Assessment of Different Experimental Setups to Determine Viral Filtration Efficiency of Face Masks
Abstract
1. Introduction
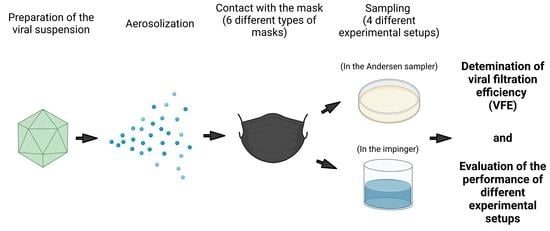
2. Materials and Methods
2.1. Types of Masks Tested
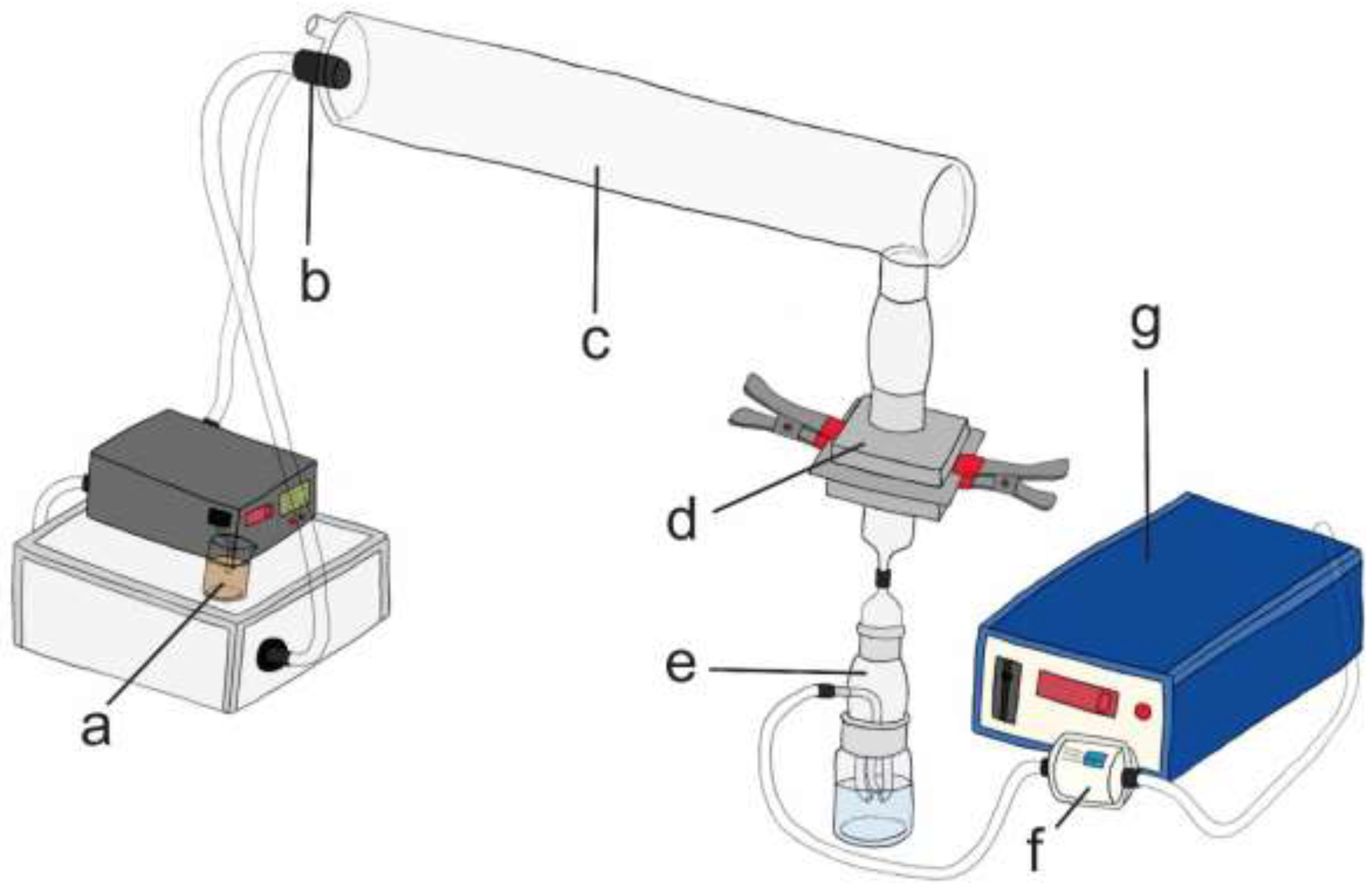
2.2. Experimental Setups and Performance of FEs
2.2.1. BFE
BFE with 6-Stage Andersen Sampler (Experimental Setup I)
BFE with Impinger Type 1 (Experimental Setup II)
2.2.2. VFE
VFE with Andersen Sampler (Experimental Setup III)
VFE with Impingers Type 1 and 2 (Experimental Setups IV, V and VI)
Double-Layer Plaque Assay
2.3. Calculation of BFE and VFE
3. Results and Discussion
3.1. Evaluation of the Experimental Setups
3.2. Determination of Filtration Efficiency of Masks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lopez, A.; Srigley, J. Transmission of SARS-CoV-2: Still up in the Air. Lancet 2022, 399, 519. [Google Scholar] [CrossRef]
- World Health Organization. Mask Use in the Context of COVID-19: Interim guidance, 1 December 2020. World Health Organization, 1–22.. Available online: https://apps.who.int/iris/handle/10665/337199 (accessed on 1 September 2022).
- Morawska, L.; Milton, D.K. It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2020, 71, 2311–2313. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Jimenez, J.L.; Prather, K.A.; Tufekci, Z.; Fisman, D.; Schooley, R. Ten Scientific Reasons in Support of Airborne Transmission of SARS-CoV-2. Lancet 2021, 397, 1603–1605. [Google Scholar] [CrossRef]
- Fennelly, K.P. Particle Sizes of Infectious Aerosols: Implications for Infection Control. Lancet Respir. Med. 2020, 8, 914–924. [Google Scholar] [CrossRef]
