Spanish Cross-Cultural Adaptation, Rasch Analysis and Validation of the Ocular Comfort Index (OCI) Questionnaire
Abstract
1. Introduction
2. Materials and Methods
2.1. Translation and Cross-Cultural Adaptation of OCI
- (1)
- Direct translation. Two bilingual (English/Spanish) translators, whose native language was Spanish, independently translated the original OCI questionnaire, including instructions, items, and grading options.
- (2)
- Consensus version of the direct translation. The two bilingual translators agreed on the translated version and a template of the instrument was constructed.
- (3)
- Back translation. Another two bilingual (English/Spanish) professional translators blind to the original version, whose mother tongue was English, independently translated the consensus version back into English.
- (4)
- Expert committee review. The panel of experts, including the four translators, two expert optometrists, and a methodologist, compared the back-translated version with the original English version to identify any discrepancies to be resolved by consensus. They consolidated the four previous translations and created the pre-final version of the Spanish OCI designated OCI-versión española (OCIVE).
- (5)
- Pre-testing of the consensus version. Interviews were conducted using a verbal probing technique with 30 native Spanish individuals aged between 18 and 70 years to ensure patient comprehension of OCIVE. The subjects completed the consensus version of the questionnaire, and then commented on words and sentences they considered difficult to understand. No issues emerged in this pre-test.
2.2. Rasch Analysis
- Item fit statistics. Infit and outfit were used to determine whether items fit the Rasch model.
- Dimensionality. Unidimensionality is a fundamental requirement of a measurement, implying that the scale measures a single concept [27]. This was determined by Principal Component Analysis (PCA) of the standardized residuals of the model using the Winsteps application. When the raw variance explained by the measures is ≤50%, multidimensionality is suggested. This indicates that the questionnaire may contain a subset of items that could be measuring a different concept.
- Person Separation Index (PSI) and OCIVE levels of performance. PSI is a measure of the discriminating capacity of a questionnaire. A PSI of two is the minimum accepted level of discrimination for an instrument to produce a valid measure [38]. The number of different levels of performance was calculated, according to a method suitable for clinical samples described by Wright [29,39].
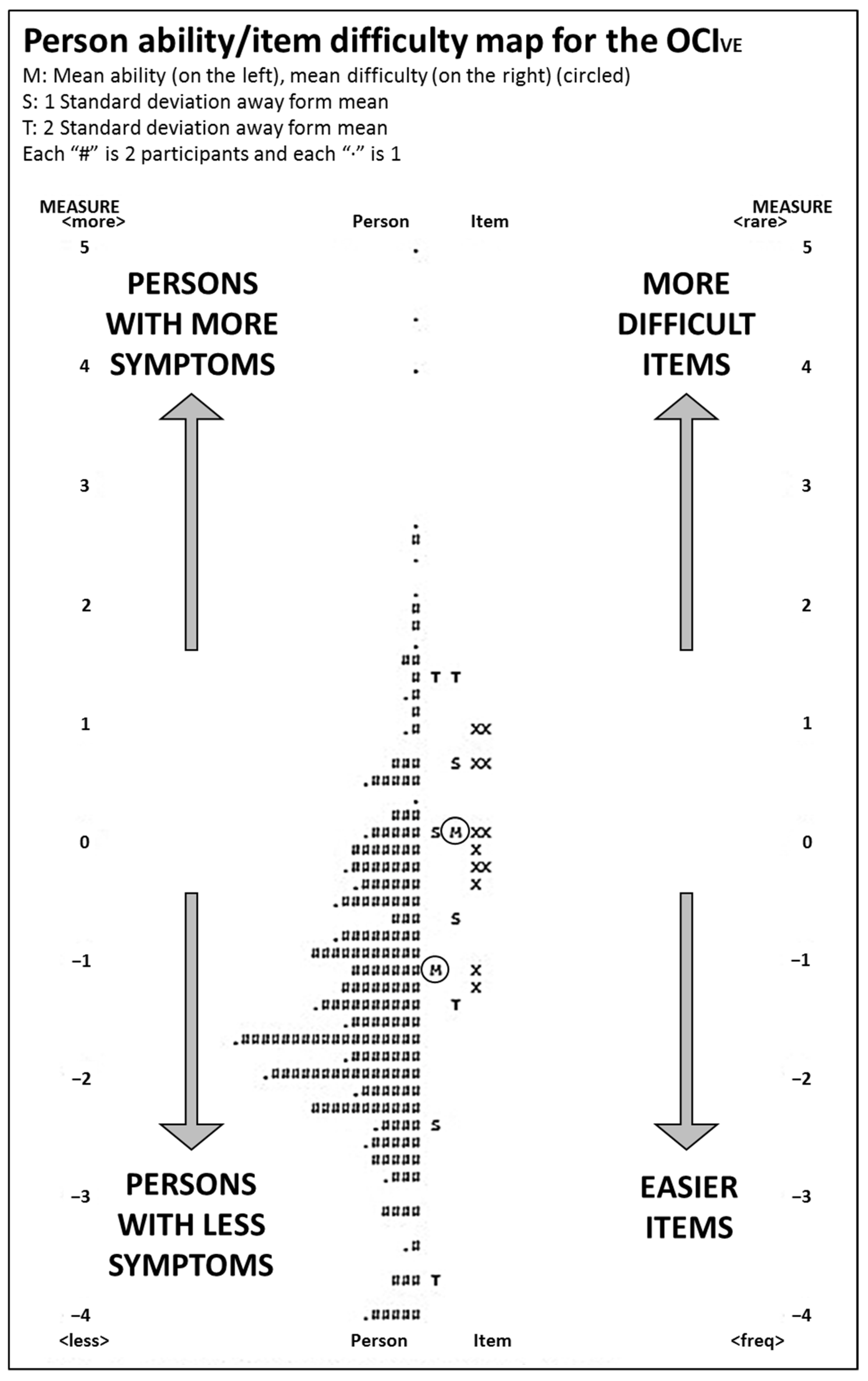
