Antibiotic Resistance in Patients with Peri-Implantitis: A Systematic Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Origin and Exploration Scheme
2.2. Selection Criteria
2.3. Research Questions
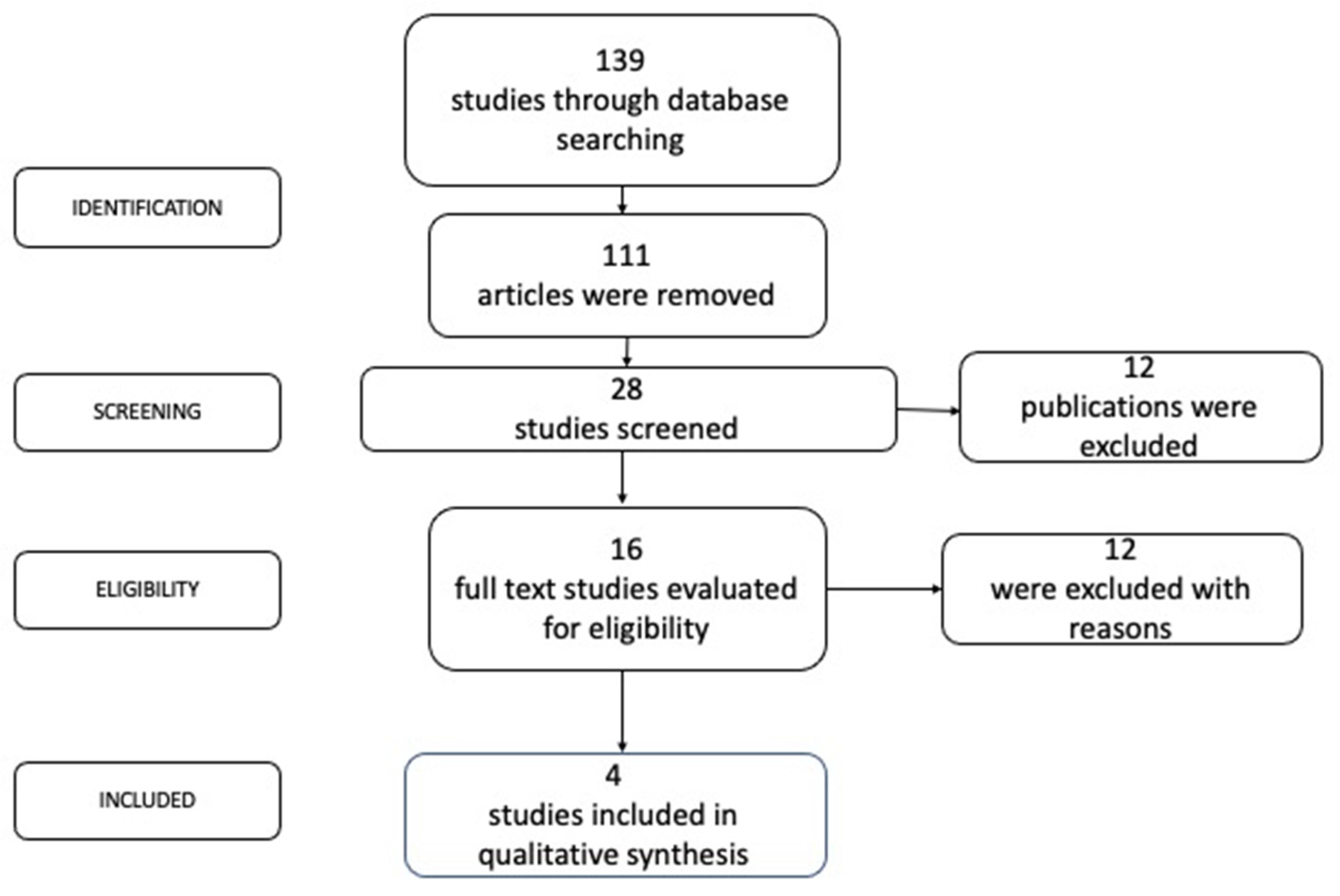
2.4. Records Choice
2.5. Information Extraction
2.6. Outcome Measures
2.7. Risk of Bias
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-implantitis. J. Clin. Periodontol. 2018, 45, 246–266. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Schaller, D.; Hakansson, J.; Wennstrom, J.L.; Tomasi, C.; Berglundh, T. Effectiveness of implant therapy analyzed in a Swedish population: Prevalence of peri-implantitis. J. Dent. Res. 2016, 95, 43–49. [Google Scholar] [CrossRef] [PubMed]
- De Waal, Y.C.; van Winkelhoff, A.J.; Meijer, H.J.; Raghoebar, G.M.; Winkel, E.G. Differences in peri-implant conditions between fully and partially edentulous subjects: A systematic review. J. Clin. Periodontol. 2013, 40, 266–286. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Berglundh, T.; Ericsson, I.; Liljenberg, B.; Marinello, C. Experimental Breakdown of Peri-Implant and Periodontal Tissues. A Study in the Beagle Dog. Clin. Oral Implant. Res. 1992, 3, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Cosgarea, R.; Sculean, A. Prevalence and Mechanisms of Peri-Implant Diseases. J. Dent. Res. 2017, 96, 31–37. [Google Scholar] [CrossRef] [PubMed]
- De Waal, Y.C.M.; Vangsted, T.E.; Van Winkelhoff, A.J. Systemic Antibiotic Therapy as an Adjunct to Non-Surgical Peri-Implantitis Treatment: A Single-Blind RCT. J. Clin. Periodontol. 2021, 48, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Dabdoub, S.M.; Tsigarida, A.A.; Kumar, P.S. Patient-Specific Analysis of Periodontal and Peri-Implant Microbiomes. J. Dent. Res. 2013, 92, 168S–175S. [Google Scholar] [CrossRef] [PubMed]
- Charalampakis, G.; Leonhardt, Å.; Rabe, P.; Dahlén, G. Clinical and Microbiological Characteristics of Peri-Implantitis Cases: A Retrospective Multicentre Study. Clin. Oral Implants Res. 2012, 23, 1045–1054. [Google Scholar] [CrossRef]
- Persson, G.R.; Renvert, S. Cluster of Bacteria Associated with Peri-Implantitis. Clin. Implant Dent. Relat. Res. 2014, 16, 783–793. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Manoil, D. Microbial Community-Driven Etiopathogenesis of Peri-Implantitis. J. Dent. Res. 2021, 100, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Roos-Jansåker, A.M.; Claffey, N. Non-Surgical Treatment of Peri-Implant Mucositis and Peri-Implantitis: A Literature Review. J. Clin. Periodontol. 2008, 35, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Berglundh, T. Peri-Implantitis-Onset and Pattern of Progression. J. Clin. Periodontol. 2016, 43, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Worthington, H.V. Treatment of peri-implantitis: What interventions are effective? A Cochrane systematic review. Eur. J. Oral Implantol. 2012, 5, S21–S41. [Google Scholar] [PubMed]
