Abstract
Pain therapy for low back pain in pregnancy is a very topical issue. In fact, it is necessary to balance the patient’s needs to control pain with the need to manage a pregnancy without negative effects on the fetus. We report a case of a 37-year-old woman with low back pain treated with neurostimulation before pregnancy. She described severe chronic low back pain unresponsive to pharmacologic treatments. We first implanted a subcutaneous stimulator into the patient, and then a definitive stimulator resulting in excellent pain control. The improvement in her quality of life allowed the woman to become pregnant. We decided to stop neurostimulation with the patient during pregnancy. The patient completed her pregnancy without complications and the baby was born healthy. During the pregnancy, the woman took only paracetamol when needed. However, this painful symptomatology, completely anecdotal, is not attributable solely to the previous spine problem but probably also to the changes occurring during pregnancy. At the end of pregnancy, the neurostimulator was reactivated without any discomfort for the patient, who is now pain free. This case report provides a first line of evidence of a possible treatment of low back pain in women intending to become pregnant, with risk-free management for both the patient and the child.
1. Introduction
Chronic low back pain represents a health, social and economic problem [1]. In fact, it is increasingly evident that this pathology, often without a clear and defined etiology, is a condition that has very high costs in terms of healthcare, lost working days and a reduction in the ability of individuals to function [2,3,4]. The new vision of chronic pain as a biopsychosocial phenomenon finds a paradigm in low back pain [5]. Despite being such a widespread condition not only in numerical terms but also by geographical spread, there is no univocity of treatment, often with guidelines that are not always in agreement [6]. However, there is consensus in recognizing chronic pain as a pathology in its own right, which has lost its protective significance (as occurs in acute pain), with relevant neurological modifications [7,8,9]. Several studies, mainly carried out in vitro and concerning possible treatments for degenerative diseases of the nervous system or cell damage, have shown how nerve cells are sensitive to electromagnetic fields and direct their growth along the lines of the field itself. Regarding the influence of electromagnetic fields in vivo on pregnancy, there is no univocal opinion [10,11]. It is reported that exposure to an electromagnetic field greater than 2 mG (0.2 microT) and using an electric thermal blanket during pregnancy is not related to a greater risk of underweight newborns or newborns with intrauterine growth retardation [12]. Mahran et al. conducted a study on the exposure of pregnant women to different electromagnetic fields produced by common household appliances and did not observe any type of effect on pregnancy or the fetus, but nevertheless recommended that women limit such exposure during this period as a precautionary measure [13]. Studies on the effects of exposure to electromagnetic fields of women in the first trimester of pregnancy have found that exposures of >16 mG increase the risk of spontaneous abortion. This type of relationship is stronger for early miscarriages (before 10 weeks) [14,15,16]. Comparing several studies in which pregnant women were subjected to electrical cardioversions or suffered accidental electric shocks, it was found that these events did not lead to problems with the pregnancy or the fetus [17,18,19]. Based on this data, and considering the much smaller amount of electrical and electromagnetic forces generated by spinal cord stimulation (SCS), it can be assumed that low voltages are reasonably safe. Furthermore, recharging the device produces electromotive forces that are much stronger than the switched system itself, but despite this, the vertebrae, pelvis, and tissues produce an insulating effect, especially when the implantable pulse generator (IPG) is implanted in the gluteal region. There is no scientific evidence to support the hypothesis that neurostimulation can stimulate uterine contractions [20].
Possible effects of neurostimulation during pregnancy are:
- -
- On the fetus: teratogenicity of fetal malformations.
- -
- On women and pregnancy: abortion, premature birth, irritation and ulceration of the skin stretched on the battery, obstetric or anesthetic difficulties or complications, pain at the electrode or implant site.
- -
- On the device: migration of the electrode, depletion of the battery, stretching of the extension following expansion of the abdomen [13,21].
The first trimester is the most critical period in which it is recommended to avoid electrical stimulation [13]. The electromotive forces produced by neurostimulation are unlikely to reach the developing fetus.
