Maximal Fat Oxidation during Incremental Upper and Lower Body Exercise in Healthy Young Males
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Exercise Tests
2.3. Measurements
2.4. Data Analysis
3. Results
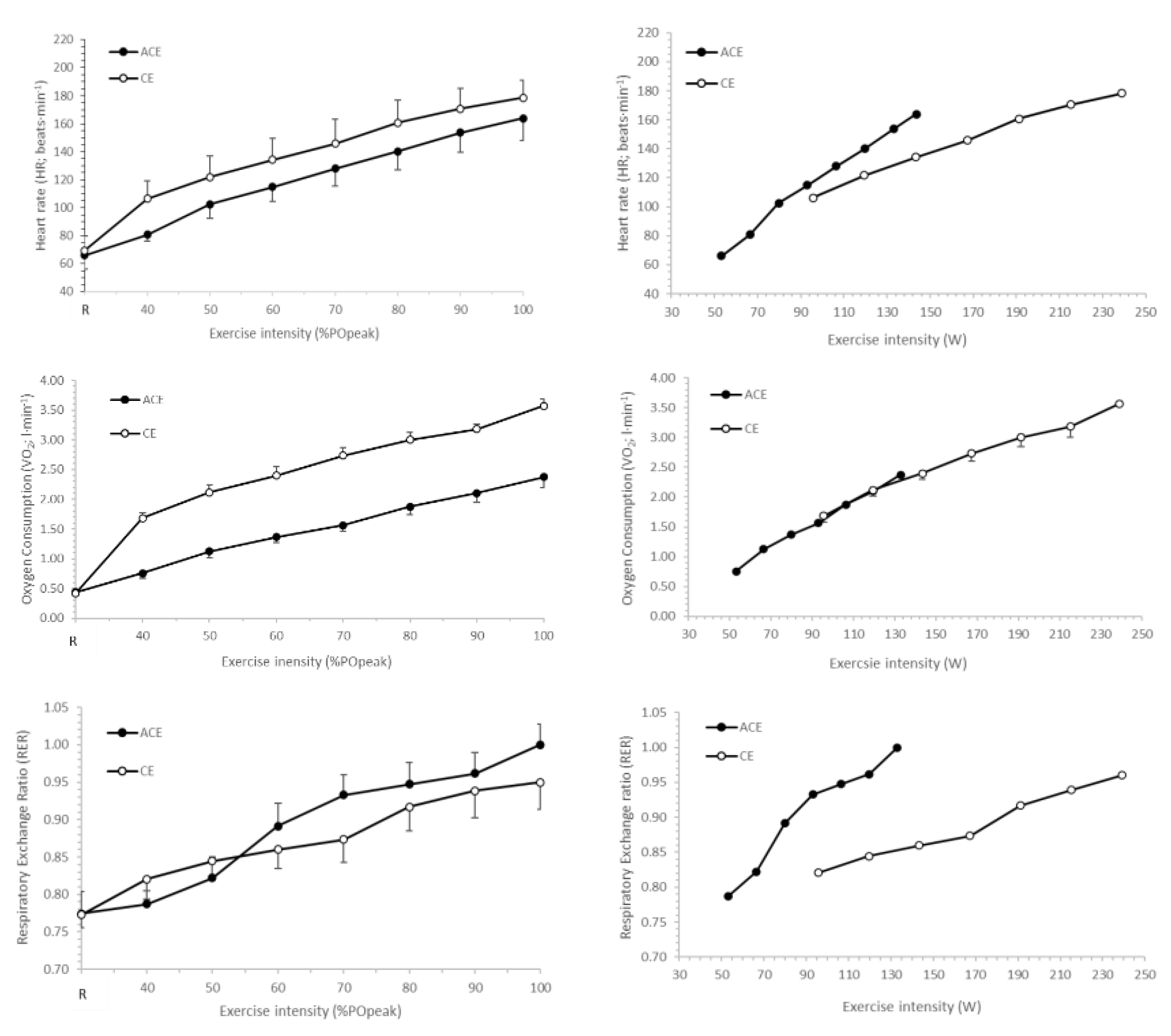
3.1. Peak Physiological Responses
3.2. Submaximal Exercise Responses
3.3. Substrate Utilisation
3.4. Comparison of 2- and 3-Minute Exercise Durations
4. Discussion
4.1. The Peak Physiological Responses
4.2. Incremental Exercise Responses
4.3. Maximal Fat Oxidation during CE
4.4. Maximal Fat Oxidation during ACE
4.5. Inter-Individual Variability
4.6. Application
4.7. Limitations
4.8. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knechtle, B.; Müller, G.; Willmann, F.; Eser, P.; Knecht, H. Comparison of fat oxidation in arm cranking in spinal cord-injured people versus ergometry in cyclists. Eur. J. Appl. Physiol. 2003, 90, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Achten, J.; Jeukendrup, A.E. Optimizing fat oxidation through exercise and diet. Nutrition 2004, 20, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Achten, J.; Gleeson, M.; Jeukendrup, A.E. Determination of the exercise intensity that elicits maximal fat oxidation. Med. Sci. Sports Exerc. 2002, 34, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Alkahtani, S.A.; King, N.A.; Hills, A.P.; Byrne, N.M. Effect of interval training intensity on fat oxidation, blood lactate and the rate of perceived exertion in obese men. SpringerPlus 2013, 2, 532. [Google Scholar] [CrossRef] [PubMed]
- Achten, J.; Jeukendrup, A.E. The effect of pre-exercise carbohydrate feedings on the intensity that elicits maximal fat oxidation. J. Sports Sci. 2003, 21, 1017–1024. [Google Scholar] [CrossRef]
- Venables, M.C.; Achten, J.; Jeukendrup, A.E. Determinants of fat oxidation during exercise in healthy men and women: A cross-sectional study. J. Appl. Physiol. 2005, 98, 160–167. [Google Scholar] [CrossRef]
- Maunder, E.; Plews, D.J.; Kilding, A.E. Contextualising maximal fat oxidation during exercise: Determinants and normative values. Front. Physiol. 2018, 9, 599. [Google Scholar] [CrossRef]
- Croci, I.; Borrani, F.; Byrne, N.M.; Wood, R.E.; Hickman, I.J.; Chenevière, X.; Malatesta, D. Reproducibility of fatmax and fat oxidation rates during exercise in recreationally trained males. PLoS ONE 2014, 9, e97930. [Google Scholar] [CrossRef] [PubMed]
- Helgerud, J.; Øiestad, B.E.; Wang, E.; Hoff, J. Prediction of upper extremity peak oxygen consumption from heart rate during submaximal arm cycling in young and middle-aged adults. Eur. J. Appl. Physiol. 2019, 119, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Price, M.; Beckford, C.; Dorricott, A.; Hill, C.; Kershaw, M.; Singh, M.; Thornton, I. Oxygen uptake during upper body and lower body Wingate anaerobic tests. Appl. Phys. Nutr. Metab. 2013, 39, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Sawka, M.N. Physiology of upper body exercise. Exerc. Sport Sci. Rev. 1986, 14, 175–211. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Vodak, P.; Wilmore, J.H.; Vodak, J.; Kurtz, P. Anaerobic threshold and maximal aerobic power for three modes of exercise. J. Appl. Physiol. 1976, 41, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.T.; Christensen, J.; Tang, L.H.; Keller, C.; Doherty, P.; Zwisler, A.D.; Taylor, R.S.; Langberg, H. A systematic review and meta-analysis comparing cardiopulmonary exercise test values obtained from the arm cycle and the leg cycle respectively in healthy adults. Int. J. Sports Phys. Ther. 2016, 11, 1006–1039. [Google Scholar] [PubMed]
- Achten, J.; Venables, M.C.; Jeukendrup, A.E. Fat oxidation rates are higher during running compared with cycling over a wide range of intensities. Metab. Clin. Exp. 2003, 52, 747–752. [Google Scholar] [CrossRef]
- Sanchís-Moysi, J.; Idoate, F.; Olmedillas, H.; Guadalupe-Grau, A.; Alayón, S.; Carreras, A.; Dorado, C.; Calbet, J.A. The upper extremity of the professional tennis player: Muscle volumes, fiber-type distribution and muscle strength. Scand. J. Med. Sci. Sports 2010, 20, 524–534. [Google Scholar] [CrossRef]
- Koppo, K.; Bouckaert, J.; Jones, A.M. Oxygen uptake kinetics during high-intensity arm and leg exercise. Resp Physio Neurobiol 2002, 133, 241–250. [Google Scholar] [CrossRef]
- Ara, I.; Larsen, S.; Stallknecht, B.; Guerra, B.; Morales-Alamo, D.; Andersen, J.L.; Ponce-González, J.G.; Guadalupe-Grau, A.; Galbo, H.; Calbet, J.A.; et al. Normal mitochondrial function and increased fat oxidation capacity in leg and arm muscles in obese humans. Int. J. Obes. 2011, 35, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.; Ara, I.; Rabøl, R.; Andersen, J.L.; Boushel, R.; Dela, F.; Helge, J.W. Are substrate use during exercise and mitochondrial respiratory capacity decreased in arm and leg muscle in type 2 diabetes? Diabetologia 2009, 52, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.A.; Burns, P.; Kressler, J.; Nash, M.S. Heavy reliance on carbohydrate across a wide range of exercise intensities during voluntary arm ergometry in persons with paraplegia. J. Spinal Cord Med. 2013, 36, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Gahete, F.; Sanchez-Delgado, G.; Jurado-Fasoli, L.; De-la-O, A.; Castillo, M.J.; Helge, J.W.; Ruiz, J.R. Assessment of maximal fat oxidation during exercise: A systematic review. Scand. J. Med. Sci. Sports 2019, 29, 910–921. [Google Scholar] [CrossRef]
- Smith, P.M.; Price, M.J. Upper body exercise. In Sport and Exercise Physiology Testing Guidelines, the British Association of Sports and Exercise Sciences Guide: Sports Testing; Winter, E.M., Jones, A.M., Davidson, R.C.R., Bromley, P.D., Mercer, T.H., Eds.; Routledge: London, UK, 2007; Volume 1, pp. 138–144. [Google Scholar]
- Lyons, S.; Richardson, M.; Bishop, P.; Smith, J.; Heath, H.; Giesen, J. Excess post-exercise oxygen consumption in untrained men following exercise of equal energy expenditure: Comparisons of upper and lower body exercise. Diab. Obes. Metab. 2007, 9, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehab. Med. 1970, 2, 92–98. [Google Scholar]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Shirreffs, S.M.; Leiper, J.B. Blood sampling. In Sport and Exercise Physiology Testing Guidelines, the British Association of Sports and Exercise Sciences Guide: Exercise and Clinical Testing; Winter, E.M., Jones, A.M., Davidson, R.C.R., Bromley, P.D., Mercer, T.H., Eds.; Routledge: London, UK, 2007; Volume 2, pp. 25–29. [Google Scholar]