- Faggion, C.M., Jr.; Chambrone, L.; Listl, S.; Tu, Y.K. Network meta-analysis for evaluating interventions in implant dentistry: The case of peri-implantitis treatment. Clin. Implant Dent. Relat. Res. 2013, 15, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Polyzois, I.; Claffey, N. Surgical therapy for the control of peri-implantitis. Clin. Oral Implant. Res. 2012, 23, 84–94. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.; Mombelli, A. The therapy of peri-implantitis: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 325–345. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, P.C.; Netti, A.; Lopez, M.A.; Giaquinto, E.F.; De Rosa, G.; Aureli, G.; Bodnarenko, A.; Papi, P.; Starzyńska, A.; Pompa, G.; et al. Local/Topical Antibiotics for Peri-Implantitis Treatment: A Systematic Review. Antibiotics 2021, 25, 1298. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Osorio, M.; Vallecillo, C.; Toledano, R.; Aguilera, F.S.; Osorio, M.T.; Muñoz-Soto, E.; García-Godoy, F.; Vallecillo-Rivas, M. A Systematic Review and Meta-Analysis of Systemic Antibiotic Therapy in the Treatment of Peri-Implantitis. Int. J. Environ. Res. Public Health 2022, 19, 6502. [Google Scholar] [CrossRef] [PubMed]
- Roca-Millan, E.; Estrugo-Devesa, A.; Merlos, A.; Jané-Salas, E.; Vinuesa, T.; López-López, J. Systemic Antibiotic Prophylaxis to Reduce Early Implant Failure: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 698. [Google Scholar] [CrossRef]
- Bolstad, A.I.; Saetre, M.M.; Aasgaard, A.S.; Bunaes, D.F. Shift in antibiotic prescription at a University Dental Clinic in Norway 2013–2017. Eur. J. Oral Sci. 2020, 128, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, P.R.; Sahrmann, P.; Ramel, C.; Imfeld, T.; Müller, J.; Roos, M.; Jung, R.E. Peri-implantitis prevalence and treatment in implant-oriented private practices: A cross-sectional postal and Internet survey. Schweiz. Monatsschr. Zahnmed. 2012, 122, 1136–1144. [Google Scholar] [PubMed]
- Busa, A.; Parrini, S.; Chisci, G.; Pozzi, T.; Burgassi, S.; Capuano, A. Local versus systemic antibiotics effectiveness: A comparative study of postoperative oral disability in lower third molar surgery. J. Craniofac. Surg. 2014, 25, 708–709. [Google Scholar] [CrossRef]
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical and surgical treatment of periodontitis: How many options for one disease? Periodontol 2000 2017, 75, 152–188. [Google Scholar] [CrossRef]
