ (accessed on 19 August 2022).
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. A classification of chronic pain for ICD-11. Pain 2015, 156, 1003. [Google Scholar] [CrossRef]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287. [Google Scholar] [CrossRef]
- Khera, T.; Rangasamy, V. Cognition and Pain: A Review. Front. Psychol. 2021, 12, 673962. [Google Scholar] [CrossRef]
- McCracken, L.M.; Iverson, G.L. Predicting complaints of impaired cognitive functioning in patients with chronic pain. J. Pain Symptom Manag. 2001, 21, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.L.; Boustani, M.A.; Skopelja, E.N.; Gao, S.; Unverzagt, F.W. Medication adherence in older adults with cognitive impairment: A systematic evidence-based review. Am. J. Geriatr. Pharmacother. 2012, 10, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, L.A.; Kilian, S.; Firek, A.; Kashner, T.M.; Firek, C.J.; Silvet, H. Cognitive impairment and medication adherence in outpatients with heart failure. Heart Lung 2012, 41, 572–582. [Google Scholar] [CrossRef]
- Timmerman, L.; Stronks, D.L.; Groeneweg, J.G.; Huygen, F.J. Prevalence and determinants of medication non-adherence in chronic pain patients: A systematic review. Acta Anaesthesiol. Scand. 2016, 60, 416–431. [Google Scholar] [CrossRef]
- Naderi, S.H.; Bestwick, J.P.; Wald, D.S. Adherence to drugs that prevent cardiovascular disease: Meta-analysis on 376,162 patients. Am. J. Med. 2012, 125, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Briesacher, B.A.; Andrade, S.E.; Fouayzi, H.; Chan, K.A. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy 2008, 28, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Osterberg, L.; Blaschke, T. Adherence to medication. N. Engl. J. Med. 2005, 353, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Jüngst, C.; Gräber, S.; Simons, S.; Wedemeyer, H.; Lammert, F. Medication adherence among patients with chronic diseases: A survey-based study in pharmacies. QJM 2019, 112, 505–512. [Google Scholar] [CrossRef]
- Sabate, E. Adherence to Long-Term Therapies. Evidence for Action; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Elliott, W.J.; Maddy, R.; Toto, R.; Bakris, G. Hypertension in patients with diabetes. Overcoming barriers to effective control. Postgrad. Med. 2000, 107, 29–32, 35–36, 38. [Google Scholar] [CrossRef]
- Vrijens, B.; De Geest, S.; Hughes, D.A.; Przemyslaw, K.; Demonceau, J.; Ruppar, T.; Dobbels, F.; Fargher, E.; Morrison, V.; Lewek, P.; et al. A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 2012, 73, 691–705. [Google Scholar] [CrossRef]
- De Geest, S.; Zullig, L.L.; Dunbar-Jacob, J.; Helmy, R.; Hughes, D.A.; Wilson, I.B.; Vrijens, B. ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Ann. Intern. Med. 2018, 169, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.L.; McKellar, J.D.; Raffa, S.D.; Clark, M.E.; Kerns, R.D.; Karlin, B.E. Cognitive Behavioral Therapy for Chronic Pain among Veterans: Therapist Manual; U.S. Department of Veterans Affairs: Washington, DC, USA, 2014. [Google Scholar]
- Lerman, F.S.; Rudich, Z.; Brill, S.; Shalev, H.I.; Shahar, G. Longitudinal Associations between Depression, Anxiety, Pain and Pain-Related Disability in Chronic Pain Patients. Psychosom. Med. 2015, 77, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Rayner, L.; Hotopf, M.; Petkova, H.; Matcham, F.; Simpson, A.; McCracken, L.M. Depression in patients with chronic pain attending a specialized pain treatment center: Prevalence and impact on health care costs. Pain 2016, 157, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Breivik, H.; Borchgrevink, P.C.; Allen, S.M.; Rosseland, L.A.; Romundstad, L.; Hals, E.K.; Kvarstein, G.; Stubhaug, A. Assessment of pain. Br. J. Anaesth. 2008, 101, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.A.; Krebs, E.E.; Bentley, T.G.; Sherbourne, C.D.; Goebel, J.R.; Zubkoff, L.; Lanto, A.B.; Asch, S.M. Exploring alternative approaches to routine outpatient pain screening. Pain Med. 2009, 10, 1291–1299. [Google Scholar] [CrossRef]
- Fejer, R.; Jordan, A.; Hartvigsen, J. Categorising the severity of neck pain: Establishment of cut points for use in clinical and epidemiological research. Pain 2005, 119, 176–182. [Google Scholar] [CrossRef]
- Paul, S.M.; Zelman, D.C.; Smith, M.; Miaskowski, C. Categorizing the severity of cancer pain: Further exploration of the establishment of cutpoints. Pain 2005, 113, 37–44. [Google Scholar] [CrossRef]
- Zelman, D.C.; Dukes, E.; Brandenburg, N.; Bostrom, A.; Gore, M. Identification of cut-points for mild, moderate and severe pain due to diabetic peripheral neuropathy. Pain 2005, 115, 29–36. [Google Scholar] [CrossRef]
- Bertolucci, P.H.F.; Sarmento, A.L.R.; Wajman, J.R. Brazilian Portuguese version for the Montreal Cognitive Assessment (MoCA) and the preliminary results. J. Alzheimer’s Dis. 2008, 4, 686. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699, Erratum in J. Am. Geriatr. Soc. 2019, 67, 1991. [Google Scholar] [CrossRef]
- Available online: https://www.mocatest.org/faq/ (accessed on 19 August 2022).