Chronic pain is a difficult field in terms of both research and patient care [22]. In fact, treatments integrating invasive methods and advanced pharmacological therapies are often required to obtain not only adequate pain control but also functional recovery [23]. The most important goal of chronic pain management is not pain control alone, but full functional recovery and improved quality of life. All this is even further complicated when it is associated with pregnancy which makes therapeutic choices very limited, even for medico-legal reasons [24]. In addition, low back pain is a fairly common condition during pregnancy (with a prevalence ranging from 24 to 90%) [25,26]. Low back pain in pregnant women can have important sequelae in her personal life and quality of life, for example, by altering the quality and duration of sleep [27,28,29]. It is a research field that still has some unclear sides, especially regarding etiology and treatment. However, among the established risk factors, a history of low back pain prior to pregnancy appears to be one of the most important [26,30]. Despite such a significant prevalence of the repercussions impacting the quality of life, less than 50% of pregnant women with low back pain receive adequate (pharmacological and/or invasive) therapies [29,31]. Neurostimulation can be an important alternative to drug treatment in a failed back surgery syndrome (FBSS) [32,33,34]. In fact, neurostimulation involves the insertion of electrical devices capable of blocking the painful signal or modulating it [35]. Neurostimulation plays an extremely important role when drug therapy fails to control pain [35]. In fact, the FBSS is a difficult-to-treat condition in which drug treatment alone may not be effective [36].
This case report shows how through adequate pain control it is possible to obtain a complete functional recovery.
2. First Clinical Evaluation and Background
The clinical case presented below concerns a female patient aged 35 at the time of delivery. The medical history of the patient reports arthrodesis for thoracolumbar scoliosis (stabilization with Harrington in 1997), with removal of the broken bar and permanent Luque threads (removal of the means of fixation in 2009). In 2015, she underwent L5 and S1 laminectomy surgery with prosthetic replacement of the L5-S1 interbody disc and posteriorly instrumented arthrodesis.
After surgery, persistence of lumbar pain radiating to the lower limbs, especially in the L5-S1 area on the left, with a numerating rating scale (NRS) score 6 was observed. The pain of medium intensity was constant with peaks occurring throughout the day. About a year after the neurosurgery, she received a course of 10 paravertebral corticosteroid infiltrations from which she gained temporary benefit. Unfortunately, we have no clinical reports of this treatment because it was performed at another center and the patient did not provide us with any documentation. On 4 October 2016, the patient was seen at the Pain Unit of Ravenna. The patient’s therapy consisted of the following: duloxetine 60 mg/day, palmitoylethanolamide (PEA) 600 mg/day, association of oxycodone + naloxone 5 mg + 2.5 mg three times a day, ibuprofen 400 mg as needed, with a multimodal approach [23,37,38]. However, this pharmacological treatment did not provide adequate pain control and decreased the patient’s quality of life. In fact, the patient presented with a 10 m claudication and had to alternate between crutches and wheelchair to be able to move around. This functional limitation greatly affected her social life, effectively preventing the possibility of becoming pregnant. In fact, chronic low back pain is a biopsychosocial disease [5,39].
The patient certainly had a desire to become pregnant, but it was impossible not only to start a pregnancy, but also to suspend the very demanding pharmacological therapy.
3. Case Presentation
In the first instance, she underwent an infiltrative cycle of the apophyseal facets of the left L3-L4 and L4-L5 on 17 and 24–31 October 2016 (on each occasion the bilateral L4-L5 lumbar joint facets were infiltrated with dexamethasone 16 mg, ropivacaine 5 mg 0.2%, triamcinolone 40 mg, which produced little benefit, numerating score scale score (NRS) 7 (Figure 1)). The patient’s condition was so poor that it would have been impossible to plan an effective physiotherapy rehabilitation intervention as the patient experienced pain too intense to do so. Indication for spinal cord stimulation (SCS) was then given [40,41].
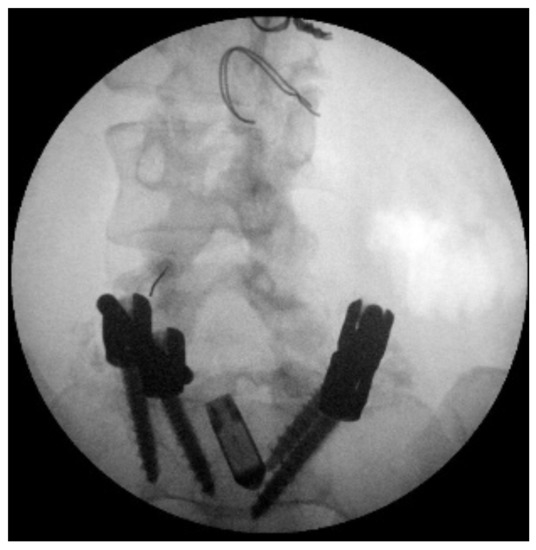
Figure 1.
X-ray image of the L4-L5 facet joints before implantation.