- Øen, M.; Leknes, K.N.; Lund, B.; Bunæs, D.F. The efficacy of systemic antibiotics as an adjunct to surgical treatment of peri-implantitis: A systematic review. BMC. Oral Health 2021, 21, 666. [Google Scholar] [CrossRef]
- Preus, H.R.; Scheie, A.A.; Baelum, V. Letter to the editor: Re: The clinical effect of scaling and root planing and the concomitant administration of systemic amoxicillin and metronidazole: A systematic review. J. Periodontol. 2014, 85, 374–384. [Google Scholar] [CrossRef]
- Edlund, C.; Hedberg, M.; Nord, C.E. Antimicrobial treatment of periodontal diseases disturbs the human ecology: A review. J. Chemother. 1996, 8, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. EFP Workshop Participants and Methodological Consultants. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Di Spirito, F.; De Caro, F.; Lanza, A.; Passarella, D.; Sbordone, L. Adherence to Antibiotic Prescription of Dental Patients: The Other Side of the Antimicrobial Resistance. Healthcare 2022, 10, 1636. [Google Scholar] [CrossRef] [PubMed]
- Chisci, G.; Hatia, A. Antibiotics in orthognathic surgery and postoperative infections. Int. J. Oral Maxillofac. Surg. 2022, 22, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Ardila, C.M.; Bedoya-García, J.A. Antimicrobial resistance in dentistry. Oral Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Int. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Sirriyeh, R.; Lawton, R.; Gardner, P.; Armitage, G. Reviewing studies with diverse designs: The development and evaluation of a new tool. J. Eval. Clin. Pract. 2012, 18, 746–752. [Google Scholar] [CrossRef]
- Rams, T.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic resistance in human peri-implantitis microbiota. Clin. Oral Implants Res. 2014, 25, 82–90. [Google Scholar] [CrossRef]
- Rams, T.E.; Balkin, B.E.; Roberts, T.W.; Molzan, A.K. Microbiological aspects of human mandibular subperiosteal dental implants. J. Oral Implantol. 2013, 39, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Karbach, J.; Callaway, A.S.; Willershausen, B.; Wagner, W.; Al-Nawas, B. Multiple resistance to betalactam antibiotics, azithromycin or moxifloxacin in implant associated bacteria. Clin. Lab. 2013, 59, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Sbordone, L.; Barone, A.; Ramaglia, L.; Ciaglia, R.N.; Iacono, V.J. Antimicrobial susceptibility of periodontopathic bacteria associated with failing implants. J. Periodontol. 1995, 66, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Approved Standard M 11-A; Reference Agar Dilution Procedures for Antimicrobial Susceptibility Testing of An-Aerobic Bacteria. National Committee for Clinical Laboratory Standards: Villanova, PA, USA, 1985.
