Impact of Physical Activity on the Characteristics and Metabolic Consequences of Alcohol Consumption: A Cross-Sectional Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Data Sources and Participants
- Sedentary activity, less than 15 min per day or no activity (681 men, 303 women);
- Low activity, 15–45 min of physical activity per day (917 men, 676 women);
- Moderate activity, 45–90 min of physical activity per day (5495 men, 5971 women);
- Vigorous activity, 90–120 min of physical activity per day (1814 men, 3033 women);
- Extreme activity, over 120 min of physical activity per day (1033 men, 1127 women).
2.2. Laboratory Analyses
2.3. Statistical Methods
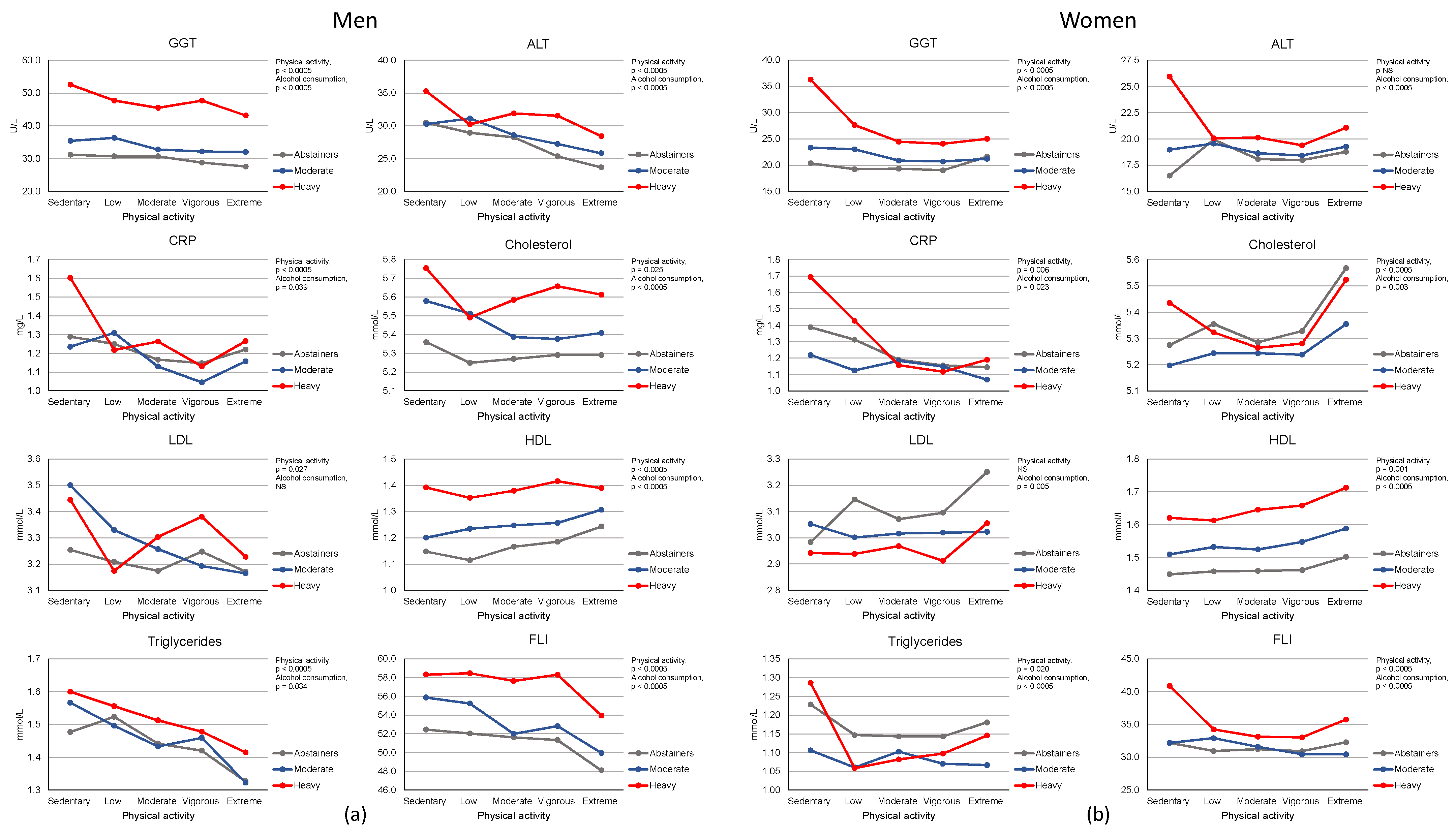
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Behrens, G.; Fischer, B.; Kohler, S.; Park, Y.; Hollenbeck, A.R.; Leitzmann, M.F. Healthy lifestyle behaviors and decreased risk of mortality in a large prospective study of U.S. women and men. Eur. J. Epidemiol. 2013, 28, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, A.; Wang, D.D.; Liu, X.; Dhana, K.; Franco, O.H.; Kaptoge, S.; Di, A.E.; Stampfer, M.; Willett, W.C.; et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation 2018, 138, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Rutten-Jacobs, L.C.; Larsson, S.C.; Malik, R.; Rannikmae, K.; Sudlow, C.L.; Dichgans, M.; Markus, H.S.; Traylor, M. Genetic risk, incident stroke, and the benefits of adhering to a healthy lifestyle: Cohort study of 306 473 UK Biobank participants. BMJ 2018, 363, k4168. [Google Scholar] [CrossRef] [PubMed]
- Nivukoski, U.; Niemelä, M.; Bloigu, A.; Bloigu, R.; Aalto, M.; Laatikainen, T.; Niemelä, O. Impacts of unfavourable lifestyle factors on biomarkers of liver function, inflammation and lipid status. PLoS ONE 2019, 14, e0218463. [Google Scholar] [CrossRef]
- McGinnis, M.; Williams-Russo, P.; Knickman, J.R. The case for more active policy attention to health promotion. Health Affairs 2002, 21, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Tamakoshi, A.; Tamakoshi, K.; Lin, Y.; Yagyu, K.; Kikuchi, S.; JACC Study Group. Healthy lifestyle and preventable death: Findings from the Japan Collaborative Cohort (JACC) study. Prev. Med. 2009, 48, 486–492. [Google Scholar] [CrossRef]
- Sundberg, C.J. Physical activity: What is already being done and how we can avert 1 million deaths annually in future. Br. J. Sports Med. 2016, 50, 319. [Google Scholar] [CrossRef][Green Version]
- Warburton, D.E.; Bredin, S.S. Reflections on physical activity and health: What should we recommend? Can. J. Cardiol. 2016, 32, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Liu, D.; Han, S.; Zhou, C. The influence of physical exercise frequency and intensity on individual entrepreneurial behavior: Evidence from China. Int. J. Environ. Res. Public Health 2022, 19, 12383. [Google Scholar] [CrossRef] [PubMed]
- Beavers, K.M.; Brinkley, T.E.; Nicklas, B.J. Effect of exercise training on chronic inflammation. Clin. Chim. Acta 2010, 411, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory effects of high and moderate intensity exercise-A systematic review. Front. Physiol. 2019, 10, 1550. [Google Scholar] [CrossRef]