The indication was then given for the placement of a peridural medullary lead for high frequency spinal cord stimulation (10,000 Hz) Nevro SenzaTM (Italy) on 24 January 2017 as the first interventional step (pulse width 30 µs; current delivered 3 mA; charging time of 40 min every 24 h). However, the intervention was technically impractical due to the anatomical conformation of the column, the presence of means of synthesis and the results of previous interventions not permitting epidural access. Therefore, during the operation, it was decided to position the lead in the paravertebral subcutaneous space. This choice proved to be feasible and the electrode was implanted (Figure 2). During the three-month follow-up period (algological visit every two weeks), the patient reported good control of the symptoms (with resumption of normal sexual life) due to which it was decided to proceed to the second interventional step and the subcutaneous positioning of the definitive implant [42]. In the second procedure, the generator was implanted in a subcutaneous pocket packed in the abdomen (right side) on 21 February 2017 (Figure 3 and Figure 4). Neurostimulation reduced pain, allowing a progressive reduction of drug therapy until its total suspension two months after the implant. About three months after the implant, the patient informed the pain therapy service that she was pregnant, raising questions about the possible effects of neurostimulation on the fetus. After extensive literature searches and discussions within our team, as well as consultation with experts, we explained to the patient that the findings in the literature were often contradictory and thus did not provide any clear conclusions [43,44,45,46,47]. In fact, there are no absolute certainties dispelling any possible doubt about the health of the fetus [47].
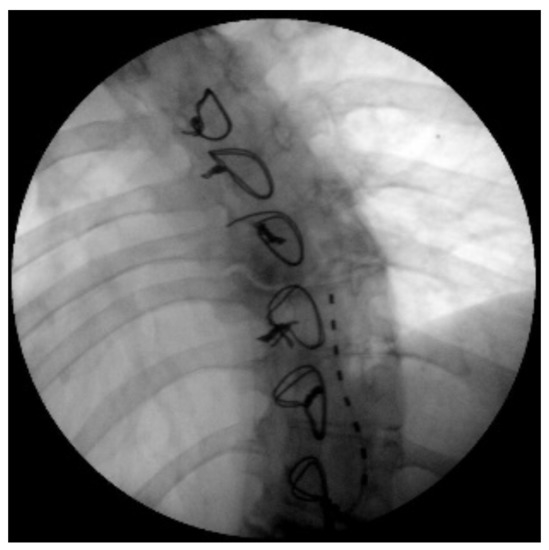
Figure 2.
X-ray image of the subcutaneous catheter positioned between D8 and D10.
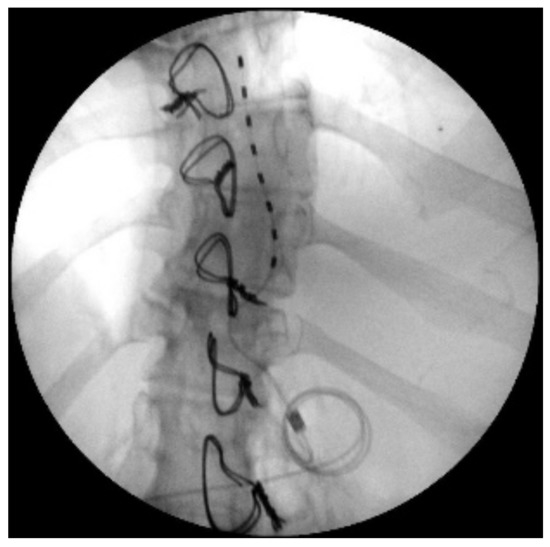
Figure 3.
X-ray image of loop for subcutaneous fixation.
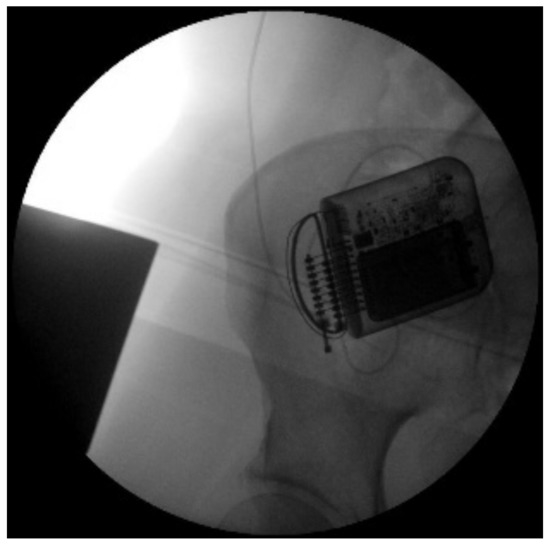
Figure 4.
X-ray image of the neurostimulator in right iliac fossa.
