Post-COVID-19 Syndrome in Outpatients and Its Association with Viral Load
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ethical Aspects
2.3. Determination of SARS-CoV-2 in Patients
2.4. Survey
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IMSS. COVID en México 2022. Available online: https://coronavirus.gob.mx/ (accessed on 5 August 2022).
- Hopkins, J. Coronavirus Research Center 2022. Available online: https://coronavirus.jhu.edu/map.html (accessed on 18 August 2022).
- Lvov, D.K.; Alkhovsky, S.V.; Kolobukhina, L.V.; Burtseva, E.I. Etiology of epidemic outbreaks COVID-19 on Wuhan, Hubei province, Chinese People Republic associated with 2019-nCoV (Nidovirales, Coronaviridae, Coronavirinae, Betacoronavirus, Subgenus Sarbecovirus): Lessons of SARS-CoV outbreak. Vopr. Virusol. 2020, 65, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Fajnzylber, J.; Regan, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Worrall, D.; Giguel, F.; Piechocka-Trocha, A.; Atyeo, C.; et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat. Commun. 2020, 11, 5493. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.; Havervall, S.; Rosell, A.; Aguilera, K.; Parv, K.; Von Meijenfeldt, F.A.; Phillipson, M. Circulating markers of neutrophil extracellular traps are of prognostic value in patients with COVID-19. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Mardani, R.; Vasmehjani, A.A.; Zali, F.; Gholami, A.; Nasab, S.D.M.; Kaghazian, H.; Kaviani, M.; Ahmadi, N. Laboratory Parameters in Detection of COVID-19 Patients with Positive RT-PCR; a Diagnostic Accuracy Study. Arch. Acad. Emerg. Med. 2020, 8, e43. [Google Scholar] [CrossRef] [PubMed]
- Alimohamadi, Y.; Sepandi, M.; Taghdir, M.; Hosamirudsari, H. Determine the most common clinical symptoms in COVID-19 patients: A systematic review and meta-analysis. J. Prev. Med. Hyg. 2020, 61, E304–E312. [Google Scholar]
- Oronsky, B.; Larson, C.; Hammond, T.C.; Oronsky, A.; Kesari, S.; Lybeck, M.; Reid, T.R. A Review of Persistent Post-COVID Syndrome (PPCS). Clin. Rev. Allergy Immunol. 2021, 1–9. [Google Scholar] [CrossRef]
- Chaloemwong, J.; Tantiworawit, A.; Rattanathammethee, T.; Hantrakool, S.; Chai-Adisaksopha, C.; Rattarittamrong, E.; Norasetthada, L. Useful clinical features and hematological parameters for the diagnosis of dengue infection in patients with acute febrile illness: A retrospective study. BMC Hematol. 2018, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.R.; Wang, K.; Yin, L.; Zhao, W.F.; Xue, Q.; Peng, M.; Min, B.Q.; Tian, Q.; Leng, H.X.; Du, J.L.; et al. Mental health and psychosocial problems of medical health workers during the COVID-19 epidemic in China. Psychother. Psychosom. 2020, 89, 242–250. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Jones, T.C.; Mühlemann, B.; Veith, T.; Biele, G.; Zuchowski, M.; Hofmann, J.; Stein, A.; Edelmann, A.; Corman, V.M.; Drosten, C. An analysis of SARS-CoV-2 viral load by patient age. MedRxiv 2020. [CrossRef]
- Salamanna, F.; Veronesi, F.; Martini, L.; Landini, M.P.; Fini, M. Post-COVID-19 syndrome: The persistent symptoms at the post-viral stage of the disease. A systematic review of the current data. Front. Med. 2021, 8, 653516. [Google Scholar] [CrossRef] [PubMed]
- Tejerina, F.; Catalan, P.; Rodriguez-Grande, C.; Adan, J.; Rodriguez-Gonzalez, C.; Muñoz, P.; Aldamiz, T.; Diez, C.; Perez, L.; Fanciulli, C.; et al. Post-COVID-19 syndrome. SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infect. Dis. 2022, 22, 211. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Buchan, B.W.; Hoff, J.S.; Gmehlin, C.G.; Perez, A.; Faron, M.L.; Munoz-Price, L.S.; Ledeboer, N.A. Distribution of SARS-CoV-2 PCR Cycle Threshold Values Provide Practical Insight Into Overall and Target-Specific Sensitivity among Symptomatic Patients. Am. J. Clin. Pathol. 2020, 154, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.H.B.; Wing, Y.K.; Yu, M.W.M.; Leung, C.M.; Ma, R.C.; Kong, A.P.; So, W.Y.; Fong, S.Y.Y.; Lam, S.P. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: Long-term follow-up. Arch. Intern. Med. 2009, 169, 2142–2147. [Google Scholar] [CrossRef]