- Targeting. This refers to the extent to which the difficulty of the items matches the abilities of the persons; ideally, they should center on the same mean. This can be assessed visually by observing the person-item map, which is a graphical representation of persons and items on the logit scale. Differences > 1 logit indicate possible mistargeting.
- Differential Item Functioning (DIF). DIF assesses whether items are responded to differently by different population subgroups. We evaluated the DIF of each item by gender and presbyopia (male-female; presbyopia-no presbyopia) using the Mantel–Haenszel method and logit-difference (logistic regression) method implemented in Winsteps. A difference in difficulty of the item measure between the two groups was that: <0.50 logits was considered as no-DIF, minimal DIF as 0.50 to 1.0 logits, and notable DIF as >1.0 logits [36].
2.3. Validity and Repeatability
3. Results
3.1. Spanish Version of OCI (OCIVE)
3.2. Rasch Analysis
3.2.1. Item Fit Statistics
3.2.2. Dimensionality
3.2.3. Person Separation Index and Performance Levels
3.2.4. Targeting
3.2.5. Differential Item Functioning (DIF) by Gender and Presbyopia
3.3. English Version versus Spanish Version
3.4. Convergent Validity, Validity for Known Groups, and Repeatability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Gothwal, V.K.; Wright, T.A.; Lamoureux, E.L.; Pesudovs, K. Activities of daily vision scale: What do the subscales measure? Investig. Ophthalmol. Vis. Sci. 2010, 51, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Liew, M.; Zhang, M.; Kim, E.; Akpek, E.K. Prevalence and predictors of Sjögren’s syndrome in a prospective cohort of patients with aqueous-deficient dry eye. Br. J. Ophthalmol. 2012, 96, 1498–1503. [Google Scholar] [CrossRef]
- Badian, R.A.; Utheim, T.P.; Chen, X.; Utheim, O.A.; Ræder, S.; Ystenæs, A.E.; Aakre, B.M.; Sundling, V. Meibomian gland dysfunction is highly prevalent among first-time visitors at a Norwegian dry eye specialist clinic. Sci. Rep. 2021, 11, 23412. [Google Scholar] [CrossRef]
- Uchino, M.; Dogru, M.; Yagi, Y.; Gotp, E.; Tomita, M.; Kon, T.; Saiki, M.; Matsumoto, Y.; Uchino, Y.; Yokoi, N.; et al. The Features of Dry Eye Disease in a Japanese Elderly Population. Optom. Vis. Sci. 2006, 83, 797–802. [Google Scholar] [CrossRef]
- Uchino, M.; Schaumberg, D.A.; Dogru, M.; Uchino, Y.; Fukagawa, K.; Shimmura, S.; Satoh, T.; Takebayashi, T.; Tsubota, K. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology 2008, 115, 1982–1988. [Google Scholar] [CrossRef]
- Fjaervoll, H.; Fjaervoll, K.; Magno, M.; Moschowits, E.; Vehof, J.; Dartt, D.A.; Utheim, T.P. The association between visual display terminal use and dry eye: A review. Acta Ophthalmol. 2022, 100, 357–375. [Google Scholar] [CrossRef]