- Ferreira Kdos, S.; Oliver, G.Z.; Thomaz, D.C.; Teixeira, C.T.; Foss, M.P. Cognitive deficits in chronic pain patients, in a brief screening test, are independent of comorbidities and medication use. Arq. Neuropsiquiatr. 2016, 74, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Lovibond, P.F.; Lovibond, S.H. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with Beck Depression and Anxiety Inventories. Behav. Res. Ther. 1995, 33, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Parkitny, L.; McAuley, J.H.; Walton, D.; Pena Costa, L.O.; Refshauge, K.M.; Wand, B.M.; Moseley, G.L. Rasch analysis supports the use of the Depression, Anxiety, and Stress Scales to measure mood in groups but not in individuals with chronic low back pain. J. Clin. Epidemiol. 2012, 65, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bazargan, M.; Loeza, M.; Ekwegh, T.; Adinkrah, E.K.; Kibe, L.W.; Cobb, S.; Assari, S.; Bazargan-Hejazi, S. Multi-Dimensional Impact of Chronic Low Back Pain among Underserved African American and Latino Older Adults. Int. J. Environ. Res. Public Health 2021, 18, 7246. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-M.; Lu, I.-C.; Chen, Y.-C.; Hsuan, C.-F.; Lin, Y.-J.; Chuang, H.-Y. Behavioral Factors Associated with Medication Nonadherence in Patients with Hypertension. Int. J. Environ. Res. Public Health 2021, 18, 9614. [Google Scholar] [CrossRef] [PubMed]
- van der Leeuw, G.; Leveille, S.G.; Dong, Z.; Shi, L.; Habtemariam, D.; Milberg, W.; Hausdorff, J.M.; Grande, L.; Gagnon, P.; McLean, R.R.; et al. Chronic Pain and Attention in Older Community-Dwelling Adults. J. Am. Geriatr. Soc. 2018, 66, 1318–1324. [Google Scholar] [CrossRef]
- Dufton, B.D. Cognitive failure and chronic pain. Int. J. Psychiatry Med. 1989, 19, 291–297. [Google Scholar] [CrossRef]
- Iezzi, T.; Archibald, Y.; Barnett, P.; Klinck, A.; Duckworth, M. Neurocognitive performance and emotional status in chronic pain patients. J. Behav. Med. 1999, 22, 205–216. [Google Scholar] [CrossRef]
- Iezzi, T.; Duckworth, M.P.; Vuong, L.N.; Archibald, Y.M.; Klinck, A. Predictors of neurocognitive performance in chronic pain patients. Int. J. Behav. Med. 2004, 11, 56–61. [Google Scholar] [CrossRef]
- McGuire, B.E. Chronic pain and cognitive function. Pain 2013, 154, 964–965. [Google Scholar] [CrossRef]
- Shuchang, H.; Mingwei, H.; Hongxiao, J.; Si, W.; Xing, Y.; Antonius, D.; Opler, M. Emotional and neurobehavioural status in chronic pain patients. Pain Res. Manag. 2011, 16, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.S.; Geisser, M.E.; Theisen-Goodvich, M.; Dixon, P.J. Cognitive complaints are associated with depression, fatigue, female sex, and pain catastrophizing in patients with chronic pain. Arch. Phys. Med. Rehabil. 2005, 86, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.; Brintz, C.E.; Zaski, A.M.; Edlund, M.J. Dialectical pain management: Feasibility of a hybrid third-wave cognitive behavioral therapy approach for adults receiving opioids for chronic pain. Pain Med. 2021, 22, 1080–1094. [Google Scholar] [CrossRef]
- Miller, R.M.; Kaiser, R.S. Psychological Characteristics of Chronic Pain: A Review of Current Evidence and Assessment Tools to Enhance Treatment. Curr. Pain Headache Rep. 2018, 22, 22. [Google Scholar] [CrossRef]
- Abdallah, C.G.; Geha, P. Chronic Pain and Chronic Stress: Two Sides of the Same Coin? Chronic stress (Thousand Oaks). Chronic Stress 2017, 1, 1–10. [Google Scholar] [CrossRef]
- The Psychology of Low Back Pain. 2019. Available online: www.health.harvard.edu/blog/psychology-low-back-pain-201604259537 (accessed on 19 August 2022).