- Eijsvogels, T.M.; Molossi, S.; Lee, D.C.; Emery, M.S.; Thompson, P.D. Exercise at the extremes: The amount of exercise to reduce cardiovascular events. J. Am. Coll. Cardiol. 2016, 67, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.G.; Øktedalen, O.; Opstad, P.K.; Lyberg, T. Plasma cytokine profiles in long-term strenuous exercise. J. Sports Med. (Hindawi. Publ. Corp.) 2016, 2016, 7186137. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Schnohr, P.; O’Keefe, J.H.; Marott, J.L.; Lange, P.; Jensen, G.B. Dose of jogging and long-term mortality: The Copenhagen City Heart Study. J. Am. Coll. Cardiol. 2015, 65, 411–419. [Google Scholar] [CrossRef]
- Kim, W.R.; Flamm, S.L.; Di Bisceglie, A.M.; Bodenheimer, H.C.; Public Policy Committee of the American Association for the Study of Liver Disease. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 2008, 47, 1363–1370. [Google Scholar] [CrossRef]
- Lau, K.; Baumeister, S.E.; Lieb, W.; Meffert, P.J.; Lerch, M.M.; Mayerle, J.; Volzke, H. The combined effects of alcohol consumption and body mass index on hepatic steatosis in a general population sample of European men and women. Aliment. Pharmacol. Ther. 2015, 41, 467–476. [Google Scholar] [CrossRef]
- Niemelä, O.; Niemelä, M.; Bloigu, R.; Aalto, M.; Laatikainen, T. Where should the safe limits of alcohol consumption stand in light of liver enzyme abnormalities in alcohol consumers? PLoS ONE 2017, 12, e0188574. [Google Scholar] [CrossRef]
- Ruhl, C.E.; Everhart, J.E. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology 2009, 136, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Åberg, F.; Luukkonen, P.K.; But, A.; Salomaa, V.; Britton, A.; Petersen, K.M.; Bojesen, S.E.; Balling, M.; Nordestgaard, B.G.; Puukka, P.; et al. Development and validation of a model to predict incident chronic liver disease in the general population: The CLivD score. J. Hepatol. 2022, 77, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Kim, W.R.; Benson, J.T.; Therneau, T.M.; Melton, L.J., III. Serum aminotransferase activity and mortality risk in a United States community. Hepatology 2008, 47, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Ruttmann, E.; Brant, L.J.; Concin, H.; Diem, G.; Rapp, K.; Ulmer, H.; Vorarlberg Health Monitoring and Promotion Program Study Group. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: An epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation 2005, 112, 2130–2137. [Google Scholar] [CrossRef]
- Borodulin, K.; Tolonen, H.; Jousilahti, P.; Jula, A.; Juolevi, A.; Koskinen, S.; Kuulasmaa, K.; Laatikainen, T.; Männistö, S.; Peltonen, M.; et al. Cohort Profile: The National FINRISK Study. Int. J. Epidemiol. 2018, 47, 696-i. [Google Scholar] [CrossRef]
- WHO MONICA Project Principal Investigators. The World Health Organization MONICA Project (Monitoring trends and determinants in cardiovascular disease): A major international collaboration. J Clin Epidemiol. 1988, 41, 105–114. [Google Scholar] [CrossRef]
- Luepker, R.V.; Evans, A.; McKeigue, P.; Srinath Reddy, K. Cardiovascular Survey Methods; World Health Organization: Geneva, Switzerland, 2004; Available online: https://apps.who.int/iris/handle/10665/42569 (accessed on 15 October 2022).