- Biryukov, J.; Boydston, J.A.; Dunning, R.A.; Yeager, J.J.; Wood, S.; Reese, A.L.; Ferris, A.; Miller, D.; Weaver, W.; Zeitouni, N.E.; et al. Increasing Temperature and Relative Humidity Accelerates Inactivation of SARS-CoV-2 on Surfaces. mSphere 2020, 5, e00441–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, T.; Deng, Y.; Liu, S.; Zhang, D.; Li, H.; Wang, X.; Jia, L.; Han, J.; Bei, Z.; et al. Stability of SARS-CoV-2 on Environmental Surfaces and in Human Excreta. J. Hosp. Infect. 2021, 107, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The Effect of Temperature on Persistence of SARS-CoV-2 on Common Surfaces. Virol. J. 2020, 17, 145. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- CDC Science Brief: SARS-CoV-2 and Surface (Fomite) Transmission for Indoor Community Environments. Available online: https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/surface-transmission.html?fbclid=IwAR3Uxjf0HuNk1qCTPNGnQtyxkB2TcfHUKftLGqTYvdH3znWbyLec3f6FJ80# (accessed on 10 November 2021).
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne Transmission of Respiratory Viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef] [PubMed]
- Božič, A.; Kanduč, M. Relative Humidity in Droplet and Airborne Transmission of Disease. J. Biol. Phys. 2021, 47, 1–29. [Google Scholar] [CrossRef]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L.; Healthcare Infection Control Practices Advisory Committee. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings. CDC. 1–206. 2022. Available online: https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html (accessed on 1 September 2022).
- World Health Organization. Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Infections in Health Care. WHO Guidelines. 2014. Available online: https://www.who.int/publications/i/item/infection-prevention-and-control-of-epidemic-and-pandemic-prone-acute-respiratory-infections-in-health-care (accessed on 1 September 2022).