- Tan, L.L.; Morgan, P.; Cai, Z.Q.; Straughan, R.A. Prevalence of and risk factors for symptomatic dry eye disease in Singapore. Clin. Exp. Optom. 2015, 98, 45–53. [Google Scholar] [CrossRef]
- Uchino, M.; Nishiwaki, Y.; Michikawa, T.; Shirakawa, K.; Kuwahara, E.; Yamada, M.; Dogru, M.; Schaumberg, D.A.; Kawakita, T.; Takebayashi, T.; et al. Prevalence and Risk Factors of Dry Eye Disease in Japan: Koumi Study. Ophthalmology 2011, 118, 2361–2367. [Google Scholar] [CrossRef]
- Paulsen, A.J.; Cruickshanks, K.J.; Fischer, M.E.; Huang, G.-H.; Klein, B.E.K.; Klein, R.; Dalton, D.S. Dry Eye in the Beaver Dam Offspring Study: Prevalence, Risk Factors, and Health-Related Quality of Life. Am. J. Ophthalmol. 2014, 157, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Versura, P.; Profazio, V.; Cellini, M.; Torreggiani, A.; Caramazza, R. Eye discomfort and air pollution. Ophthalmologica 1999, 213, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Novaes, P.; do Nascimento Saldiva, P.H.; Matsuda, M.; Macchione, M.; Rangel, M.P.; Kara-José, N.; Berra, A. The effects of chronic exposure to traffic derived air pollution on the ocular surface. Environ. Res. 2010, 110, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.A.; Cheon, J.J.; Lee, J.S.; Kim, S.Y.; Chang, S.S. Prevalence of dry eye syndrome after a three-year exposure to a clean room. Ann. Occup. Environ. Med. 2014, 26, 26. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Kumar, N.; Feuer, W.; Lee, D.J. Environmental factors affect the risk of dry eye syndrome in a United States veteran population. Ophthalmology 2014, 121, 972–973. [Google Scholar] [CrossRef]
- Gupta, N.; Prasad, I.; Himashree, G.; D’Souza, P. Prevalence of dry eye at high altitude: A case controlled comparative study. High Alt. Med. Biol. 2008, 9, 327–334. [Google Scholar] [CrossRef]
- Schein, O.D.; Tielsch, J.M.; Munoz, B.; Bandeen-Roche, K.; West, S. Relation between signs and symptoms of dry eye in the elderly: A population-based perspective. Ophthalmology 1997, 104, 1395–1401. [Google Scholar] [CrossRef]
- Begley, C.G.; Chalmers, R.L.; Abetz, L.; Venkataraman, K.; Mertzanis, P.; Caffery, B.A.; Snyder, C.; Edrington, T.; Nelson, D.; Simpson, T. The Relationship between Habitual Patient-Reported Symptoms and Clinical Signs among Patients with Dry Eye of Varying Severity. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4753–4761. [Google Scholar] [CrossRef]
- Nichols, K.K.; Nichols, J.J.; MPH, M.S.; Mitchell, G.L. The Lack of Association Between Signs and Symptoms in Patients With Dry Eye Disease. Cornea 2004, 23, 762–770. [Google Scholar] [CrossRef]