- Nivukoski, U.; Bloigu, A.; Bloigu, R.; Aalto, M.; Laatikainen, T.; Niemelä, O. Liver enzymes in alcohol consumers with or without binge drinking. Alcohol 2019, 78, 13–19. [Google Scholar] [CrossRef]
- Alcohol Research: Current Reviews Editorial Staff. Drinking patterns and their definitions. Alcohol Res. 2018, 39, 17–18. [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism. NIAAA Council Approves Definition of Binge Drinking. NIAAA Newsl. 2004, 3, 3. Available online: https://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf (accessed on 18 October 2022).
- World Health Organization. International Guide for Monitoring Alcohol Consumption and Related Harm; World Health Organization: Geneva, Switzerland, 2000; Available online: https://apps.who.int/iris/handle/10665/66529 (accessed on 18 October 2022).
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Nivukoski, U.; Niemelä, M.; Bloigu, A.; Bloigu, R.; Aalto, M.; Laatikainen, T.; Niemelä, O. Combined effects of lifestyle risk factors on fatty liver index. BMC Gastroenterol. 2020, 20, 109. [Google Scholar] [CrossRef] [PubMed]
- Kyu, H.H.; Bachman, V.F.; Alexander, L.T.; Mumford, J.E.; Afshin, A.; Estep, K.; Veerman, J.L.; Delwiche, K.; Iannarone, M.L.; Moyer, M.L.; et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: Systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ 2016, 354, i3857. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef]
- Smith, A.D.; Crippa, A.; Woodcock, J.; Brage, S. Physical activity and incident type 2 diabetes mellitus: A systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia 2016, 59, 2527–2545. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, N.; Kojima, N.; Osuka, Y.; Sasai, H. Factors associated with passive sedentary behavior among community-dwelling older women with and without knee osteoarthritis: The Otassha Study. Int. J. Environ. Res. Public Health 2022, 19, 13765. [Google Scholar] [CrossRef]
- Hu, S.; Tucker, L.; Wu, C.; Yang, L. Beneficial effects of exercise on depression and anxiety during the Covid-19 pandemic: A narrative review. Front. Psychiatry 2020, 11, 587557. [Google Scholar] [CrossRef]
- Connor, J.P.; Haber, P.S.; Hall, W.D. Alcohol use disorders. Lancet 2016, 387, 988–998. [Google Scholar] [CrossRef]
- Holahan, C.J.; Holahan, C.K.; Moos, R.H. Binge drinking and alcohol problems among moderate average-level drinkers. Am. J. Prev. Med. 2022, 63, 324–330. [Google Scholar] [CrossRef]
- Niemelä, O.; Aalto, M.; Bloigu, A.; Bloigu, R.; Halkola, A.S.; Laatikainen, T. Alcohol drinking patterns and laboratory indices of health: Does type of alcohol preferred make a difference? Nutrients 2022, 14, 4529. [Google Scholar] [CrossRef]
- Borodulin, K.; Tuomilehto, J.; Peltonen, M.; Lakka, T.A.; Sundvall, J.; Jousilahti, P. Association of leisure time physical activity and abdominal obesity with fasting serum insulin and 2-h postchallenge plasma glucose levels. Diabet. Med. 2006, 23, 1025–1028. [Google Scholar] [CrossRef]