- Alidjinou, E.; Poissy, J.; Ouafi, M.; Caplan, M.; Benhalima, I.; Goutay, J.; Tinez, C.; Faure, K.; Chopin, M.-C.; Yelnik, C.; et al. Spatial and Temporal Virus Load Dynamics of SARS-CoV-2: A Single-Center Cohort Study. Diagnostics 2021, 11, 427. [Google Scholar] [CrossRef]
- Weiss, A.; Jellingsø, M.; Sommer, M.O.A. Spatial and Temporal Dynamics of SARS-CoV-2 in COVID-19 Patients: A Systematic Review and Meta-Analysis. EBioMedicine 2020, 58, 102916. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.T.J.; Boisvert, L.N.; Zuo, Y.Y. Face Masks against COVID-19: Standards, Efficacy, Testing and Decontamination Methods. Adv. Colloid Interface Sci. 2021, 292, 102435. [Google Scholar] [CrossRef]
- CEN, the European Committee for Standardization. EN 149:2001+A1:2009, Respiratory protective devices-Filtering half masks to protect against particles-Requirements, testing, marking. European Committee for Standardization 1–44 (2009). Available online: https://standards.iteh.ai/catalog/standards/cen/f440f60a-91c1-497b-815e-4e9d46436256/en-149-2001a1-2009 (accessed on 1 September 2022).
- CEN, European Committee for Standardization. EN 14683:2019+AC:2019, Medical face masks-requirements and test methods. European Committee for Standardization 1–23 (2019). Available online: https://standards.iteh.ai/catalog/standards/cen/4bdef56d-7660-4a66-ba96-8e287fbc7d8c/en-14683-2019ac-2019 (accessed on 1 September 2022).
- Das, S.; Sarkar, S.; Das, A.; Das, S.; Chakraborty, P.; Sarkar, J. A Comprehensive Review of Various Categories of Face Masks Resistant to Covid-19. Clin. Epidemiol. Glob. Heal. 2021, 12, 100835. [Google Scholar] [CrossRef]
- Konda, A.; Prakash, A.; Moss, G.A.; Schmoldt, M.; Grant, G.D.; Guha, S. Aerosol Filtration Efficiency of Common Fabrics Used in Respiratory Cloth Masks. ACS Nano 2020, 14, 6339–6347. [Google Scholar] [CrossRef]
- Nelson Labs Bacterial & Viral Filtration Efficiency (BFE/VFE). Available online: https://www.nelsonlabs.com/testing/bacterial-viral-filtration-efficiency-bfe-vfe/ (accessed on 15 June 2022).
- Tang, S.; Li, X.; Ding, P.; Mao, Y.; Deng, F.; Cha, Y.; Zhuang, S.; Ding, C.; Wang, J.; Wang, Y.; et al. Filtration Efficiency of Face Masks against Aerosolized Surrogate SARS-CoV-2 at Different Social Distances. Sci. Bull. 2021, 67, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Whiley, H.; Keerthirathne, T.P.; Nisar, M.A.; White, M.A.F.; Ross, K.E. Viral Filtration Efficiency of Fabric Masks Compared with Surgical and N95 Masks. Pathogens 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, S.; Shaffer, R.; Williams, B.; Smit, S. A Comparison of Facemask and Respirator Filtration Test Methods. J. Occup. Environ. Hyg. 2017, 14, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Košir, T.; Fric, K.; Filipić, A.; Kogovšek, P. Bacterial Filtration Efficiency of Different Masks. Strojniški Vestn.-J. Mech. Eng. 2022, 68, 225–232. [Google Scholar] [CrossRef]
- Andersen, A.A. New Sampler for the Collection, Sizing, and Enumeration of Viable Airborne Particles. J. Bacteriol. 1958, 76, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, C.; Mocé-Llivina, L.; Lucena, F.; Jofre, J. Evaluation of Escherichia Coli Host Strain CB390 for Simultaneous Detection of Somatic and F-Specific Coliphages. Appl. Environ. Microbiol. 2008, 74, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, M.L.; Shaffer, R. Do We Need to Challenge Respirator Filters With Biological Aerosols? Available online: https://blogs.cdc.gov/niosh-science-blog/2014/04/02/respirator-filter-testing/ (accessed on 13 June 2022).