- Vincent, W.J. Statistics in Kinesiology, 2nd ed.; Human Kinetics: Champaign, IL, USA, 1999; p. 163. [Google Scholar]
- Sawka, M.N.; Pimental, N.A.; Pandolf, K.B. Thermoregulatory responses to upper body exercise. Eur. J. Appl. Physiol. 1984, 52, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Robertson, R.J.; Goss, F.L.; Dasilva, S.G.; Suminski, R.R.; Utter, A.C.; Zoeller, R.F.; Metz, K.F. Metabolic efficiency during arm and leg exercise at the same relative intensities. Med. Sci. Sports Exerc. 1997, 29, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Sedlock, D.A.; Schneider, D.A.; Gass, E.; Gass, G. Excess post-exercise oxygen consumption in spinal cord-injured men. Eur. J. Appl. Physiol. 2004, 93, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Calbet, J.A.; González-Alonso, J.; Helge, J.W.; Søndergaard, H.; Munch-Andersen, T.; Saltin, B.; Boushel, R. Central and peripheral hemodynamics in exercising humans: Leg vs. arm exercise. Scand. J. Med Sci. Sports 2015, 25 (Suppl. 4), 144–157. [Google Scholar] [CrossRef] [PubMed]
- Sanada, K.; Kearns, C.F.; Kojima, K.; Abe, T. Peak oxygen uptake during running and arm cranking normalized to total and regional skeletal muscle mass measured by magnetic resonance imaging. Eur. J. Appl. Physiol. 2005, 93, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Eston, R.G.; Brodie, D.A. Responses to arm and leg ergometry. Br. J. Sports Med. 1986, 20, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Randell, R.K.; Rollo, I.; Roberts, T.J.; Dalrymple, K.J.; Jeukendrup, A.E.; Carter, J.M. Maximal fat oxidation rates in an athletic population. Med. Sci. Sports Exerc. 2017, 49, 133–140. [Google Scholar] [CrossRef]
- Robinson, S.L.; Hattersley, J.; Frost, G.S.; Chambers, E.S.; Wallis, G.A. Maximal fat oxidation during exercise is positively associated with 24-hour fat oxidation and insulin sensitivity in young, healthy men. J. Appl. Physiol. 2015, 118, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, G.; Eves, F.F.; Glover, E.I.; Robinson, S.L.; Vernooij, C.A.; Thompson, J.L.; Wallis, G.A. Dietary intake is independently associated with the maximal capacity for fat oxidation during exercise. Am. J. Clin. Nutr. 2017, 105, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Karppinen, J.E.; Rottensteiner, M.; Wiklund, P.; Hämäläinen, K.; Laakkonen, E.K.; Kaprio, J.; Kainulainen, H.; Kujala, U.M. Fat oxidation at rest and during exercise in male monozygotic twins. Eur. J. Appl. Physiol. 2019, 119, 2711–2722. [Google Scholar] [CrossRef] [PubMed]