Prudently, the patient chose to suspend the stimulation. The timing of the suspension of the stimulation was at the end of the third month of gestation. This timing is due to the time needed to diagnose pregnancy, the time needed to develop a therapeutic strategy, and finally, but much more importantly, the time needed by the patient to make a decision based on the information received from the physicians. However, the pregnancy proceeded normally, with no obstetric problems. Pain control was optimal as the patient took paracetamol 1000 mg orally when needed. It should be emphasized that the painful episodes that required paracetamol intake cannot be attributed solely to the underlying pathology, but could also be a consequence of the change in load on the spine due to pregnancy. A caesarean section was performed at the 37th week of gestation in January 2018 and the baby was born healthy with Apgar score of 9/10 at birth, 10/10 at 1 min; weight 3300 g and 50 cm. From March 2018 to today, the patient maintains excellent pain control with no problems in the operation of the implant.
4. Discussion
The orientation that several authors agree on is to use an upper lumbar or thoracic medullary access for the positioning of the lead for women of childbearing age and, if possible, to place the implantable pulse generator (IPG) in the gluteal region rather than the abdominal one. The aim of these two suggestions is to not compromise any anesthetic maneuvers such as the placement of an epidural catheter for analgesic delivery or the choice of performing a subarachnoid locoregional anesthesia for a caesarean section, reducing the risk of stretching the extension (with growth abdominal diameter) and not creating any surgical difficulties.
It has been highlighted by several authors that subarachnoid anesthesia does not involve any additional risk for patients with a neurostimulation implant, provided that the puncture is situated in a region below the device. Even epidural anesthesia and catheter placement were not related to migration problems of the already implanted lead, with the usual foresight of keeping lower than the device when positioning the epidural catheter. However, maximum care must be taken during the administration of anesthetic boluses to ensure absolute sterility, as the risk of implant infections, albeit rare, is present. In pregnant women with an implant, the choice of elective caesarean section is often considered as the only option. Some authors justify this choice by explaining that, especially in regard to the sacral stimulators, they could be damaged by the thrusts of the woman in the gynecological position who rests all her weight and thrusts on the buttocks. However, this is not supported by the evidence. Studies on malfunctioning report a higher frequency in cases of caesarean section (38%) than in vaginal deliveries (25%) [48]. Therefore, the indication for a caesarean section should only concern obstetric problems and not just the fact of involving a neurostimulator. In our case, the caesarean section was indicated not due to the presence of the electro stimulator, but on the basis of two other reasons. The first, of an obstetric nature, provided for the impossibility of risk-free labor given the particular situation of the patient’s spine. It should be mentioned that obstetric surgery is one of the fields subject to the greatest number of legal medical complaints in Italy [24]. The second reason was the choice of the patient. In fact, in Italy, one of the indications for caesarean section is the so-called “self-determination”, that is, the choice of the pregnant woman who prefers to undergo a caesarean section rather than face a difficult and dangerous labor. The implantation of the stimulator created a means of avoiding potentially teratogenic drugs, allowing the onset of pregnancy for two reasons. The first undoubtedly concerns the improvement in the quality of life, with the resumption of normal personal life and a normal gait. The second reason was that of the suspension of risky drugs, necessary before implantation for pain control, albeit inadequate. It should be emphasized that the patient, after the implantation of the stimulator, no longer used drugs, except for paracetamol as needed, preserving the health of the fetus and giving birth to a healthy child.
A special mention should be given to the rehabilitation option which is also important in chronic low back pain treatment [49]. We offered the patient physiotherapy and rehabilitation treatments which were also indicated [50]. However, the patient’s denial of such treatments was due to her very poor quality of life which prevented her from undertaking any physical activity. Therefore, although aware of the extreme importance of a multidisciplinary treatment, we opted for an attack therapy that could quickly restore an acceptable quality of life. In addition, the evidence of rehabilitation is there, but it is not of high quality, and we were not allowed to waste any more time on a patient who needed immediate improvement [51].
5. Conclusions
An important aspect highlighted by the clinical case described is the issue of using drugs with a possible teratogenic effect. Many drugs commonly used for pain control, such as anticonvulsants, antidepressants, opioids and NMDA antagonists, result in diverse side effects on the fetus and fall into category C of Food and Drug Administration classification (FDA), i.e., “studies carried out on animals have shown adverse effects on the fetus, but there are no adequate control studies in humans and the potential benefits guarantee the use of the drug in pregnant women despite a potential risk“. Patients who have to undergo treatments with these drugs usually choose to use contraceptives so as to avoid pregnancy and risks to the fetus. The patient described took advantage of a treatment-free window to successfully attempt conception. This case report presents a first line of evidence of the possibility to treat low back pain in women intending to become pregnant, with risk-free management for the patient and the child.