- Lee, S.M.; Kang, W.S.; Cho, A.-R.; Kim, T.; Park, J.K. Psychological impact of the 2015 MERS outbreak on hospital workers and quarantined hemodialysis patients. Compr. Psychiatry 2018, 87, 123–127. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Caronna, E.; Ballvé, A.; Llauradó, A.; Gallardo, V.J.; Ariton, D.M.; Lallana, S.; Lopez Maza, S.; Olive Gadea, M.; Quibus, L.; Restrepo, J.L.; et al. Headache: A striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia 2020, 40, 1410–1421. [Google Scholar] [CrossRef]
- Vaira, L.A.; Hopkins, C.; Petrocelli, M.; Lechien, J.R.; Chiesa-Estomba, C.M.; Salzano, G.; Cucurullo, M.; Salzano, F.A.; Saussez, S.; Boscolo-Rizzo, P.; et al. Smell and taste recovery in coronavirus disease 2019 patients: A 60-day objective and prospective study. J. Laryngol. Otol. 2020, 134, 703–709. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Polesel, J.; Vaira, L.A. Smell and Taste Dysfunction after COVID-19; British Medical Journal Publishing Group: London, UK, 2022. [Google Scholar]
- da Silva Júnior, R.T.; Apolonio, J.S.; Cuzzuol, B.R.; da Costa, B.T.; Silva, C.S.; Araújo, G.R.L.; Luz, M.S.; Marques, H.S.; de Sá Santos, L.K.; Pinheiro, S.L.R.; et al. COVID-19 neuropsychiatric repercussions: Current evidence on the subject. World J. Methodol. 2022, 12, 365–380. [Google Scholar] [CrossRef]
- Villa, D.; Ardolino, G.; Borellini, L.; Cogiamanian, F.; Vergari, M.; Savojardo, V.; Peyvandi, F.; Barbieri, S. Subclinical myopathic changes in COVID-19. Neurol. Sci. 2021, 42, 3973–3979. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.H. Cerebral venous sinus thrombosis and coronavirus infection (COVID-19): A multicenter Asian study. J. Neurol. Sci. 2021, 429, 29–39. [Google Scholar]
- Athamneh, M.; Sa’Di, Q.; Aldabbour, B.; Khader, Y.; Batayha, W. Knowledge, attitudes, and impact of COVID-19 pandemic among neurology patients in Jordan: A cross-sectional study. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 104. [Google Scholar] [CrossRef] [PubMed]
- Del Carpio-Orantes, L.; García-Méndez, S.; Solís-Sánchez, I.; Perfecto-Arroyo, M.A.; Moreno-Aldama, N.P.; Sánchez-Díaz, J.S. Neurological symptoms in patients with COVID-19 as manifestation of severity and prognosis, the case of anosmia and dysgeusia. J. Neurol. Sci. 2021, 429, 2–9. [Google Scholar] [CrossRef]
- Jeong, H.; Yim, H.W.; Song, Y.-J.; Ki, M.; Min, J.-A.; Cho, J.; Chae, J.-H. Mental health status of people isolated due to Middle East Respiratory Syndrome. Epidemiol. Health 2016, 38, e2016048. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Rodríguez-Hidalgo, A.J.; Pantaleón, Y.; Dios, I.; Falla, D. Fear of COVID-19, stress, and anxiety in university undergraduate students: A predictive model for depression. Front. Psychol. 2020, 11, 591797. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Shi, L.; Li, J.; Jiang, Z.; Xie, C.; Luo, S.; Ling, L.; Lin, H.; Chen, Z.; Zhao, Y.; et al. The Trends of Psychological Status of People Entering from High-Risk Areas of COVID-19 Coronavirus During the Quarantine in Dedicated Hotels: A Longitudinal Survey Study from Guangzhou, China. Risk Manag. Health Policy 2021, 14, 5005–5014. [Google Scholar] [CrossRef]
- WHO. Vías de Transmisión del Virus de la COVID-19: Repercusiones para las Recomendaciones Relativas a las Precauciones en Materia de Prevención y Control de las Infecciones: World Health Organization. 2022. Available online: https://www.who.int/es/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (accessed on 15 September 2022).