- Qian, Y.; Willeke, K.; Grinshpun, S.A.; Donnelly, J.; Coffey, C.C. Performance of N95 Respirators: Filtration Efficiency for Airborne Microbial and Inert Particles. Am. Ind. Hyg. Assoc. J. 1998, 59, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Tcharkhtchi, A.; Abbasnezhad, N.; Zarbini Seydani, M.; Zirak, N.; Farzaneh, S.; Shirinbayan, M. An Overview of Filtration Efficiency through the Masks: Mechanisms of the Aerosols Penetration. Bioact. Mater. 2021, 6, 106–122. [Google Scholar] [CrossRef]
- Santarpia, J.L.; Herrera, V.L.; Rivera, D.N.; Ratnesar-Shumate, S.; Reid, S.P.; Ackerman, D.N.; Denton, P.W.; Martens, J.W.S.; Fang, Y.; Conoan, N.; et al. The Size and Culturability of Patient-Generated SARS-CoV-2 Aerosol. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Morawska, L.; Johnson, G.R.; Ristovski, Z.D.; Hargreaves, M.; Mengersen, K.; Corbett, S.; Chao, C.Y.H.; Li, Y.; Katoshevski, D. Size Distribution and Sites of Origin of Droplets Expelled from the Human Respiratory Tract during Expiratory Activities. J. Aerosol Sci. 2009, 40, 256–269. [Google Scholar] [CrossRef]
- Shen, J.; Kong, M.; Dong, B.; Birnkrant, M.J.; Zhang, J. Airborne Transmission of SARS-CoV-2 in Indoor Environments: A Comprehensive Review. Sci. Technol. Built Environ. 2021, 27, 1331–1367. [Google Scholar] [CrossRef]
- Farzaneh, S.; Shirinbayan, M. Processing and Quality Control of Masks: A Review. Polymers 2022, 14, 291. [Google Scholar] [CrossRef]
- Li, X.; Ding, P.; Deng, F.; Mao, Y.; Zhou, L.; Ding, C.; Wang, Y.; Luo, Y.; Zhou, Y.; MacIntyre, C.R.; et al. Wearing Time and Respiratory Volume Affect the Filtration Efficiency of Masks against Aerosols at Different Sizes. Environ. Technol. Innov. 2021, 25, 102165. [Google Scholar] [CrossRef]
- Blachere, F.M.; Lemons, A.R.; Coyle, J.P.; Derk, R.C.; Lindsley, W.G.; Beezhold, D.H.; Woodfork, K.; Duling, M.G.; Boutin, B.; Boots, T.; et al. Face Mask Fit Modifications That Improve Source Control Performance. Am. J. Infect. Control 2021, 50, 133–140. [Google Scholar] [CrossRef]
- Kwong, L.H.; Wilson, R.; Kumar, S.; Crider, Y.S.; Reyes Sanchez, Y.; Rempel, D.; Pillarisetti, A. Review of the Breathability and Filtration Efficiency of Common Household Materials for Face Masks. ACS Nano 2021, 15, 5904–5924. [Google Scholar] [CrossRef]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J. Physical Distancing, Face Masks, and Eye Protection to Prevent Person-to-Person Transmission of SARS-CoV-2 and COVID-19: A Systematic Review and Meta-Analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, H.; Zhang, L.; Zhang, M.; Guo, D.; Wu, W.; Zhang, X.; Kan, G.L.; Jia, L.; Huo, D.; et al. Reduction of Secondary Transmission of SARS-CoV-2 in Households by Face Mask Use, Disinfection and Social Distancing: A Cohort Study in Beijing, China. BMJ Glob. Health 2020, 5, e002794. [Google Scholar] [CrossRef] [PubMed]
- Andrejko, K.; Pry, J.; Myers, J.; Fukui, N.; DeGuzman, J.; Openshaw, J.; Watt, J.; Lewnard, J.; Jain, S. Effectiveness of Face Mask or Respirator Use in Indoor Public Settings for Prevention of SARS-CoV-2 Infection. Morb. Mortal. Wkly. Rep. 2022, 71, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Leffler, C.T.; Ing, E.; Lykins, J.D.; Hogan, M.C.; McKeown, C.A.; Grzybowski, A. Association of Country-Wide Coronavirus Mortality with Demographics, Testing, Lockdowns, and Public Wearing of Masks. Am. J. Trop. Med. Hyg. 2020, 103, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.C.; Wong, S.C.; Chuang, V.W.M.; So, S.Y.C.; Chen, J.H.K.; Sridhar, S.; To, K.K.W.; Chan, J.F.W.; Hung, I.F.N.; Ho, P.L.; et al. The Role of Community-Wide Wearing of Face Mask for Control of Coronavirus Disease 2019 (COVID-19) Epidemic Due to SARS-CoV-2. J. Infect. 2020, 81, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ma, N.; Witt, C.; Rapp, S.; Wild, P.S.; Andreae, M.O.; Pöschl, U.; Su, H. Face Masks Effectively Limit the Probability of SARS-CoV-2 Transmission. Science 2021, 372, 1439–1443. [Google Scholar] [CrossRef]
- CDC Science Brief: Community Use of Masks to Control the Spread of SARS-CoV-2. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/masking-science-sars-cov2.html (accessed on 10 November 2022).