- Knaster, P.; Karlsson, H.; Estlander, A.M.; Kalso, E. Psychiatric disorders as assessed with SCID in chronic pain patients: The anxiety disorders precede the onset of pain. Gen. Hosp. Psychiatry 2012, 34, 46–52. [Google Scholar] [CrossRef]
- Lee, T.H. Zero Pain Is Not the Goal. JAMA 2016, 315, 1575–1577. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Wilt, T.J.; McLean, R.M.; Forciea, M.A. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2017, 166, 514–530. [Google Scholar] [CrossRef]
- Bruns, D.; Disorbio, J.M. The psychological evaluation of patients with chronic pain: A review of BHI 2 clinical and forensic interpretive considerations. Psychol. Inj. Law 2014, 7, 335–361. [Google Scholar] [CrossRef]

| Variables | Categories | No. (%) | p |
|---|---|---|---|
| Gender | Male | 26 (37.1) | 0.06 * |
| Female | 44 (62.9) | ||
| MoCA test | Normal cognitive functioning | 32 (45.7) | 0.03 * |
| Mild cognitive impairment (MCI) | 28 (40.0) | ||
| Dementia (AD) | 10 (14.3) | ||
| Adherence to pharmacotherapy | Followed | 45 (64.3) | 0.09 * |
| Did not follow | 25 (35.7) | ||
| Categories | Me (25–75%) | p | |
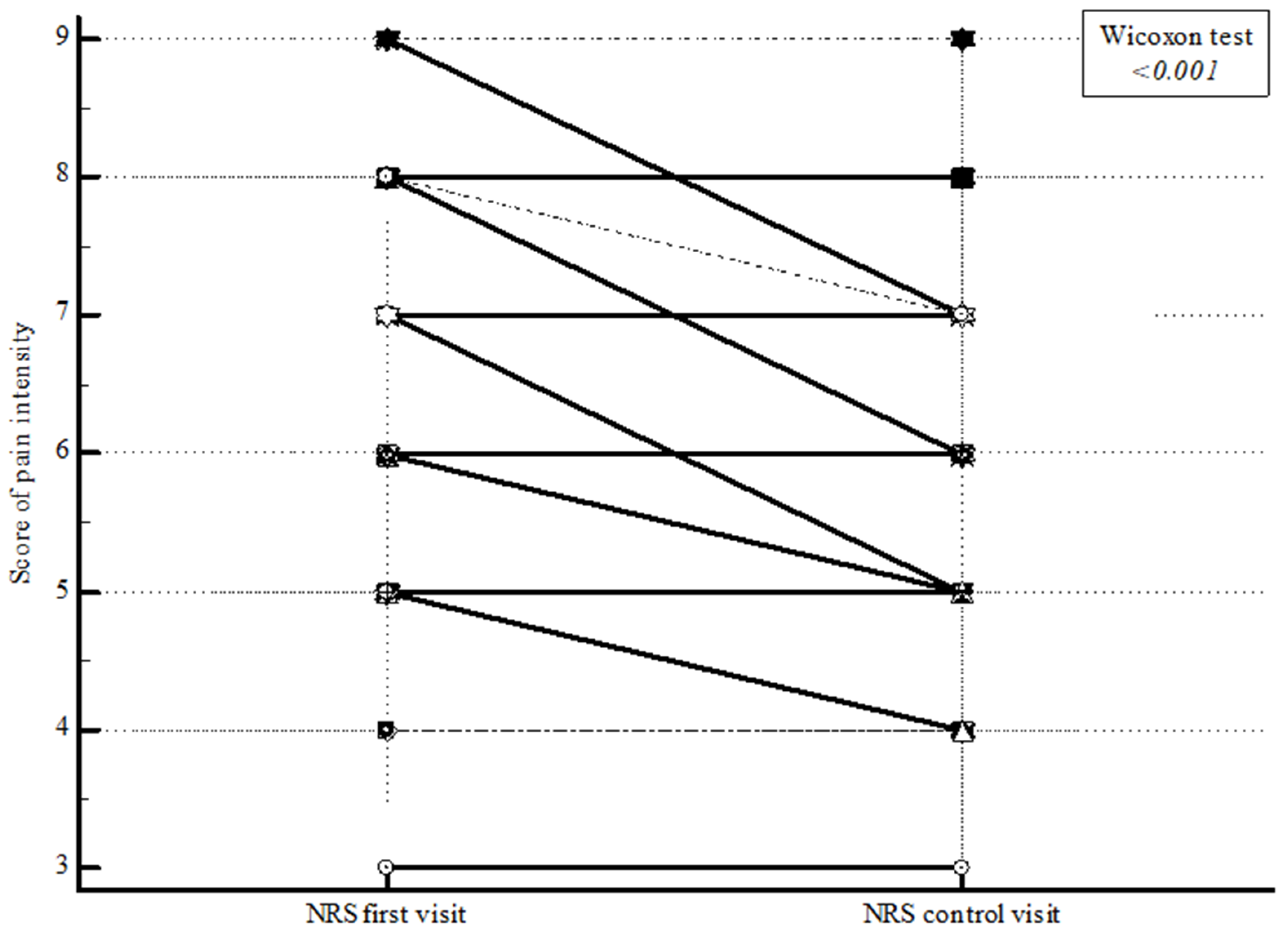
| NRS | First measurement point | 6.0 (5.0 to 8.0) | <0.001 † |
| Second measurement point | 5.0 (5.0 to 7.0) | ||
| Me | (25–75%) | Min–Max | |
| Age | 59.0 | 51.0 to 72.0 | 29.0 to 80.0 |
| MoCA test | 25.0 | 22.0 to 26.0 | 14.0 to 29.0 |
| Depression DASS-21 | 5.0 | 1.0 to 8.0 | 0 to 21.0 |
| Anxiety DASS-21 | 5.0 | 1.0 to 11.0 | 0 to 20.0 |
| Stress DASS-21 | 6.0 | 4.0 to 8.0 | 0 to 21.0 |
| Variables | Followed Instructions | Did Not Follow | p |
|---|---|---|---|
| n = 45 | n = 25 | ||
| No. (%) | |||
| Male | 19 (42.2) | 7 (28.0) | 0.24 * |
| Female | 26 (57.8) | 18 (72.0) | |
| Me (25–75%) | |||
| Age | 53.0 (49.0 to 68.0) | 70.0 (57.3 to 74.5) | 0.01 † |
| NRS first visit | 6.0 (5.0 to 8.0) | 5.0 (5.0 to 7.0) | 0.13 † |