- Sinha, M.; Pande, B.; Sinha, R. Impact of Covid-19 Lockdown on Sleep-Wake Schedule and Associated Lifestyle Related Behavior: A National Survey. J. Public Health Res. 2020, 9, 1826. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Moreira, T.C.L.; de Araújo, A.L.; Imamura, M.; Damiano, R.F.; Garcia, M.L.; Sawamura, M.V.; Pinna, F.R.; Guedes, B.F.; Gonçalves, F.A.R.; et al. Clinical, sociodemographic and environmental factors impact post-COVID-19 syndrome. J. Glob. Health 2022, 12, 05029. [Google Scholar] [CrossRef]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef] [PubMed]
- Katoto, P.D.M.C.; Brand, A.S.; Bakan, B.; Obadia, P.M.; Kuhangana, C.; Kayembe-Kitenge, T.; Kitenge, J.P.; Nkulu, C.B.L.; Vanoirbeek, J.; Nawrot, T.S.; et al. Acute and chronic exposure to air pollution in relation with incidence, prevalence, severity and mortality of COVID-19: A rapid systematic review. Environ. Health 2021, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.S.; Jerrett, M.; Kawachi, I.; Levy, J.I.; Cohen, A.J.; Gouveia, N.; Wilkinson, P.; Fletcher, T.; Cifuentes, L.; Schwartz, J.; et al. Health, wealth, and air pollution: Advancing theory and methods. Environ. Health Perspect. 2003, 111, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.J.; Billet, S.; Landkocz, Y.; Fougère, B. Inflammation at the Crossroads: The Combined Effects of COVID-19, Ageing, and Air Pollution. J. Frailty Aging 2021, 10, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Argyropoulos, K.V.; Serrano, A.; Hu, J.; Black, M.; Feng, X.; Shen, G.; Call, M.; Kim, M.J.; Lytle, A.; Belovarac, B.; et al. Association of initial viral load in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients with outcome and symptoms. Am. J. Pathol. 2020, 190, 1881–1887. [Google Scholar] [CrossRef]