- Lemp, M.; Baudouin, C.; Baum, J. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul. Surf. 2007, 5, 75–92. [Google Scholar]
- Chalmers, R.L.; Begley, C.G.; Edrington, T.; Caffery, B.; Nelson, D.; Snyder, C.; Simpson, T. The agreement between self-assessment and clinician assessment of dry eye severity. Cornea 2005, 24, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Miller, W.L.; Prager, T.C.; Jackson, J.A.; Leach, N.E.; McDermott, A.M.; Christensen, M.T.; Bergmanson, J.P.G. The diagnosis and characteristics of moderate dry eye in non-contact lens wearers. Eye Contact Lens 2005, 31, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Zeev, M.S.B.; Miller, D.D.; Latkany, R. Diagnosis of dry eye disease and emerging technologies. Clin. Ophthalmol. 2014, 8, 581–590. [Google Scholar] [CrossRef]
- Okumura, Y.; Inomata, T.; Iwata, N.; Sung, J.; Fujimoto, K.; Fujio, K.; Midorikawa-Inomata, A.; Miura, M.; Akasaki, Y.; Murakami, A. A review of dry eye questionnaires: Measuring patient-reported outcomes and health-related quality of life. Diagnostics 2020, 10, 559. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health Qual. Life Outcomes 2006, 4, 79. [Google Scholar] [CrossRef]
- Johnson, M.E.; Murphy, P.J. Measurement of ocular surface irritation on a linear interval scale with the ocular comfort index. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4451–4458. [Google Scholar] [CrossRef] [PubMed]
- Khadka, J.; McAlinden, C.; Pesudovs, K. Quality assessment of ophthalmic questionnaires: Review and recommendations. Optom. Vis. Sci. 2013, 90, 720–744. [Google Scholar] [CrossRef] [PubMed]
- McAlinden, C.; Gao, R.; Wang, Q.; Zhu, S.; Yang, J.; Yu, A.; Bron, A.J.; Huang, J. Rasch analysis of three dry eye questionnaires and correlates with objective clinical tests. Ocul. Surf. 2017, 15, 202–210. [Google Scholar] [CrossRef]
- Chao, C.; Golebiowski, B.; Cui, Y.; Stapleton, F. Development of a Chinese version of the ocular comfort index. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3562–3571. [Google Scholar] [CrossRef]
- Pastor, C. El español en el mundo. In Anuario del Instituto Cervantes 2022; Instituto Cervantes: Madrid, Spain, 2022. [Google Scholar]
- Wu, M.; Adams, R.J. Applying the Rasch Model to Psycho-Social Measurement: A Practical Approach; Educational Measurement Solutions: Melbourne, Australia, 2007. [Google Scholar]
- Prolific What Is Prolific and How does It Work? Available online: https://participant-help.prolific.co/hc/en-gb/articles/360022523613-What-is-Prolific-and-how-does-it-work- (accessed on 8 November 2022).