- Oh, S.; Shida, T.; Yamagishi, K.; Tanaka, K.; So, R.; Tsujimoto, T.; Shoda, J. Moderate to vigorous physical activity volume is an important factor for managing nonalcoholic fatty liver disease: A retrospective study. Hepatology 2015, 61, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Zaccardi, F.; Davies, M.J.; Khunti, K.; Yates, T. Comparative relevance of physical fitness and adiposity on life expectancy: A UK Biobank observational study. Mayo Clin. Proc. 2019, 94, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, M.; Vancampfort, D.; Schuch, F.; Lundin, A.; Stubbs, B. More reasons to move: Exercise in the treatment of alcohol use disorders. Front. Psychiatry 2017, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Sattar, N.; Smith, G.D.; Ebrahim, S. The associations of physical activity and adiposity with alanine aminotransferase and gamma-glutamyltransferase. Am. J. Epidemiol. 2005, 161, 1081–1088. [Google Scholar] [CrossRef]
- Perreault, K.; Bauman, A.; Johnson, N.; Britton, A.; Rangul, V.; Stamatakis, E. Does physical activity moderate the association between alcohol drinking and all-cause, cancer and cardiovascular diseases mortality? A pooled analysis of eight British population cohorts. Br. J. Sports Med. 2017, 51, 651–657. [Google Scholar] [CrossRef]
- Pälve, K.S.; Pahkala, K.; Magnussen, C.G.; Koivistoinen, T.; Juonala, M.; Kähönen, M.; Lehtimäki, T.; Rönnemaa, T.; Viikari, J.S.; Raitakari, O.T. Association of physical activity in childhood and early adulthood with carotid artery elasticity 21 years later: The cardiovascular risk in Young Finns Study. J. Am. Heart Assoc. 2014, 3, e000594. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Tapper, E.B.; Parikh, N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: Observational study. BMJ 2018, 362, k2817. [Google Scholar] [CrossRef]
- Breitling, L.P.; Raum, E.; Muller, H.; Rothenbacher, D.; Brenner, H. Synergism between smoking and alcohol consumption with respect to serum gamma-glutamyltransferase. Hepatology 2009, 49, 802–808. [Google Scholar] [CrossRef]
- Park, E.Y.; Lim, M.K.; Oh, J.K.; Cho, H.; Bae, M.J.; Yun, E.H.; Kim, D.I.; Shin, H.R. Independent and supra-additive effects of alcohol consumption, cigarette smoking, and metabolic syndrome on the elevation of serum liver enzyme levels. PLoS ONE 2013, 8, e63439. [Google Scholar] [CrossRef]
- Zheng, J.S.; Sharp, S.J.; Imamura, F.; Koulman, A.; Schulze, M.B.; Ye, Z.; Griffin, J.; Guevara, M.; Huerta, J.M.; Kröger, J.; et al. Association between plasma phospholipid saturated fatty acids and metabolic markers of lipid, hepatic, inflammation and glycaemic pathways in eight European countries: A cross-sectional analysis in the EPIC-InterAct study. BMC Med. 2017, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Hüsing, A.; Kaaks, R. Lifestyle risk factors and residual life expectancy at age 40: A German cohort study. BMC Med. 2014, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Manuel, D.G.; Perez, R.; Sanmartin, C.; Taljaard, M.; Hennessy, D.; Wilson, K.; Tanuseputro, P.; Manson, H.; Bennett, C.; Tuna, M.; et al. Measuring burden of unhealthy behaviours using a multivariable predictive approach: Life expectancy lost in Canada attributable to smoking, alcohol, physical inactivity, and diet. PLoS Med. 2016, 13, e1002082. [Google Scholar] [CrossRef] [PubMed]