Author Contributions
Conceptualization, M.A.I., M.C. and A.V.; methodology, M.A.I., E.P., P.P.; validation, M.A.I., P.P. and F.M.; formal analysis, M.C. and A.V.; investigation, M.A.I. and P.P.; resources, M.A.I. and P.P.; data curation, M.A.I. and P.P.; writing—original draft preparation, M.A.I., P.P. and A.V.; writing—review and editing, E.P., M.C., E.G.B., F.M. and A.V.; visualization, M.A.I. and P.P.; supervision, F.M. and A.V.; project administration, M.A.I. and A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from the patient to publish this paper.
Data Availability Statement
The data can be requested from the corresponding author for a reasonable purpose.
Acknowledgments
The authors would like to thank the patient for her availability.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buchbinder, R.; van Tulder, M.; Öberg, B.; Costa, L.M.; Woolf, A.; Schoene, M.; Croft, P.; Lancet Low Back Pain Series Working Group. Low Back Pain: A Call for Action. Lancet 2018, 391, 2384–2388. [Google Scholar] [CrossRef]
- Smith, E.; Hoy, D.G.; Cross, M.; Vos, T.; Naghavi, M.; Buchbinder, R.; Woolf, A.D.; March, L. The Global Burden of Other Musculoskeletal Disorders: Estimates from the Global Burden of Disease 2010 Study. Ann. Rheum Dis. 2014, 73, 1462–1469. [Google Scholar] [CrossRef]
- De David, C.N.; de Deligne, L.M.C.; da Silva, R.S.; Malta, D.C.; Duncan, B.B.; de Passos, V.M.A.; Cousin, E. The Burden of Low Back Pain in Brazil: Estimates from the Global Burden of Disease 2017 Study. Popul. Health Metr. 2020, 18, 12. [Google Scholar] [CrossRef]
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vos, T.; Barendregt, J.; et al. The Global Burden of Low Back Pain: Estimates from the Global Burden of Disease 2010 Study. Ann. Rheum. Dis. 2014, 73, 968–974. [Google Scholar] [CrossRef]
- Cuomo, A.; Cascella, M.; Vittori, A.; Marinangeli, F. Chronic Low Back Pain as a Biopsychosocial Disease: Time to Change Our Point of View. J. Anesth. Analg. Crit. Care 2021, 1, 7. [Google Scholar] [CrossRef]
- Corp, N.; Mansell, G.; Stynes, S.; Wynne-Jones, G.; Morsø, L.; Hill, J.C.; van der Windt, D.A. Evidence-Based Treatment Recommendations for Neck and Low Back Pain across Europe: A Systematic Review of Guidelines. Eur. J. Pain 2021, 25, 275–295. [Google Scholar] [CrossRef]
- Yang, S.; Chang, M.C. Chronic Pain: Structural and Functional Changes in Brain Structures and Associated Negative Affective States. Int. J. Mol. Sci. 2019, 20, 3130. [Google Scholar] [CrossRef]
- Li, W.; Gong, Y.; Liu, J.; Guo, Y.; Tang, H.; Qin, S.; Zhao, Y.; Wang, S.; Xu, Z.; Chen, B. Peripheral and Central Pathological Mechanisms of Chronic Low Back Pain: A Narrative Review. J. Pain Res. 2021, 14, 1483–1494. [Google Scholar] [CrossRef]
- Kregel, J.; Meeus, M.; Malfliet, A.; Dolphens, M.; Danneels, L.; Nijs, J.; Cagnie, B. Structural and Functional Brain Abnormalities in Chronic Low Back Pain: A Systematic Review. Semin. Arthritis Rheum. 2015, 45, 229–237. [Google Scholar] [CrossRef]
- Krewski, D.; Glickman, B.W.; Habash, R.W.Y.; Habbick, B.; Lotz, W.G.; Mandeville, R.; Prato, F.S.; Salem, T.; Weaver, D.F. Recent Advances in Research on Radiofrequency Fields and Health: 2001–2003. J. Toxicol. Environ. Health B Crit. Rev. 2007, 10, 287–318. [Google Scholar] [CrossRef]
- Habash, R.W.Y.; Elwood, J.M.; Krewski, D.; Lotz, W.G.; McNamee, J.P.; Prato, F.S. Recent Advances in Research on Radiofrequency Fields and Health: 2004–2007. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 250–288. [Google Scholar] [CrossRef]