- Ardila, C.M.; Bedoya-García, J.A.; Arrubla-Escobar, D.E. Antibiotic resistance in periodontitis patients: A systematic scoping review of randomized clinical trials. Oral Dis. 2022. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.M.; Gonçalves, C.; Souto, R.; Feres-Filho, E.J.; Uzeda, M.; Colombo, A.P. Antibiotic resistance profile of the subgingival microbiota following systemic or local tetracycline therapy. J. Clin. Periodontol. 2004, 31, 420–427. [Google Scholar] [CrossRef]
- Feres, M.; Haffajee, A.D.; Allard, K.; Som, S.; Goodson, J.M.; Socransky, S.S. Antibiotic resistance of subgingival species during and after antibiotic therapy. J. Clin. Periodontol. 2002, 29, 724–735. [Google Scholar] [CrossRef]
- Ardila, C.M.; Bedoya-García, J.A.; González-Arroyave, D. Antimicrobial resistance in patients with endodontic infections: A systematic scoping review of observational studies. Aust. Endod. J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.P.; Jacinto, R.C.; Montagner, F.; Sousa, E.L.; Ferraz, C.C. Analysis of the antimicrobial susceptibility of anaerobic bacteria isolated from endodontic infections in Brazil during a period of nine years. J. Endod. 2011, 37, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Ardila, C.M.; Bedoya-García, J.A. Antimicrobial resistance of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Tannerella forsythia in periodontitis patients. J. Glob. Antimicrob. Resist. 2020, 22, 215–218. [Google Scholar] [CrossRef]
- Carr, V.R.; Witherden, E.A.; Lee, S.; Shoaie, S.; Mullany, P.; Proctor, G.B.; Gomez-Cabrero, D.; Moyes, D.L. Abundance and diversity of resistomes differ between healthy human oral cavities and gut. Nat. Commun. 2020, 11, 693. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Sun, B.; Chen, Y.; Lou, Y.; Zheng, M.; Li, Z. Dental Plaque Microbial Resistomes of Periodontal Health and Disease and Their Changes after Scaling and Root Planing Therapy. mSphere 2021, 6, e0016221. [Google Scholar] [CrossRef] [PubMed]
- Verspecht, T.; Rodriguez Herrero, E.; Khodaparast, L.; Khodaparast, L.; Boon, N.; Bernaerts, K.; Quirynen, M.; Teughels, W. Development of antiseptic adaptation and cross-adapatation in selected oral pathogens in vitro. Sci. Rep. 2019, 9, 8326. [Google Scholar] [CrossRef] [PubMed]
- Crighton, A. Prescribing in primary dental care: General principles. Prim. Dent. J. 2014, 3, 65–69. [Google Scholar] [CrossRef]
- Jepsen, K.; Falk, W.; Brune, F.; Fimmers, R.; Jepsen, S.; Bekeredjian-Ding, I. Prevalence and antibiotic susceptibility trends of periodontal pathogens in the subgingival microbiota of German periodontitis patients: A retrospective surveillance study. J. Clin. Periodontol. 2021, 48, 1216–1227. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Salvi, G.E.; Mombelli, A.; Faddy, M.; Lang, N.P. Anti-infective surgical therapy of peri-implantitis. A 12-month prospective clinical study. Clin. Oral Implant. Res. 2012, 23, 205–210. [Google Scholar] [CrossRef]
- Mombelli, A. Antimicrobial advances in treating periodontal diseases. Front. Oral Biol. 2012, 15, 133–148. [Google Scholar]
- Stratton, C.W. In vitro susceptibility testing versus in vivo effectiveness. Med. Clin. N. Am. 2006, 90, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Ardila, C.M.; Granada, M.I.; Guzmán, I.C. Antibiotic resistance of subgingival species in chronic periodontitis patients. J. Periodontal Res. 2010, 45, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Ardila, C.M.; Bedoya-García, J.A. Clinical and Microbiological Efficacy of Adjunctive Systemic Quinolones to Mechanical Therapy in Periodontitis: A Systematic Review of the Literature. Int. J. Dent. 2022, 2022, 4334269. [Google Scholar] [CrossRef] [PubMed]
- Ardila, C.M.; Bedoya-García, J.A. Antimicrobial resistance in patients with odontogenic infections: A systematic scoping review of prospective and experimental studies. J. Clin. Exp. Dent. 2022, 14, e834–e845. [Google Scholar] [CrossRef] [PubMed]
| Authors | Participants/Implants | Mean Age | Methodology | Prevalence of Antibiotic-Resistant Species | Antibiotic |
|---|---|---|---|---|---|