- Rosenkilde, M.; Nordby, P.; Nielsen, L.B.; Stallknecht, B.M.; Helge, J.W. Fat oxidation at rest predicts peak fat oxidation during exercise and metabolic phenotype in overweight men. Int. J. Obes. 2010, 34, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Venables, M.C.; Jeukendrup, A.E. Endurance training and obesity: Effect on substrate metabolism and insulin sensitivity. Med. Sci. Sports Exerc. 2008, 40, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wang, X.; Wang, J. Effects of supervised exercise training at the intensity of maximal fat oxidation in overweight young women. J. Exerc. Sci. Fit. 2012, 10, 64–69. [Google Scholar] [CrossRef]
- Wang, J.; Tan, S.; Cao, L. Exercise training at the maximal fat oxidation intensity improved health-related physical fitness in overweight middle-aged women. J. Exerc. Sci. Fit. 2015, 13, 111–116. [Google Scholar] [CrossRef]
- Botero, J.P.; Prado, W.L.; Guerra, R.L.; Speretta, G.F.; Leite, R.D.; Prestes, J.; Sanz, A.V.; Lyons, S.; de Azevedo, P.H.; Baldissera, V.; et al. Does aerobic exercise intensity affect health-related parameters in overweight women? Clin. Physiol. Funct. Imag. 2014, 34, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Bottoms, L.M. Thermoregulatory Responses during Upper Body Exercise, Thermal Stress, Training and Heat Acclimation. Ph.D. Thesis, Coventry University, Coventry, UK, 2008. [Google Scholar]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, E380–E391. [Google Scholar] [CrossRef]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Rosenblatt, J.; Wolfe, R.R. Substrate metabolism during different exercise intensities in endurance-trained women. J. Appl. Physiol. 2000, 88, 1707–1714. [Google Scholar] [CrossRef]
- Frandsen, J.; Poggi, A.I.; Ritz, C.; Larsen, S.; Dela, F.; Helge, J.W. Peak fat oxidation rate is closely associated with plasma free fatty acid concentrations in Women; Similar to men. Front. Physiol. 2021, 12, 696261. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Gahete, F.; Guillermo Sanchez-Delgado, G.; Ruiz, J. Commentary: Contextualising maximal fat oxidation during exercise: Determinants and normative values. Front. Physiol. 2018, 9, 599. [Google Scholar] [CrossRef]
- Orr, J.L.; Williamson, P.; Anderson, W.; Ross, R.; McCafferty, S.; Fettes, P. Cardiopulmonary exercise testing: Arm crank vs cycle ergometry. Anaesth 2013, 68, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Bhambhani, Y.N. Prediction of stroke volume during upper and lower body exercise in men and women. Arch. Phys. Med. Rehab. 1995, 76, 713–718. [Google Scholar] [CrossRef]
- Miller, A.E.; MacDougall, J.D.; Tarnopolsky, M.A.; Sale, D.G. Gender differences in strength and muscle fiber characteristics. Eur. J. Appl. Physiol. 1993, 66, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C. Sex-based differences in endurance exercise muscle metabolism: Impact on exercise and nutritional strategies to optimize health and performance in women. Exp. Physiol. 2016, 101, 243–249. [Google Scholar] [CrossRef] [PubMed]

| ACE | CE | p | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| O2 peak (L·min−1) | 2.73 ± 0.64 | 3.62 ± 0.46 | <0.001 |
| O2peak (mL·kg·−1min−1) | 36 ± 7 | 48 ± 5 | <0.001 |