| Number of Experiments | |||||||
|---|---|---|---|---|---|---|---|
| BFE | VFE | ||||||
| Experimental Setup | I | II | III | IV | V | VI | |
| Sampler Type | Andersen | Impinger Type 1 | Andersen | Impinger Type 1 | Impinger Type 2 | ||
| Mask Sample | + Different Pump | ||||||
| A | 3 | 3 | 2 | 3 | 3 | 3 | |
| B | 4 | 3 | 2 | 2 | 3 | n.t. | |
| C | 1 | n.t. | n.t. | 1 | n.t. | n.t. | |
| D | 1 | n.t. | n.t. | n.t. | 1 | n.t. | |
| E | 1 | n.t. | n.t. | n.t. | 1 | n.t. | |
| F | 1 | n.t. | n.t. | n.t. | 1 | n.t. | |
| Mask Sample | Experimental Setup | Coefficient of Variation (%) |
|---|---|---|
| A | I | 0.16 |
| II | 0.07 | |
| III | 0.26 | |
| IV | 0.15 | |
| V | 0.17 | |
| VI | 0.14 | |
| B | I | 1.00 |
| II | 0.94 | |
| III | 0.48 | |
| IV | 0.09 | |
| V | 0.61 | |
| C | I | 2.14 |
| IV | 0.73 | |
| D | I | 5.34 |
| V | 3.15 | |
| E | I | 0.06 |
| V | 0.002 | |
| F | I | 2.11 |
| V | 1.63 |
| Experimental Setup | I | II | III | IV | V | VI | Type a |
|---|---|---|---|---|---|---|---|
| Mask Sample | BFE (%) b | VFE (%) b | |||||
| A | 99.8 | 99.9 | 99.4 | 99.9 | 99.8 | 99.8 | II |
| B | 96 | 98 | 98 | 99.3 | 99 | - | I or II |
| C | 91 | - | - | - | 92 | - | NA |
| D | 79 | - | - | - | 87 | - | NA |
| E | 99.9 | - | - | - | 99.999 | - | II |
| F | 91 | - | - | 97 | - | - | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipić, A.; Fric, K.; Ravnikar, M.; Kogovšek, P. Assessment of Different Experimental Setups to Determine Viral Filtration Efficiency of Face Masks. Int. J. Environ. Res. Public Health 2022, 19, 15353. https://doi.org/10.3390/ijerph192215353
Filipić A, Fric K, Ravnikar M, Kogovšek P. Assessment of Different Experimental Setups to Determine Viral Filtration Efficiency of Face Masks. International Journal of Environmental Research and Public Health. 2022; 19(22):15353. https://doi.org/10.3390/ijerph192215353
Chicago/Turabian StyleFilipić, Arijana, Katja Fric, Maja Ravnikar, and Polona Kogovšek. 2022. "Assessment of Different Experimental Setups to Determine Viral Filtration Efficiency of Face Masks" International Journal of Environmental Research and Public Health 19, no. 22: 15353. https://doi.org/10.3390/ijerph192215353
APA StyleFilipić, A., Fric, K., Ravnikar, M., & Kogovšek, P. (2022). Assessment of Different Experimental Setups to Determine Viral Filtration Efficiency of Face Masks. International Journal of Environmental Research and Public Health, 19(22), 15353. https://doi.org/10.3390/ijerph192215353