- Gjersing, L.; Caplehom, J.R.; Clausen, T. Cross-cultural adaptation of research instruments: Language, setting, time and statistical considerations. BMC Med. Res. Methodol. 2018, 10, 13. [Google Scholar] [CrossRef]
- Beaton, D.; Bombardier, C.; Guillemin, F.; Ferraz, M. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine 2000, 25, 3186–3191. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Grove, A.; Martin, M.; Eremenco, S.; McElroy, S.; Verjee-Lorenz, A.; Erikson, P. Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-Reported Outcomes (PRO) Measures: Report of the ISPOR task force for translating adaptation. Value Health 2005, 8, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Linacre, J.M. Winsteps® Rasch Measurement Computer Program User’s Guide; Winsteps.com: Beaverton, Oregon, 2020; ISBN 0941938034. [Google Scholar]
- Andrich, D. Rating scales and Rasch measurement. Pharm. Outcomes Res. 2011, 11, 571–585. [Google Scholar] [CrossRef] [PubMed]
- McAlinden, C.; Jonsson, M.; Kugelberg, M.; Lundström, M.; Khadka, J.; Pesudovs, K. Establishing levels of indications for cataract surgery: Combining clinical and questionnaire data into a measure of cataract impact. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Rouse, M.W.; Borsting, E.J.; Lynn Mitchell, G.; Scheiman, M.; Cotter, S.A.; Cooper, J.; Kulp, M.T.; London, R.; Wensveen, J.; Convergence Insufficiency Treatment Trial Group. Validity and realiability of the revised convergence insifficiency symptom survey in adults. Opthalmic Physiol. Opt. 2004, 24, 384–390. [Google Scholar] [CrossRef]
- Linacre, J.M. Sample Size and Item Calibration Stability. Rasch Meas. Trans. 1994, 7, 328. [Google Scholar]
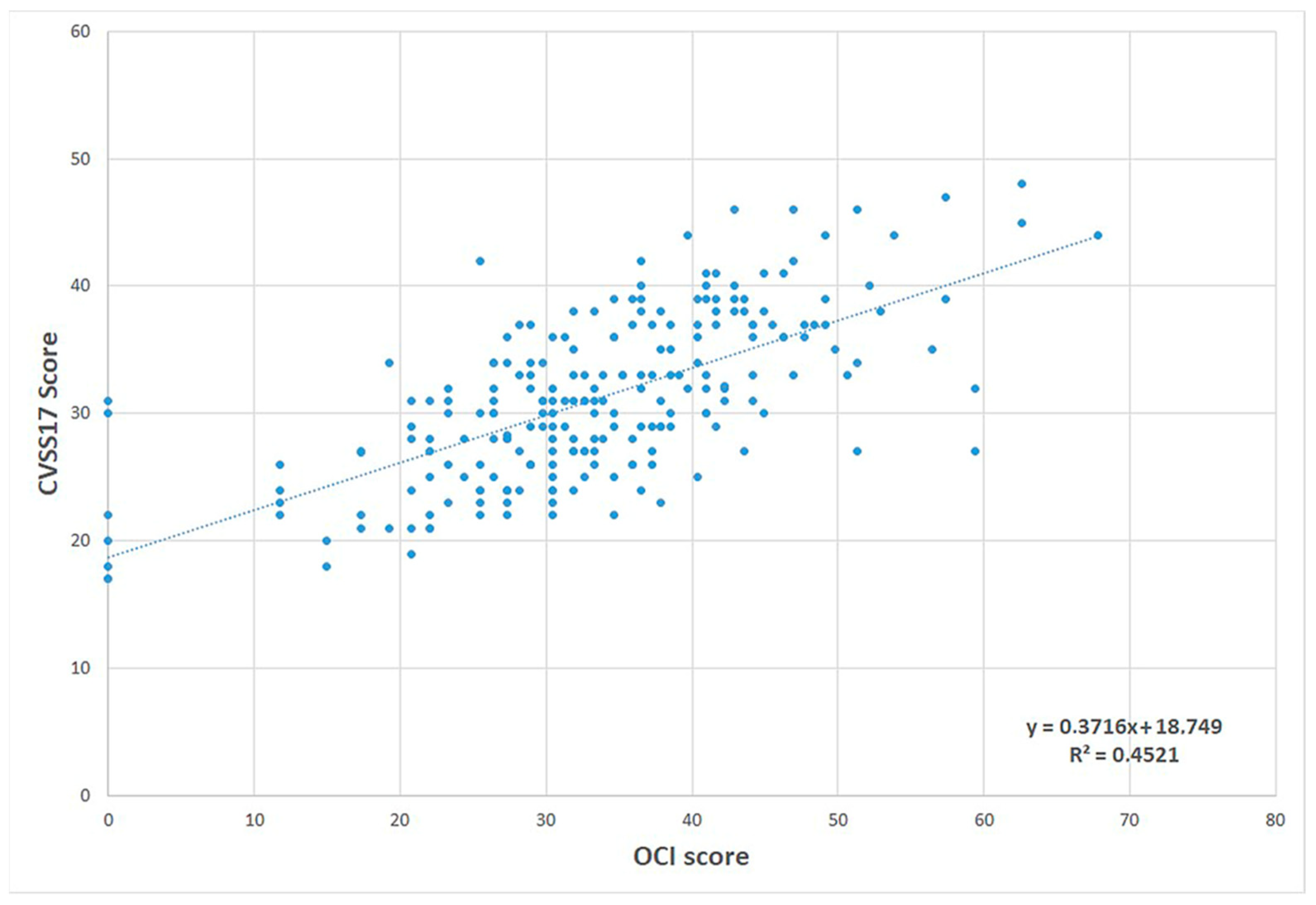
- González-Pérez, M.; Susi, R.; Antona, B.; Barrio, A.; González, E. The Computer-Vision Symptom Scale (CVSS17): Development and initial validation. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4504–4511. [Google Scholar] [CrossRef]
- Bron, A.J. The Doyne Lecture reflections on the tears. Eye 1997, 11, 583–602. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Lamoureux, E.L.; Pallant, J.F.; Pesudovs, K.; Rees, G.; Hassell, J.B.; Keeffe, J.E. The impact of vision impairment questionnaire: An assessment of its domain structure using confirmatory factor analysis and rasch analysis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1001–1006. [Google Scholar] [CrossRef]
- Pesudovs, K.; Burr, J.M.; Harley, C.; Elliott, D.B. The development Assessment, and selection of questionnaires. Optom. Vis. Sci. 2007, 84, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Khadka, J.; Gothwal, V.K.; McAlinden, C.; Lamoureux, E.L.; Pesudovs, K. The importance of rating scales in measuring patient-reported outcomes. Health Qual. Life Outcomes 2012, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- McNeely, R.N.; Moutari, S.; Arba-Mosquera, S.; Verma, S.; Moore, J.E. An alternative application of Rasch analysis to assess data from ophthalmic patient-reported outcome instruments. PLoS ONE 2018, 13, e0197503. [Google Scholar] [CrossRef]