| NRS control visit | 5.0 (5.0 to 7.0) | 5.0 (5.0 to 6.3) | 0.56 † |
| Reduction in pain intensity | 0 (0 to 1.0) | 0 (0 to 0) | 0.04 † |
| MoCA test | 26.0 (23.0 to 27.0) | 22.0 (16.0 to 24.0) | <0.001 † |
| Depression DASS-21 | 5.0 (1.0 to 7.0) | 6.0 (1.8 to 8.0) | 0.40 † |
| Anxiety DASS-21 | 4.0 (1.0 to 9.0) | 6.0 (2.0 to 12.0) | 0.32 † |
| Stress DASS-21 | 6.0 (4.0 to 8.0) | 4.0 (3.0 to 12.0) | 0.54 † |
| Predictor | β | Wald | p * | OR | 95% CI of OR | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| MoCA | 0.28 | 6.17 | 0.01 | 1.33 | 1.06 | 1.66 |
| Age | −0.002 | 0.004 | 0.95 | 1.0 | 0.93 | 1.07 |
| NRS first visit | 0.32 | 1.18 | 0.28 | 1.38 | 0.77 | 2.49 |
| Anxiety DASS-21 | −0.11 | 1.34 | 0.25 | 0.89 | 0.74 | 1.08 |
| Depression DASS-21 | −0.11 | 0.54 | 0.46 | 0.89 | 0.66 | 1.21 |
| Stress DASS-21 | 0.17 | 0.92 | 0.34 | 1.18 | 0.84 | 1.66 |
| Male gender | 0.11 | 0.02 | 0.88 | 1.11 | 0.28 | 4.36 |
| Constant | −7.64 | 3.77 | 0.05 | |||
| Matching Variables | Tau | 95% CI | p | |
|---|---|---|---|---|
| Reduction in pain intensity | MoCA test | 0.003 | −0.18 to 0.19 | 0.98 * |
| Depression DASS-21 | 0.007 | −0.16 to 0.17 | 0.93 * | |
| Anxiety DASS-21 | −0.21 | −0.36 to −0.04 | 0.01 * | |
| Stress DASS-21 | −0.04 | −0.21 to 0.13 | 0.59 * | |
| Age | 0.24 | 0.06 to 0.39 | 0.004 * | |
| NRS first measurement point | 0.23 | 0.03 to 0.40 | 0.006 * | |
| Gender | Me (25–75%) | |||
| Male | Female | |||
| n = 26 | n = 44 | |||
| 0 (0 to 1.0) | 0 (0 to 1.0) | 0.49 † | ||
| Predictor | β | Wald | p * | OR | 95% CI of OR | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| MoCA | 0.42 | 5.14 | 0.02 | 1.53 | 1.06 | 2.21 |
| Age | 0.24 | 6.96 | 0.008 | 1.28 | 1.06 | 1.53 |
| NRS first visit | 0.08 | 0.04 | 0.83 | 1.08 | 0.53 | 2.17 |
| Anxiety DASS-21 | −0.78 | 6.54 | 0.01 | 0.46 | 0.25 | 0.83 |
| Depression DASS-21 | 0.03 | 0.03 | 0.87 | 1.03 | 0.69 | 1.54 |
| Stress DASS-21 | 0.67 | 2.67 | 0.10 | 1.96 | 0.87 | 4.39 |