- Teeriniemi, A.M.; Salonurmi, T.; Jokelainen, T.; Vähänikkilä, H.; Alahäivälä, T.; Karppinen, P.; Enwald, H.; Huotari, M.L.; Laitinen, J.; Oinas-Kukkonen, H.; et al. A randomized clinical trial of the effectiveness of a Web-based health behaviour change support system and group lifestyle counselling on body weight loss in overweight and obese subjects: 2-year outcomes. J. Intern. Med. 2018, 284, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Shirazi, L.; Endler, G.; Winkler, S.; Schickbauer, T.; Wagner, O.; Marsik, C. Gamma glutamyltransferase and long-term survival: Is it just the liver? Clin. Chem. 2007, 53, 940–946. [Google Scholar] [CrossRef]
- Niemelä, O. Biomarker-based approaches for assessing alcohol use disorders. Int. J. Environ. Res. Public Health 2016, 13, 166. [Google Scholar] [CrossRef]
- Kozakova, M.; Palombo, C.; Eng, M.P.; Dekker, J.; Flyvbjerg, A.; Mitrakou, A.; Gastaldelli, A.; Ferrannini, E. Fatty liver index, gamma-glutamyltransferase, and early carotid plaques. Hepatology 2012, 55, 1406–1415. [Google Scholar] [CrossRef]
- Mascaró, C.M.; Bouzas, C.; Montemayor, S.; García, S.; Mateos, D.; Casares, M.; Gómez, C.; Ugarriza, L.; Borràs, P.A.; Martinez, J.A.; et al. Impact of physical activity differences due to COVID-19 pandemic lockdown on non-alcoholic fatty liver parameters in adults with metabolic syndrome. Nutrients 2022, 14, 2370. [Google Scholar] [CrossRef]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Light alcohol drinking and cancer: A meta-analysis. Ann. Oncol. 2013, 24, 301–308. [Google Scholar] [CrossRef]
- Cao, Y.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J.; Giovannucci, E.L. Light to moderate intake of alcohol, drinking patterns, and risk of cancer: Results from two prospective US cohort studies. BMJ 2015, 351, h4238. [Google Scholar] [CrossRef]
- Choi, Y.J.; Myung, S.K.; Lee, J.H. Light alcohol drinking and risk of cancer: A meta-analysis of cohort studies. Cancer Res. Treat. 2018, 50, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Schwarzinger, M.; Pollock, B.G.; Hasan, O.S.M.; Dufouil, C.; Rehm, J. Contribution of alcohol use disorders to the burden of dementia in France 2008–2013: A nationwide retrospective cohort study. Lancet Public Health 2018, 3, e124–e132. [Google Scholar] [CrossRef]
- Topiwala, A.; Allan, C.L.; Valkanova, V.; Zsoldos, E.; Filippini, N.; Sexton, C.; Mahmood, A.; Fooks, P.; Singh-Manoux, A.; Mackay, C.E.; et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: Longitudinal cohort study. BMJ 2017, 357, j2353. [Google Scholar] [CrossRef] [PubMed]
- Catena, C.; Colussi, G.; Verheyen, N.D.; Novello, M.; Fagotto, V.; Soardo, G.; Sechi, L.A. Moderate alcohol consumption is associated with left ventricular diastolic dysfunction in nonalcoholic hypertensive patients. Hypertension 2016, 68, 1208–1216. [Google Scholar] [CrossRef][Green Version]
- Klatsky, A.L. Alcohol and cardiovascular diseases: Where do we stand today? J. Intern. Med. 2015, 278, 238–250. [Google Scholar] [CrossRef]
- McManus, D.D.; Yin, X.; Gladstone, R.; Vittinghoff, E.; Vasan, R.S.; Larson, M.G.; Benjamin, E.J.; Marcus, G.M. Alcohol consumption, left atrial diameter, and atrial fibrillation. J. Am. Heart Assoc. 2016, 5, e004060. [Google Scholar] [CrossRef]