- Shah, S.; Singhal, T.; Davar, N.; Thakkar, P. No correlation between Ct values and severity of disease or mortality in patients with COVID 19 disease. Indian J. Med. Microbiol. 2020, 39, 116–117. [Google Scholar] [CrossRef]
- Kirby, T. COVID-19 survivor experiencing long-term symptoms. Lancet Respir. Med. 2021, 9, 570–572. [Google Scholar] [CrossRef]
- Bitker, L.; Dhelft, F.; Chauvelot, L.; Frobert, E.; Folliet, L.; Mezidi, M.; Trouillet-Assant, S.; Belot, A.; Lina, B.; Wallet, F.; et al. Protracted viral shedding and viral load are associated with ICU mortality in Covid-19 patients with acute respiratory failure. Ann. Intensiv. Care 2020, 10, 167. [Google Scholar] [CrossRef]
- Koopmans, P.C.; Bakhtali, R.; Katan, A.A.; Groothoff, J.W.; Roelen, C.A.M. Return to work following sickness absence due to infectious mononucleosis. Occup. Med. 2010, 60, 249–254. [Google Scholar] [CrossRef][Green Version]
- Schürks, M.; Rist, P.M.; Shapiro, R.E.; Kurth, T. Migraine and mortality: A systematic review and meta-analysis. Cephalalgia 2011, 31, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Raman, B.; Bluemke, D.A.; Lüscher, T.F.; Neubauer, S. Long COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef] [PubMed]

| Total | Male | Females | |
|---|---|---|---|
| Patients | 76 | 40 | 36 |
| Age (years) | 20–70 | 45 ± 25 | 30 ± 25 |
| Patients with no symptoms after COVID-19 infection | 6 | 5 | 1 |