- Abad, M.; Malekafzali, H.; Simbar, M.; Seyed Mosaavi, H.; Merghati Khoei, E. Association between Electromagnetic Field Exposure and Abortion in Pregnant Women Living in Tehran. Int. J. Reprod. Biomed. 2016, 14, 347–354. [Google Scholar] [CrossRef]
- Mahran, A.; Soriano, A.; Safwat, A.S.; Hijaz, A.; Mahajan, S.T.; Trabuco, E.C.; Siegel, S.W.; El-Nashar, S.A. The Effect of Sacral Neuromodulation on Pregnancy: A Systematic Review. Int. Urogynecol. J. 2017, 28, 1357–1365. [Google Scholar] [CrossRef]
- Ghazanfarpour, M.; Kashani, Z.A.; Pakzad, R.; Abdi, F.; Rahnemaei, F.A.; Akbari, P.A.; Roozbeh, N. Effect of Electromagnetic Field on Abortion: A Systematic Review and Meta-Analysis. Open Med. 2021, 16, 1628–1641. [Google Scholar] [CrossRef]
- Pacchierotti, F.; Ardoino, L.; Benassi, B.; Consales, C.; Cordelli, E.; Eleuteri, P.; Marino, C.; Sciortino, M.; Brinkworth, M.H.; Chen, G.; et al. Effects of Radiofrequency Electromagnetic Field (RF-EMF) Exposure on Male Fertility and Pregnancy and Birth Outcomes: Protocols for a Systematic Review of Experimental Studies in Non-Human Mammals and in Human Sperm Exposed in Vitro. Environ. Int. 2021, 157, 106806. [Google Scholar] [CrossRef]
- Regan, L.; Rai, R. Epidemiology and the Medical Causes of Miscarriage. Baillieres Best Pract. Res. Clin. Obs. Gynaecol. 2000, 14, 839–854. [Google Scholar] [CrossRef]
- Tromp, C.H.N.; Nanne, A.C.M.; Pernet, P.J.M.; Tukkie, R.; Bolte, A.C. Electrical Cardioversion during Pregnancy: Safe or Not? Neth. Heart J. 2011, 19, 134–136. [Google Scholar] [CrossRef]
- Wong, G.R.; Ang, M.; Jayarajan, J.; Walker, F.; Lambiase, P.D. Pregnancy in Patients with Implantable Cardiac Defibrillators. Herzschrittmacherther. Elektrophysiol. 2021, 32, 214–220. [Google Scholar] [CrossRef]
- Einarson, A.; Bailey, B.; Inocencion, G.; Ormond, K.; Koren, G. Accidental Electric Shock in Pregnancy: A Prospective Cohort Study. Am. J. Obs. Gynecol. 1997, 176, 678–681. [Google Scholar] [CrossRef]
- Li, Y.; Eliashiv, D.; LaHue, S.C.; Rao, V.R.; Martini, M.L.; Panov, F.; Oster, J.M.; Yoshii-Contreras, J.; Skidmore, C.T.; Kalayjian, L.A.; et al. Pregnancy Outcomes of Refractory Epilepsy Patients Treated with Brain-Responsive Neurostimulation. Epilepsy Res. 2021, 169, 106532. [Google Scholar] [CrossRef]
- El-Khawand, D.; Montgomery, O.C.; Wehbe, S.A.; Whitmore, K.E. Sacral Nerve Stimulation during Pregnancy: Case Report and Review of the Literature. Female Pelvic Med. Reconstr. Surg. 2012, 18, 127–129. [Google Scholar] [CrossRef]
- Gewandter, J.S.; Dworkin, R.H.; Turk, D.C.; Farrar, J.T.; Fillingim, R.B.; Gilron, I.; Markman, J.D.; Oaklander, A.L.; Polydefkis, M.J.; Raja, S.N.; et al. Research Design Considerations for Chronic Pain Prevention Clinical Trials: IMMPACT Recommendations. Pain 2015, 156, 1184–1197. [Google Scholar] [CrossRef]
- Vittori, A.; Petrucci, E.; Cascella, M.; Innamorato, M.; Cuomo, A.; Giarratano, A.; Petrini, F.; Marinangeli, F. Pursuing the Recovery of Severe Chronic Musculoskeletal Pain in Italy: Clinical and Organizational Perspectives from a SIAARTI Survey. J. Pain Res. 2021, 14, 3401–3410. [Google Scholar] [CrossRef]
- Petrucci, E.; Vittori, A.; Cascella, M.; Vergallo, A.; Fiore, G.; Luciani, A.; Pizzi, B.; Degan, G.; Fineschi, V.; Marinangeli, F. Litigation in Anesthesia and Intensive Care Units: An Italian Retrospective Study. Healthcare 2021, 9, 1012. [Google Scholar] [CrossRef]