- González-Pérez, M.; Susi, R.; Barrio, A.; Antona, B. Five levels of performance and two subscales identified in the computer-vision symptom scale (CVSS17) by Rasch, factor, and discriminant analysis. PLoS ONE 2018, 13, e0202173. [Google Scholar] [CrossRef]
- Streiner, D.L. Starting at the beginning: An introduction to coefficient alpha and internal consistency. J. Personal. Assess. 2003, 80, 99–103. [Google Scholar] [CrossRef] [PubMed]
- McAlinden, C.; Pesudovs, K.; Moore, J.E. The development of an instrument to measure quality of vision: The quality of vision (QoV) questionnaire. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5537–5545. [Google Scholar] [CrossRef] [PubMed]
- Begley, C.G.; Chalmers, R.L.; Mitchell, G.L.; Nichols, K.K.; Caffery, B.; Simpson, T.; DuToit, R.; Portello, J.; Davis, L. Characterization of ocular surface symptoms from optometric practices in North America. Cornea 2001, 20, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.; Massof, R.W. Validating Translations of Rating Scale Questionnaires Using Rasch Analysis. Ophthalmic Epidemiol. 2017, 24, 1–2. [Google Scholar] [CrossRef]


| Ocular Comfort Index (OCI) Original Version (British English) | Escala Índice Confort Ocular (OCIVE) Translated Version to Spanish | |
|---|---|---|
| Instructions | This questionnaire was designed to grade the comfort of your eyes. For each question, please circle your answer. Example: In the last week, how often were your eyes red? (Never) 0, 1, 2, 3, 4, ⑤, 6 (Always) There are no right or wrong answers. Do not spend too long on any one question. | Este cuestionario está diseñado para medir el confort de sus ojos. En cada pregunta, por favor, rodee su respuesta. Ejemplo: En la última semana, ¿con qué frecuencia estuvieron tus ojos rojos? (Nunca) 0, 1, 2, 3, 4, 5, ⑥ (Siempre) No hay respuestas correctas o incorrectas. No tarde mucho tiempo en responder ninguna. |
| 1 | In the last week, how often did your eyes feel dry? | En la última semana, ¿con qué frecuencia sintió sequedad en los ojos? |
| 2 | When your eyes felt dry, typically, how intense was the dryness? | Cuando sintió los ojos secos ¿cómo era de intensa la sensación de sequedad normalmente? |
| 3 | In the last week, how often did your eyes feel gritty? | En la última semana, ¿con qué frecuencia sintió sensación de arenilla en los ojos? |
| 4 | When your eyes felt gritty, typically, how intense was the grittiness? | Cuando sintió sensación de arenilla en los ojos ¿cómo era de intensa la sensación normalmente? |
| 5 | In the last week, how often did your eyes feel gritty? | En la última semana, ¿con qué frecuencia sintió sensación de arenilla en los ojos? |