| Persistent clinical symptomatology | |||
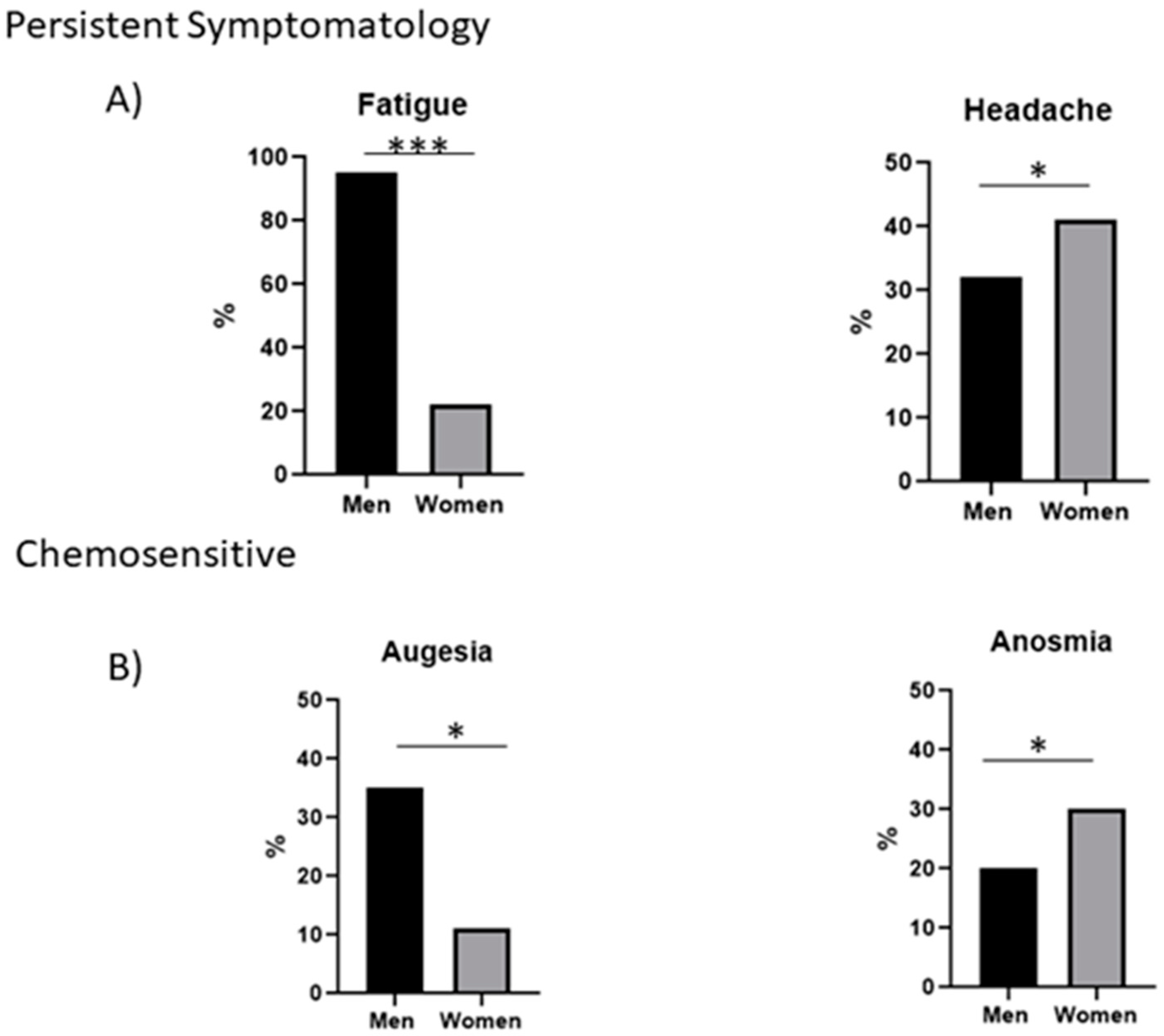
| Fatigue | 46 | 38 | 8 |
| Muscle pain | 22 | 13 | 9 |
| Headache | 28 | 13 | 15 |
| Cough | 15 | 10 | 5 |
| Diarrhea | 12 | 7 | 5 |
| Nausea | 2 | 0 | 2 |
| Chemosensitive problems | |||
| Smell | 19 | 8 | 11 |
| Taste | 18 | 14 | 4 |
| Vision | 10 | 6 | 4 |
| Audition | 2 | 1 | 1 |
| Cognitive skill changes | |||
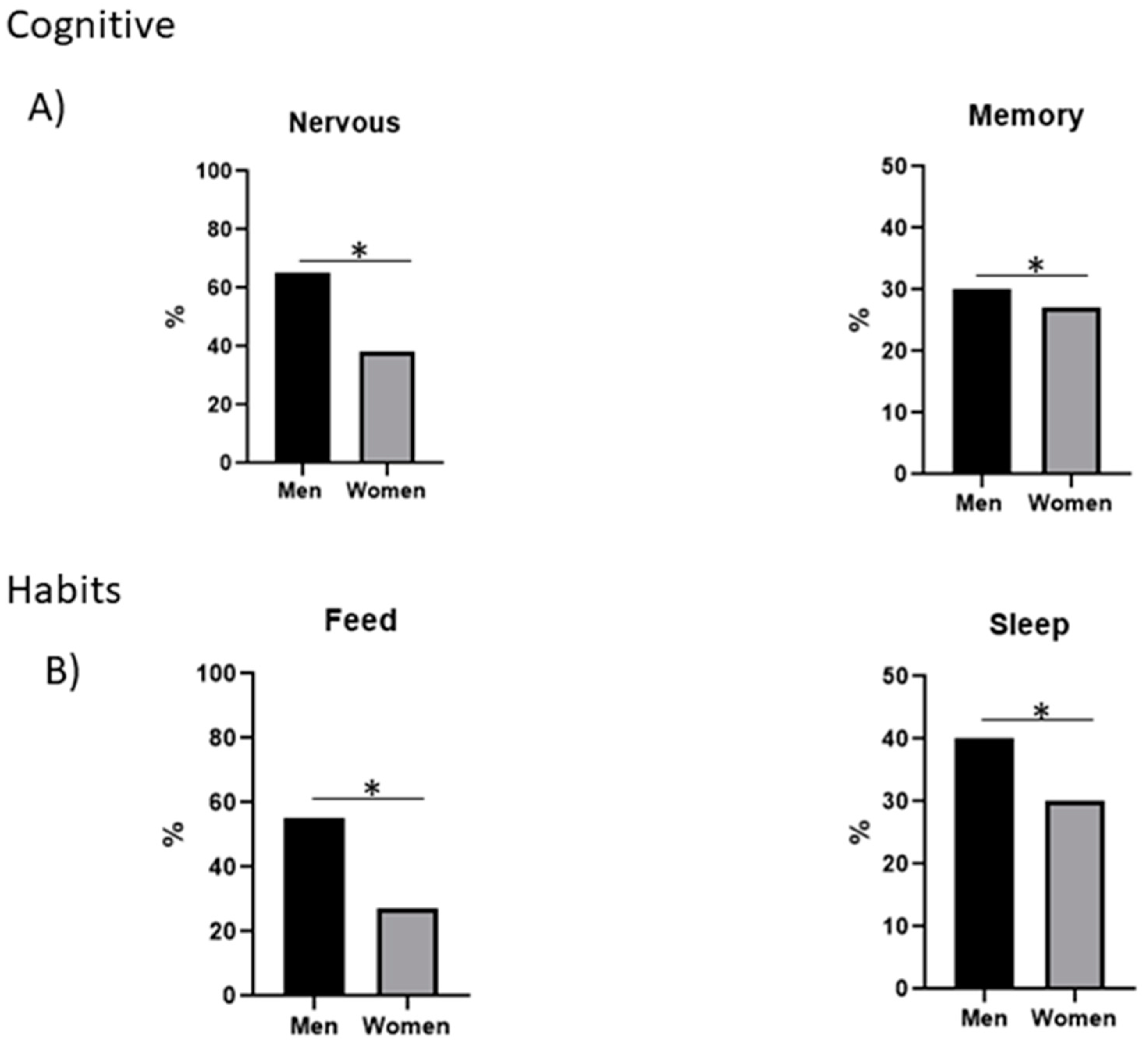
| Nervousness | 40 | 26 | 14 |
| Remembering problems | 22 | 12 | 10 |
| Confusion | 19 | 11 | 8 |
| Happiness | 10 | 7 | 3 |
| Sadness | 16 | 8 | 8 |
| Sudden discomfort | 10 | 6 | 4 |
| Mood | 10 | 6 | 4 |
| Fear | 16 | 9 | 7 |
| The feeling of change (partner, house) | 11 | 7 | 4 |
| Decision-making problems | 7 | 6 | 1 |
| Problems in expressing oneself | 4 | 4 | 0 |
| Mathematical troubleshooting problems. | 8 | 6 | 2 |
| Impulsive | 4 | 4 | 0 |
| Habit changes | |||
| Feeding | 32 | 22 | 10 |
| Sleep | 27 | 16 | 11 |
| Load Viral of SARS-CoV-2 Based on E Gene | |
|---|---|
| Ct | Log Copy/µL |
| 20< | <451,743 |
| 20 | 451,743 |
| 21 | 241,631 |
| 22 | 129,245 |
| 23 | 69,131 |
| 24 | 36,977 |
| 25 | 19,779 |
| 26 | 10,579 |
| 27 | 5659 |
| 28 | 3027 |
| 29 | 1619 |
| 30 | 866 |
| 31 | 463 |
| 32 | 248 |
| 33 | 133 |
| 34 | 71 |
| 35 | 38 |
| 36 | 20 |
| 37 | 11 |
| 38 | 6 |
| 39 | 3 |
| 40 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girón Pérez, D.A.; Fonseca-Agüero, A.; Toledo-Ibarra, G.A.; Gomez-Valdivia, J.d.J.; Díaz-Resendiz, K.J.G.; Benitez-Trinidad, A.B.; Razura-Carmona, F.F.; Navidad-Murrieta, M.S.; Covantes-Rosales, C.E.; Giron-Pérez, M.I. Post-COVID-19 Syndrome in Outpatients and Its Association with Viral Load. Int. J. Environ. Res. Public Health 2022, 19, 15145. https://doi.org/10.3390/ijerph192215145