| Did not follow instructions | −1.08 | 1.16 | 0.28 | 0.34 | 0.05 | 2.42 |
| Male gender | 1.20 | 1.21 | 0.27 | 3.32 | 0.39 | 28.15 |
| Constant | −26.35 | 8.59 | 0.003 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hnatešen, D.; Radoš, I.; Dimitrijević, I.; Budrovac, D.; Omrčen, I.; Pavić, R.; Gusar, I.; Čebohin, M.; Šolić, K. Influence of the Cognitive and Emotional Status of Patients with Chronic Pain on Treatment Success (Reduction in Pain Intensity and Adherence to Pharmacotherapy): A Prospective Study. Int. J. Environ. Res. Public Health 2022, 19, 15968. https://doi.org/10.3390/ijerph192315968
Hnatešen D, Radoš I, Dimitrijević I, Budrovac D, Omrčen I, Pavić R, Gusar I, Čebohin M, Šolić K. Influence of the Cognitive and Emotional Status of Patients with Chronic Pain on Treatment Success (Reduction in Pain Intensity and Adherence to Pharmacotherapy): A Prospective Study. International Journal of Environmental Research and Public Health. 2022; 19(23):15968. https://doi.org/10.3390/ijerph192315968
Chicago/Turabian StyleHnatešen, Dijana, Ivan Radoš, Iva Dimitrijević, Dino Budrovac, Ivan Omrčen, Roman Pavić, Ivana Gusar, Maja Čebohin, and Krešimir Šolić. 2022. "Influence of the Cognitive and Emotional Status of Patients with Chronic Pain on Treatment Success (Reduction in Pain Intensity and Adherence to Pharmacotherapy): A Prospective Study" International Journal of Environmental Research and Public Health 19, no. 23: 15968. https://doi.org/10.3390/ijerph192315968
APA StyleHnatešen, D., Radoš, I., Dimitrijević, I., Budrovac, D., Omrčen, I., Pavić, R., Gusar, I., Čebohin, M., & Šolić, K. (2022). Influence of the Cognitive and Emotional Status of Patients with Chronic Pain on Treatment Success (Reduction in Pain Intensity and Adherence to Pharmacotherapy): A Prospective Study. International Journal of Environmental Research and Public Health, 19(23), 15968. https://doi.org/10.3390/ijerph192315968