- Vermani, E.; Mittal, R.; Weeks, A. Pelvic Girdle Pain and Low Back Pain in Pregnancy: A Review. Pain Pract. 2010, 10, 60–71. [Google Scholar] [CrossRef]
- Vleeming, A.; Albert, H.B.; Ostgaard, H.C.; Sturesson, B.; Stuge, B. European Guidelines for the Diagnosis and Treatment of Pelvic Girdle Pain. Eur. Spine J. 2008, 17, 794–819. [Google Scholar] [CrossRef]
- Kalus, S.M.; Kornman, L.H.; Quinlivan, J.A. Managing Back Pain in Pregnancy Using a Support Garment: A Randomised Trial. BJOG 2008, 115, 68–75. [Google Scholar] [CrossRef]
- Mogren, I. Perceived Health, Sick Leave, Psychosocial Situation, and Sexual Life in Women with Low-Back Pain and Pelvic Pain during Pregnancy. Acta Obs. Gynecol. Scand. 2006, 85, 647–656. [Google Scholar] [CrossRef]
- Skaggs, C.D.; Prather, H.; Gross, G.; George, J.W.; Thompson, P.A.; Nelson, D.M. Back and Pelvic Pain in an Underserved United States Pregnant Population: A Preliminary Descriptive Survey. J. Manip. Physiol. Ther. 2007, 30, 130–134. [Google Scholar] [CrossRef]
- Albert, H.B.; Godskesen, M.; Korsholm, L.; Westergaard, J.G. Risk Factors in Developing Pregnancy-Related Pelvic Girdle Pain. Acta Obs. Gynecol. Scand. 2006, 85, 539–544. [Google Scholar] [CrossRef]
- Greenwood, C.J.; Stainton, M.C. Back Pain/Discomfort in Pregnancy: Invisible and Forgotten. J. Perinat Educ. 2001, 10, 1–12. [Google Scholar] [CrossRef]
- Deer, T.R.; Mekhail, N.; Provenzano, D.; Pope, J.; Krames, E.; Thomson, S.; Raso, L.; Burton, A.; DeAndres, J.; Buchser, E.; et al. The Appropriate Use of Neurostimulation: Avoidance and Treatment of Complications of Neurostimulation Therapies for the Treatment of Chronic Pain. Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014, 17, 571–597, discussion 597–598. [Google Scholar] [CrossRef]
- Deer, T.R.; Mekhail, N.; Provenzano, D.; Pope, J.; Krames, E.; Leong, M.; Levy, R.M.; Abejon, D.; Buchser, E.; Burton, A.; et al. The Appropriate Use of Neurostimulation of the Spinal Cord and Peripheral Nervous System for the Treatment of Chronic Pain and Ischemic Diseases: The Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014, 17, 515–550, discussion 550. [Google Scholar] [CrossRef]
- Deer, T.R.; Krames, E.; Mekhail, N.; Pope, J.; Leong, M.; Stanton-Hicks, M.; Golovac, S.; Kapural, L.; Alo, K.; Anderson, J.; et al. The Appropriate Use of Neurostimulation: New and Evolving Neurostimulation Therapies and Applicable Treatment for Chronic Pain and Selected Disease States. Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014, 17, 599–615, discussion 615. [Google Scholar] [CrossRef]
- Moisset, X.; Lanteri-Minet, M.; Fontaine, D. Neurostimulation Methods in the Treatment of Chronic Pain. J. Neural Transm. 2020, 127, 673–686. [Google Scholar] [CrossRef]
- Sebaaly, A.; Lahoud, M.-J.; Rizkallah, M.; Kreichati, G.; Kharrat, K. Etiology, Evaluation, and Treatment of Failed Back Surgery Syndrome. Asian Spine J. 2018, 12, 574–585. [Google Scholar] [CrossRef]
- Cuomo, A.; Bimonte, S.; Forte, C.A.; Botti, G.; Cascella, M. Multimodal Approaches and Tailored Therapies for Pain Management: The Trolley Analgesic Model. J. Pain Res. 2019, 12, 711–714. [Google Scholar] [CrossRef]
- Polati, E.; Nizzero, M.; Rama, J.; Martini, A.; Gottin, L.; Donadello, K.; Del Balzo, G.; Varrassi, G.; Marinangeli, F.; Vittori, A.; et al. Oxycodone-Naloxone Combination Hinders Opioid Consumption in Osteoarthritic Chronic Low Back Pain: A Retrospective Study with Two Years of Follow-Up. Int. J. Environ. Res. Public Health 2022, 19, 13354. [Google Scholar] [CrossRef]