| 6 | When your eyes stung, typically, how intense was the stinging? | Cuando sintió ardor o escozor en los ojos ¿Cómo era de intenso normalmente? |
| 7 | In the last week, how often did your eyes feel tired? | En la última semana, ¿con qué frecuencia notó los ojos cansados? |
| 8 | When your eyes felt tired, typically, how intense was the tiredness? | Cuando sintió los ojos cansados ¿Cómo de intenso era el cansancio normalmente? |
| 9 | In the last week, how often did your eyes feel painful? | En la última semana, ¿con qué frecuencia sintió dolor en los ojos? |
| 10 | When your eyes felt painful, typically, how intense was the pain? | Cuando sintió dolor en los ojos ¿Cómo de intenso era normalmente? |
| 11 | In the last week, how often did your eyes itch? | En la última semana, ¿con qué frecuencia le picaron los ojos? |
| 12 | When your eyes itched, typically, how intense was the itching? | Cuando le picaron los ojos ¿Cómo de intenso era el picor normalmente? |
| Response | Never (0), 1, 2, 3, 4, 5 (6) Always Never had it (0), 1, 2 3, 4, 5 (6) Severe | Nunca (0), 1, 2, 3, 4, 5 (6) Siempre Nunca lo he tenido (0), 1, 2 3, 4, 5 (6) Muy intenso |
| Item Number | Item | Infit (MNSQ) | Outfit (MNSQ) | Measure (Logits) |
|---|---|---|---|---|
| 1 | En la última semana, ¿con qué frecuencia sintió sequedad en los ojos? | 1.00 | 0.99 | −0.27 |
| 2 | Cuando sintió los ojos secos ¿cómo era de intensa la sensación de sequedad normalmente? | 0.77 | 0.75 | −0.18 |
| 3 | En la última semana, ¿con qué frecuencia sintió sensación de arenilla en los ojos? | 1.40 | 1.16 | 1.00 |
| 4 | Cuando sintió sensación de arenilla en los ojos ¿cómo era de intensa la sensación normalmente? | 1.28 | 1.12 | 0.97 |
| 5 | En la última semana, ¿con qué frecuencia sintió ardor o escozor en los ojos? | 0.87 | 0.82 | 0.01 |
| 6 | Cuando sintió ardor o escozor en los ojos ¿Cómo era de intenso normalmente? | 0.80 | 0.77 | 0.03 |
| 7 | En la última semana, ¿con qué frecuencia notó los ojos cansados? | 0.91 | 0.96 | −1.29 |
| 8 | Cuando sintió los ojos cansados ¿Cómo de intenso era el cansancio normalmente? | 0.82 | 0.88 | −1.04 |
| 9 | En la última semana, ¿con qué frecuencia sintió dolor en los ojos? | 1.19 | 1.03 | 0.66 |
| 10 | Cuando sintió dolor en los ojos ¿Cómo de intenso era normalmente? | 1.16 | 1.08 | 0.63 |
| 11 | En la última semana, ¿con qué frecuencia le picaron los ojos? | 1.08 | 1.04 | −0.37 |
| 12 | Cuando le picaron los ojos ¿Cómo de intenso era el picor normalmente? | 0.91 | 0.91 | −0.15 |
| OCIVE Levels of Performance | |||||||
|---|---|---|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | Level 6 | Level 7 | |
| Score range | ≥0 to ≤29 | >29 to ≤36 | >36 to ≤43 | >43 to ≤51 | >51 to ≤59 | >59 to ≤70 | >70 to ≤100 |
| Parameter | Rasch Model Expectation | OCIVE |
|---|---|---|
| Number of items | - | 12 |
| Person separation index PSI (reliability) | >2.0 (>0.80) | 3.42 (0.90) |