Girón Pérez DA, Fonseca-Agüero A, Toledo-Ibarra GA, Gomez-Valdivia JdJ, Díaz-Resendiz KJG, Benitez-Trinidad AB, Razura-Carmona FF, Navidad-Murrieta MS, Covantes-Rosales CE, Giron-Pérez MI. Post-COVID-19 Syndrome in Outpatients and Its Association with Viral Load. International Journal of Environmental Research and Public Health. 2022; 19(22):15145. https://doi.org/10.3390/ijerph192215145
Chicago/Turabian StyleGirón Pérez, Daniel Alberto, Aimee Fonseca-Agüero, Gladys Alejandra Toledo-Ibarra, Jaqueline de Jesus Gomez-Valdivia, Karina Janice Guadaluope Díaz-Resendiz, Alma Benitez Benitez-Trinidad, Francisco Fabian Razura-Carmona, Migdalia Sarahy Navidad-Murrieta, Carlos Eduardo Covantes-Rosales, and Manuel Ivan Giron-Pérez. 2022. "Post-COVID-19 Syndrome in Outpatients and Its Association with Viral Load" International Journal of Environmental Research and Public Health 19, no. 22: 15145. https://doi.org/10.3390/ijerph192215145
APA StyleGirón Pérez, D. A., Fonseca-Agüero, A., Toledo-Ibarra, G. A., Gomez-Valdivia, J. d. J., Díaz-Resendiz, K. J. G., Benitez-Trinidad, A. B., Razura-Carmona, F. F., Navidad-Murrieta, M. S., Covantes-Rosales, C. E., & Giron-Pérez, M. I. (2022). Post-COVID-19 Syndrome in Outpatients and Its Association with Viral Load. International Journal of Environmental Research and Public Health, 19(22), 15145. https://doi.org/10.3390/ijerph192215145