| Rams et al. 2014 [33] | 120/160 | 61 years | Complete anaerobically viable amounts were determined on EBBA primary isolation plates, implementing reasonable phenotypic devices formerly defined. Resistance to the drug breakpoint intensities was documented when test bacteria growth was noticed on the antibiotic-supplemented EBBA plates. | Four Pg strains (3.3% of patients) presented resistance to clindamycin. Pi/n was resistant to amoxicillin among 38 strains (40% of patients), and clindamycin in 35 strains (47% of patients). Strains also showed resistance to doxycycline (25%) and metronidazole (22%). Six Tf strains showed resistance to amoxicillin (5% of patients) and clindamycin among seven strains (6% of patients). Sc strains had resistance to metronidazole (22%), 25% to doxycycline, 40% to amoxicillin, and 47% to clindamycin. All Aa strains presented resistance to clindamycin, and 83% to doxycycline. | Clindamycin Amoxicillin Metronidazole Doxycycline |
| Rams et al. 2013 [34] | 11/11 | 74 years | Entire anaerobically viable amounts were recognized on EBBA plates utilizing probable phenotypic processes and standards formerly presented. Resistance to the drug breakpoint intensities was detailed when level of test bacteria growth was high on the antimicrobial-supplemented EBBA plates. | Fn exhibited resistance to doxycycline in one patient (9%). | Doxycycline |
| Karbach et al. 2013 [35] | 24/24 | NR | Microbial isolates were divided according to their cellular morphology. All isolates were Gram-stained. Aliquots of 0.1 mL were marked on agar plates to establish the probable resistance of the diverse samples. The sensitivity of the samples was confirmed utilizing the E-test. | Pm, Hs, and Ss were resistant to azithromycin, moxifloxacin, penicillin G, ampicillin, and ampicillin+sulbactam (28%). Two Aa (8%) and two As (8%) isolates presented resistance to all five tested antibiotics. | Azithromycin Moxifloxacin Penicillin G Ampicillin Ampicillin+Sulbactam |
| Sbordone et al. 1995 [36] | 13/19 | NR | Bacterial cultural examination of the species was achieved implementing continuous anaerobic procedures. The MICs of antimicrobials were established for predominant cultivable microflora. MICs were completed by the agar dilution system. | Pg (90%) presented resistance to tetracycline, metronidazole, and erythromycin. Pi was resistant to erythromycin (100%). Fn (90%) showed resistance to tetracycline, metronidazole, and erythromycin. | Tetracycline Metronidazole Erythromycin. |
| Investigation | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | Result |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rams et al. [33] | 3 | 3 | 3 | 0 | 0 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 0 | 3 | 81% |
| Rams et al. [34] | 3 | 3 | 3 | 0 | 0 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 0 | 3 | 81% |
| Karbach et al. [35] | 3 | 3 | 3 | 0 | 0 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 0 | 0 | 75% |
| Sbordone et al. [36] | 3 | 3 | 3 | 0 | 0 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 0 | 3 | 81% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardila, C.M.; Vivares-Builes, A.M. Antibiotic Resistance in Patients with Peri-Implantitis: A Systematic Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 15609. https://doi.org/10.3390/ijerph192315609
Ardila CM, Vivares-Builes AM. Antibiotic Resistance in Patients with Peri-Implantitis: A Systematic Scoping Review. International Journal of Environmental Research and Public Health. 2022; 19(23):15609. https://doi.org/10.3390/ijerph192315609
Chicago/Turabian StyleArdila, Carlos M., and Annie Marcela Vivares-Builes. 2022. "Antibiotic Resistance in Patients with Peri-Implantitis: A Systematic Scoping Review" International Journal of Environmental Research and Public Health 19, no. 23: 15609. https://doi.org/10.3390/ijerph192315609
APA StyleArdila, C. M., & Vivares-Builes, A. M. (2022). Antibiotic Resistance in Patients with Peri-Implantitis: A Systematic Scoping Review. International Journal of Environmental Research and Public Health, 19(23), 15609. https://doi.org/10.3390/ijerph192315609