- Sipilä, P.; Rose, R.J.; Kaprio, J. Drinking and mortality: Long-term follow-up of drinking-discordant twin pairs. Addiction 2016, 111, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.M.; Kaptoge, S.; Butterworth, A.S.; Willeit, P.; Warnakula, S.; Bolton, T.; Paige, E.; Paul, D.S.; Sweeting, M.; Burgess, S.; et al. Risk thresholds for alcohol consumption: Combined analysis of individual-participant data for 599,912 current drinkers in 83 prospective studies. Lancet 2018, 391, 1513–1523. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K.; Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: From pathophysiology to practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef]
- Moradi, H.; Streja, E.; Kalantar-Zadeh, K. Serum high density lipoprotein cholesterol level and risk of death: Let’s avoid the extremes. J. Thorac. Dis. 2017, 9, 4849–4852. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Anti-inflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W. C-reactive protein and cardiovascular risk: Will the controversy end after CANTOS? Clin. Chem. 2017, 63, 1897–1898. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic review: Exercise-induced gastrointestinal syndrome-implications for health and intestinal disease. Aliment. Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef]
- Duggal, N.A.; Niemiro, G.; Harridge, S.D.R.; Simpson, R.J.; Lord, J.M. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat. Rev. Immunol. 2019, 19, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Niemelä, M.; Kangastupa, P.; Niemelä, O.; Bloigu, R.; Juvonen, T. Acute changes in inflammatory biomarker levels in recreational runners participating in a marathon or half-marathon. Sports Med. Open 2016, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Neubauer, O.; Walsh, N.P.; Simpson, R.J. Recovery of the immune system after exercise. J. Appl. Physiol 2017, 122, 1077–1087. [Google Scholar] [CrossRef]
- Sellami, M.; Gasmi, M.; Denham, J.; Hayes, L.D.; Stratton, D.; Padulo, J.; Bragazzi, N. Effects of acute and chronic exercise on immunological parameters in the elderly aged: Can physical activity counteract the effects of aging? Front. Immunol. 2018, 9, 2187. [Google Scholar] [CrossRef]
- Ezzatvar, Y.; Ramírez-Vélez, R.; Izquierdo, M.; Garcia-Hermoso, A. Physical activity and risk of infection, severity and mortality of COVID-19: A systematic review and non-linear dose-response meta-analysis of data from 1,853,610 adults. Br. J. Sports Med. 2022, 56, 1188–1193. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet (London, England) 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Del Pozo Cruz, B.; Ahmadi, M.; Naismith, S.L.; Stamatakis, E. Association of daily step count and intensity with incident dementia in 78 430 adults living in the UK. JAMA Neurol. 2022, 79, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
| Physical Activity Level | ||||||
|---|---|---|---|---|---|---|
| Men, N = 9940 | Sedentary | Low | Moderate | Vigorous | Extreme | p-Value * |
| n (%) | 681 (6.9) | 917 (9.2) | 5495 (55.3) | 1814 (18.2) | 1033 (10.4) | |