- Biancuzzi, H.; Dal Mas, F.; Brescia, V.; Campostrini, S.; Cascella, M.; Cuomo, A.; Cobianchi, L.; Dorken-Gallastegi, A.; Gebran, A.; Kaafarani, H.M.; et al. Opioid Misuse: A Review of the Main Issues, Challenges, and Strategies. Int. J. Environ. Res. Public Health 2022, 19, 11754. [Google Scholar] [CrossRef]
- Wong, S.S.; Chan, C.W.; Cheung, C.W. Spinal Cord Stimulation for Chronic Non-Cancer Pain: A Review of Current Evidence and Practice. Hong Kong Med. J. 2017, 23, 517–523. [Google Scholar] [CrossRef]
- Bocci, T.; De Carolis, G.; Paroli, M.; Barloscio, D.; Parenti, L.; Tollapi, L.; Valeriani, M.; Sartucci, F. Neurophysiological Comparison Among Tonic, High Frequency, and Burst Spinal Cord Stimulation: Novel Insights Into Spinal and Brain Mechanisms of Action. Neuromodulation 2018, 21, 480–488. [Google Scholar] [CrossRef]
- Xu, J.; Liu, A.; Cheng, J. New Advancements in Spinal Cord Stimulation for Chronic Pain Management. Curr. Opin. Anaesthesiol. 2017, 30, 710–717. [Google Scholar] [CrossRef]
- Khunda, A.; Karmarkar, R.; Abtahi, B.; Gonzales, G.; Elneil, S. Pregnancy in Women with Fowler’s Syndrome Treated with Sacral Neuromodulation. Int. Urogynecol. J. 2013, 24, 1201–1204. [Google Scholar] [CrossRef]
- Alghazwani, Y.; Alghafees, M.A.; Alfraidi, O.; Aldarrab, R. Sacral Neuromodulation in a Pregnant Patient With Fowler’s Syndrome: A Case Report. Cureus 2020, 12, e11796. [Google Scholar] [CrossRef]
- Agnello, M.; Vottero, M.; Bertapelle, P. Do You Really Want to Deactivate Your Sacral Neuromodulation Device during Pregnancy? A Single Center Case Series. Int. Urogynecol. J. 2021, 32, 709–717. [Google Scholar] [CrossRef]
- Roulette, P.; Castel-Lacanal, E.; Sanson, S.; Caremel, R.; Phé, V.; Bart, S.; Duchêne, F.; De Sèze, M.; Even, A.; Manunta, A.; et al. Sacral Neuromodulation and Pregnancy: Results of a National Survey Carried out for the Neuro-Urology Committee of the French Association of Urology (AFU). Neurourol. Urodyn. 2018, 37, 792–798. [Google Scholar] [CrossRef]
- Szymański, J.K.; Słabuszewska-Jóźwiak, A.; Jakiel, G. Sacral Neuromodulation in Pregnant Women-A Case Report and Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 8340. [Google Scholar] [CrossRef]
- Sommerfield, D.; Hu, P.; O’Keeffe, D.; McKeating, A.K. Caesarean Section in a Parturient with a Spinal Cord Stimulator. Int. J. Obs. Anesth. 2010, 19, 114–117. [Google Scholar] [CrossRef]
- Van Middelkoop, M.; Rubinstein, S.M.; Kuijpers, T.; Verhagen, A.P.; Ostelo, R.; Koes, B.W.; van Tulder, M.W. A Systematic Review on the Effectiveness of Physical and Rehabilitation Interventions for Chronic Non-Specific Low Back Pain. Eur. Spine J. 2011, 20, 19–39. [Google Scholar] [CrossRef]
- Kamper, S.J.; Apeldoorn, A.T.; Chiarotto, A.; Smeets, R.J.E.M.; Ostelo, R.W.J.G.; Guzman, J.; van Tulder, M.W. Multidisciplinary Biopsychosocial Rehabilitation for Chronic Low Back Pain. Cochrane Database Syst. Rev. 2014, 1, CD000963. [Google Scholar] [CrossRef]
- Kamper, S.J.; Apeldoorn, A.T.; Chiarotto, A.; Smeets, R.J.E.M.; Ostelo, R.W.J.G.; Guzman, J.; van Tulder, M.W. Multidisciplinary Biopsychosocial Rehabilitation for Chronic Low Back Pain: Cochrane Systematic Review and Meta-Analysis. BMJ 2015, 350, h444. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).