| PCA. Raw variance explained by measure | >50 | 67.3 |
| Number of items with infit outside range 0.7 to 1.3 | 0 | 1 |
| Number of items with outfit outside range 0.7 to 1.3 | 0 | 0 |
| Number of items with DIF-gender > 0.5 logits | 0 | 0 |
| Number of items with DIF-presbyopia > 0.5 logits | 0 | 2 |
| Targeting | ≥−1.0 | −1.15 |
| Parameter | OCIVE | OCI |
|---|---|---|
| Number of items | 12 | 12 |
| Person separation index PSI (reliability) | 3.42 (0.90) | 3.22 (0.91) |
| PCA. Raw variance explained by measure | 67.3% | 65% |
| Number of items with infit outside range 0.7 to 1.3 | 1 | 0 |
| Number of items with outfit outside range 0.7 to 1.3 | 0 | 1 |
| Number of items with DIF-gender > 0.5 logits | 0 | 0 |
| Number of items with DIF-presbyopia > 0.5 logits | 2 | 0 |
| Targeting | 12 | 12 |
| Item Number | Item | Dry Eye (n = 53) | No Dry Eye (n = 53) | p-Value |
|---|---|---|---|---|
| 1. | Dryness-Frequency | 5 (2) | 1 (3) | |
| 2. | Dryness-Intensity | 5 (1) | 2 (2) | |
| 3. | Grittiness-Frequency | 4 (3) | 0 (2) | |
| 4. | Grittiness-Intensity | 4 (2) | 0 (2) | |
| 5. | Eye stinging-Frequency | 4 (3) | 1 (2) | <0.001 |
| 6. | Eye stinging-Intensity | 4 (2) | 1 (3) | |
| 7. | Eye tiredness-Frequency | 5 (2) | 2 (1) | |
| 8. | Eye tiredness-Intensity | 5 (2) | 2 (2) | |
| 9. | Eye pain-Frequency | 2 (3) | 0 (2) | |
| 10. | Eye pain-Intensity | 4 (4) | 0 (2) | |
| 11. | Eye itchiness-Frequency | 4 (3) | 2 (2) | |
| 12. | Eye itchiness-Intensity | 3 (3) | 2 (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrio, A.R.; González-Pérez, M.; Heredia-Pastor, C.; Enríquez-Fuentes, J.; Antona, B. Spanish Cross-Cultural Adaptation, Rasch Analysis and Validation of the Ocular Comfort Index (OCI) Questionnaire. Int. J. Environ. Res. Public Health 2022, 19, 15142. https://doi.org/10.3390/ijerph192215142
Barrio AR, González-Pérez M, Heredia-Pastor C, Enríquez-Fuentes J, Antona B. Spanish Cross-Cultural Adaptation, Rasch Analysis and Validation of the Ocular Comfort Index (OCI) Questionnaire. International Journal of Environmental Research and Public Health. 2022; 19(22):15142. https://doi.org/10.3390/ijerph192215142
Chicago/Turabian StyleBarrio, Ana Rosa, Mariano González-Pérez, Clara Heredia-Pastor, Jacobo Enríquez-Fuentes, and Beatriz Antona. 2022. "Spanish Cross-Cultural Adaptation, Rasch Analysis and Validation of the Ocular Comfort Index (OCI) Questionnaire" International Journal of Environmental Research and Public Health 19, no. 22: 15142. https://doi.org/10.3390/ijerph192215142
APA StyleBarrio, A. R., González-Pérez, M., Heredia-Pastor, C., Enríquez-Fuentes, J., & Antona, B. (2022). Spanish Cross-Cultural Adaptation, Rasch Analysis and Validation of the Ocular Comfort Index (OCI) Questionnaire. International Journal of Environmental Research and Public Health, 19(22), 15142. https://doi.org/10.3390/ijerph192215142