| Age, years, mean ± SD | 47.5 ± 12.2 | 47.9 ± 12.7 | 47.9 ± 13.1 | 52.2 ± 13.5 | 53.1 ± 14.5 | <0.0005 |
| BMI, kg/m2, mean ± SD | 28.0 ± 4.9 | 27.5 ± 4.6 | 27.0 ± 3.9 | 27.0 ± 3.8 | 26.7 ± 3.6 | <0.0005 |
| Waist circumference, cm, mean ± SD | 98.6 ± 13.6 | 97.3 ± 12.6 | 95.3 ± 11.3 | 95.6 ± 11.1 | 93.7 ± 10.8 | <0.0005 |
| Women, N = 11,110 | ||||||
| n (%) | 303 (2.7) | 676 (6.1) | 5971 (53.7) | 3033 (27.3) | 1127 (10.1) | |
| Age, years, mean ± SD | 47.4 ± 12.0 | 46.8 ± 12.5 | 46.1 ± 12.9 | 48.6 ± 13.5 | 51.1 ± 13.4 | <0.0005 ** |
| BMI, kg/m2, mean ± SD | 27.7 ± 6.1 | 27.7 ± 6.2 | 26.3 ± 5.1 | 26.1 ± 4.6 | 26.2 ± 4.4 | <0.0005 |
| Waist circumference, cm, mean ± SD | 87.4 ± 15.4 | 87.0 ± 14.8 | 83.6 ± 13.0 | 83.4 ± 12.1 | 83.0 ± 11.7 | <0.0005 |
| Physical Activity Level | ||||||
|---|---|---|---|---|---|---|
| Men, N = 9940 | Sedentary | Low | Moderate | Vigorous | Extreme | p-Value * |
| Alcohol intake, g/day, mean ± SD | 18.9 ± 28.4 | 15.3 ± 22.5 | 13.2 ± 16.9 | 11.3 ± 15.3 | 10.6 ± 15.5 | <0.0005 |
| Average level of drinking | ||||||
| abstainers, total 2721 | 194 (28.5%) | 277 (30.2%) | 1410 (25.7%) | 500 (27.6%) | 340 (32.9%) | 0.075 |
| moderate, total 5529 | 298 (43.8%) | 449 (49.0%) | 3135 (57.1%) | 1079 (59.5%) | 568 (55.0%) | <0.0005 |
| heavy, total 1690 | 189 (27.8%) | 191 (20.8%) | 950 (17.3%) | 235 (13.0%) | 125 (12.1%) | <0.0005 |
| Total 9940 | 681 (100%) | 917 (100%) | 5495 (100%) | 1814 (100%) | 1033 (100%) | |
| Drinking pattern | ||||||
| regular, total 4777 | 268 (55.0%) | 380 (59.4%) | 2685 (65.7%) | 948 (72.1%) | 496 (71.6%) | <0.0005 |
| binge, total 2442 | 219 (45.0%) | 260 (40.6%) | 1400 (34.3%) | 366 (27.9%) | 197 (28.4%) | <0.0005 |
| Total 7219 | 487 (100%) | 640 (100%) | 4085 (100%) | 1314 (100%) | 693 (100%) | |
| Type of alcohol preferred | ||||||
| wine, total 951 | 47 (9.7%) | 67 (10.5%) | 564 (13.8%) | 190 (14.5%) | 83 (12.0%) | 0.057 |
| beer, total 3535 | 249 (51.1%) | 334 (52.2%) | 2014 (49.3%) | 616 (46.9%) | 322 (46.5%) | 0.010 |
| cider/long drink, total 139 | 18 (3.7%) | 15 (2.3%) | 77 (1.9%) | 19 (1.4%) | 10 (1.4%) | 0.003 |
| spirit, total 1311 | 109 (22. %) | 123 (19.2%) | 692 (16.9%) | 240 (18.3%) | 147 (21.2%) | 0.892 |
| mixed, total 1283 | 64 (13.1%) | 101 (15.8%) | 738 (18.1%) | 249 (18.9%) | 131 (18.9%) | 0.004 |
| Total 7219 | 487 (100%) | 640 (100%) | 4085 (100%) | 1314 (100%) | 693 (100%) | |
| Smoking | ||||||
| no, total 6930 | 369 (54.7%) | 538 (59.1%) | 3869 (70.9%) | 1361 (75.2%) | 793 (77.5%) | <0.0005 |
| yes, total 2943 | 306 (45.3%) | 373 (40.9%) | 1586 (29.1%) | 448 (24.8%) | 230 (22.5%) | <0.0005 |
| Total 9873 | 675 (100%) | 911 (100%) | 5455 (100%) | 1809 (100%) | 1023 (100%) | |
| cigarettes/day, mean ± SD | 9.5 ± 13.0 | 7.3 ± 10.8 | 4.4 ± 8.4 | 3.8 ± 8.0 | 3.3 ± 7.3 | <0.0005 |
| Coffee, cups/day, mean ± SD | 5.5 ± 4.2 | 4.9 ± 3.5 | 4.5 ± 3.0 | 4.5 ± 3.0 | 4.5 ± 3.5 | <0.0005 |
| Women, N = 11,110 | ||||||
| Alcohol intake, g/day, mean ± SD | 6.4 ± 11.5 | 6.0 ± 9.7 | 5.3 ± 8.4 | 4.2 ± 6.6 | 4.1 ± 6.6 | <0.0005 |
| Average level of drinking | ||||||
| abstainers, total 4707 | 147 (48.5%) | 303 (44.8%) | 2360 (39.5%) | 1369 (45.1%) | 528 (46.9%) | 0.003 |
| moderate, total 5099 | 107 (35.3%) | 266 (39.3%) | 2844 (47.6%) | 1386 (45.7%) | 496 (44.0%) | 0.179 |