| Epeak (L·min−1) | 100.4 ± 19.6 | 120.1 ± 19.4 | 0.003 |
| POpeak (W) | 133 ± 21 | 239 ± 33 | <0.001 |
| HRpeak (beats·min−1) | 170 ± 16 | 182 ± 11 | 0.020 |
| RPEC (Borg Scale) | 16 ± 3 | 18 ± 2 | 0.065 |
| RPEL (Borg Scale) | 19 ± 1 | 19 ± 1 | 0.120 |
| BLapeak (mmol·L−1) | 7.6 ± 1.9 | 9.0 ± 2.9 | 0.134 |
| BLa+5 (mmol·L−1) | 8.0 ± 1.7 | 8.4 ± 3.0 | 0.179 |
| RERpeak | 1.00 ± 0.10 | 0.96 ± 0.13 | 0.328 |
| Stage | %POpeak | p | ||||||
|---|---|---|---|---|---|---|---|---|
| 40 | 50 | 60 | 70 | %POpeak | Stage | Int. | ||
| HR | 2 min | 103 ± 22 | 122 ± 19 | 135 ± 22 | 151 ± 22 | <0.05 | 0.556 | 0.997 |
| (beats·min−1) | 3 min | 107 ± 23 | 125 ± 19 | 138 ± 22 | 152 ± 23 | |||
| O2 | 2 min | 0.65 ± 0.11 | 1.04 ± 0.30 | 1.34 ± 0.31 | 1.58 ± 0.33 | <0.05 | 0.983 | 0.991 |
| (L·min−1) | 3 min | 0.62 ± 0.11 | 1.07 ± 0.35 | 1.34 ± 0.33 | 1.58 ± 0.35 | |||
| CO2 | 2 min | 0.57 ± 0.16 | 0.94 ± 0.21 | 1.23 ± 0.25 | 1.43 ± 0.31 | <0.05 | 0.773 | 0.919 |
| (L·min−1) | 3 min | 0.57 ± 0.15 | 1.03 ± 0.22 | 1.23 ± 0.26 | 1.42 ± 0.41 | |||
| RER | 2 min | 0.83 ± 0.03 | 0.88 ± 0.03 | 0.95 ± 0.03 | 0.96 ± 0.03 | <0.05 | 0.145 | 0.774 |
| 3-min | 0.83 ± 0.03 | 0.92 ± 0.03 | 0.96 ± 0.04 | 0.95 ± 0.04 | ||||
| FOx | 2 min | 0.16 ± 0.04 | 0.20 ± 0.06 | 0.22 ± 0.08 | 0.31 ± 0.14 | 0.324 | 0.963 | 0.986 |
| (g·min−1) | 3 min | 0.16 ± 0.04 | 0.17 ± 0.06 | 0.25 ± 0.10 | 0.33 ± 0.15 | |||
| CHO | 2 min | 0.81 ± 0.78 | 1.49 ± 0.63 | 2.00 ± 0.82 | 2.20 ± 1.57 | 0.003 | 0.692 | 0.918 |
| (g·min−1) | 3 min | 0.92 ± 0.96 | 1.88 ± 0.83 | 1.95 ± 1.02 | 2.15 ± 1.62 | |||
| RPEC | 2 min | 7.2 ± 0.7 | 8.8 ± 1.0 | 10.6 ± 1.3 | 12.6 ± 1.1 | <0.05 | 0.145 | 0.774 |
| (Borg Scale) | 3 min | 7.2 ± 0.7 | 9.1 ± 1.1 | 10.2 ± 1.3 | 13.0 ± 1.6 | |||
| RPEL | 2 min | 8.4 ± 1.2 | 11.0 ± 1.2) | 13.1 ± 0.8 | 14.3 ± 0.9 | <0.05 | 0.088 | 0.968 |
| (Borg Scale) | 3 min | 8.9 ± 1.1 | 12.0 ± 1.0 | 14.3 ± 0.8 | 14.8 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Price, M.; Bottoms, L.; Hill, M.; Eston, R. Maximal Fat Oxidation during Incremental Upper and Lower Body Exercise in Healthy Young Males. Int. J. Environ. Res. Public Health 2022, 19, 15311. https://doi.org/10.3390/ijerph192215311
Price M, Bottoms L, Hill M, Eston R. Maximal Fat Oxidation during Incremental Upper and Lower Body Exercise in Healthy Young Males. International Journal of Environmental Research and Public Health. 2022; 19(22):15311. https://doi.org/10.3390/ijerph192215311
Chicago/Turabian StylePrice, Mike, Lindsay Bottoms, Matthew Hill, and Roger Eston. 2022. "Maximal Fat Oxidation during Incremental Upper and Lower Body Exercise in Healthy Young Males" International Journal of Environmental Research and Public Health 19, no. 22: 15311. https://doi.org/10.3390/ijerph192215311
APA StylePrice, M., Bottoms, L., Hill, M., & Eston, R. (2022). Maximal Fat Oxidation during Incremental Upper and Lower Body Exercise in Healthy Young Males. International Journal of Environmental Research and Public Health, 19(22), 15311. https://doi.org/10.3390/ijerph192215311