| heavy, total 1304 | 49 (16.2%) | 107 (15.8%) | 767 (12.8%) | 278 (9.2%) | 103 (9.1%) | <0.0005 |
| Total 11,110 | 303 (100%) | 676 (100%) | 5971 (100%) | 3033 (100%) | 1127 (100%) | |
| Drinking pattern | ||||||
| regular, total 5663 | 126 (80.8%) | 313 (83.9%) | 3161 (87.5%) | 1522 (91.5%) | 541 (90.3%) | <0.0005 |
| binge, total 740 | 30 (19.2%) | 60 (16.1%) | 450 (12.5%) | 142 (8.5%) | 58 (9.7%) | <0.0005 |
| Total 6403 | 156 (100%) | 373 (100%) | 3611 (100%) | 1664 (100%) | 599 (100%) | |
| Type of alcohol preferred | ||||||
| wine, total 2217 | 37 (23.7%) | 110 (29.5%) | 1275 (35.3%) | 587 (35.3%) | 208 (34.7%) | 0.037 |
| beer, total 2127 | 72 (46.2%) | 128 (34.3%) | 1198 (33.2%) | 530 (31.9%) | 199 (33.2%) | 0.030 |
| cider/long drink, total 413 | 10 (6.4%) | 30 (8.0%) | 224 (6.2%) | 108 (6.5%) | 41 (6.8%) | 0.979 |
| spirit, total 565 | 21 (13.5%) | 45 (12.1%) | 287 (7.9%) | 151 (9.1%) | 61 (10.2%) | 0.744 |
| mixed, total 1081 | 16 (10.3%) | 60 (16.1%) | 627 (17.4%) | 288 (17.3%) | 90 (15.0%) | 0.731 |
| Total 6403 | 156 (100%) | 373 (100%) | 3611 (100%) | 1664 (100%) | 599 (100%) | |
| Smoking | ||||||
| no, total 8824 | 182 (60.3%) | 480 (71.2%) | 4769 (80.2%) | 2462 (81.6%) | 931 (82.8%) | <0.0005 |
| yes, total 2243 | 120 (39.7%) | 194 (28.8%) | 1179 (19.8%) | 557 (18.4%) | 193 (17.2%) | <0.0005 |
| Total 11,067 | 302 (100%) | 674 (100%) | 5948 (100%) | 3019 (100%) | 1124 (100%) | |
| cigarettes/day, mean ± SD | 6.4 ± 9.4 | 4.1 ± 7.7 | 2.2 ± 5.3 | 2.0 ± 5.0 | 1.9 ± 5.0 | <0.0005 |
| Coffee, cups/day, mean ± SD | 4.1 ± 3.2 | 3.8 ± 2.5 | 3.6 ± 2.5 | 3.8 ± 2.4 | 3.9 ± 2.6 | <0.0005 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niemelä, O.; Bloigu, A.; Bloigu, R.; Halkola, A.S.; Niemelä, M.; Aalto, M.; Laatikainen, T. Impact of Physical Activity on the Characteristics and Metabolic Consequences of Alcohol Consumption: A Cross-Sectional Population-Based Study. Int. J. Environ. Res. Public Health 2022, 19, 15048. https://doi.org/10.3390/ijerph192215048
Niemelä O, Bloigu A, Bloigu R, Halkola AS, Niemelä M, Aalto M, Laatikainen T. Impact of Physical Activity on the Characteristics and Metabolic Consequences of Alcohol Consumption: A Cross-Sectional Population-Based Study. International Journal of Environmental Research and Public Health. 2022; 19(22):15048. https://doi.org/10.3390/ijerph192215048
Chicago/Turabian StyleNiemelä, Onni, Aini Bloigu, Risto Bloigu, Anni S. Halkola, Markus Niemelä, Mauri Aalto, and Tiina Laatikainen. 2022. "Impact of Physical Activity on the Characteristics and Metabolic Consequences of Alcohol Consumption: A Cross-Sectional Population-Based Study" International Journal of Environmental Research and Public Health 19, no. 22: 15048. https://doi.org/10.3390/ijerph192215048
APA StyleNiemelä, O., Bloigu, A., Bloigu, R., Halkola, A. S., Niemelä, M., Aalto, M., & Laatikainen, T. (2022). Impact of Physical Activity on the Characteristics and Metabolic Consequences of Alcohol Consumption: A Cross-Sectional Population-Based Study. International Journal of Environmental Research and Public Health, 19(22), 15048. https://doi.org/10.3390/ijerph192215048





